Abstract
The aim of this study was to understand the polymer degradation and drug release mechanism from PLGA microspheres embedded in a PVA hydrogel. Two types of microspheres were prepared with different molecular weight PLGA polymers (approximately 25 and 7 kDa) to achieve different drug release profiles, with a 9-day lag phase and without a lag phase, respectively. The kinetics of water uptake into the microspheres coincided with the drug release profiles for both formulations. For the 25 kDa microspheres, minimal water uptake was observed in the early part of the lag phase followed by substantial water uptake at the later stages and in the drug release phase. For the 7 kDa microspheres, water uptake occurred simultaneously with drug release. Water uptake was approximately 2–3 times that of the initial microsphere weight for both formulations. The internal structure of the PLGA microspheres was evaluated using low temperature scanning electron microscopy (cryo-SEM). Burst drug release occurred followed by pore forming from the exterior to the core of both microspheres. A well-defined hydrogel/microsphere interface was observed. For the 25 kDa microspheres, internal pore formation and swelling occurred before the second drug release phase. The surface layer of the microspheres remained intact whereas swelling, and degradation of the core continued throughout the drug release period. In addition, microsphere swelling reduced glucose transport through the coatings in PBS media and this was considered to be a as a consequence of the increased thickness of the coatings. The combination of the swelling and microdialysis results provides a fresh understanding on the competing processes affecting molecular transport of bioanalytes (i.e. glucose) through these composite coatings during prolonged exposure in PBS.
Keywords: swelling, release mechanism, heterogeneous degradation, outside-in pore formation, cryo-SEM, glucose diffusion
Graphical abstract
1. Introduction
Poly (lactic-co-glycolic acid) (PLGA) based parenteral formulations are widely used for sustained delivery of various therapeutic entities such as small molecules, peptides as well as proteins [1–3]. There are currently twelve PLGA/PLA based microsphere products available on the market, including Risperdal® Consta®, Sandostatin® LAR, Zoladex®, and Lupron Depot®. This type of formulation is suitable for achieving long-term efficacy with reduced dosing frequency and is typically administered via the intramascular (I.M.) and subcutaneous (S.C.) routes. It is critical to understand the drug release mechanism from microspheres in order to design and develop formulations with controlled release kinetics. Diffusion and degradation/erosion are two main release mechanisms associated with this type of formulation. The initial phase of drug release is usually considered to be controlled by drug diffusion from the surface and the later stage of drug release is associated with degradation and erosion[4]. Hydration takes place with great speed relative to erosion when the microspheres are immersed in an aqueous buffer. Microsphere hydration is followed by polymer chain degradation which occurs throughout the polymer matrix in the form of random hydrolysis and leads to the formation of internal pores [5].
However, the detailed underlying mechanisms/processes of drug release from PLGA microspheres are not yet fully understood. Complex mechanisms/processes have been proposed by researchers regarding degradation and drug release from various types of PLGA microspheres [5, 6]. Heterogeneous bulk degradation was proposed as two distinctive glass transition temperatures were observed in different PLGA microspheres with varied copolymer compositions [7]. Such heterogeneity can be partly attributed to a pH gradient from the interior (low pH) to the surface (high pH) of the microspheres, due to accumulation of hydrolyzed lactic/glycolic acid monomers and oligomers within the microspheres [8, 9]. This occurs as a result of the slower diffusion of lactic/glycolic acid monomers and oligomers compared to buffer components [8]. An often neglected fact is that water diffusion from the outside to the inside (swelling) due to the increased osmotic pressure can lead to a higher degree of dilution of the acidic components close to the surface. Swelling is an important process during PLGA microsphere degradation. Initial microsphere swelling has been reported to form a skin layer on the surface as a result of pore-closing [10] and therefore delay the initial drug release which may cause an apparent lag phase. Initial microsphere swelling has also been reported to cause burst release [11]. Swelling and water uptake in clonidine-loaded PLGA microspheres during the second drug release phase was reported by Gaignaux et al[12]. However, the method used by these researchers which involved measuring filtered wet microspheres may have resulted in significant error due to adsorbed water on the microsphere surfaces. More recent findings have indicated that microsphere swelling with significant volume increase coincides with the onset of the second drug burst release phase [13, 14]. Microsphere swelling is possibly a result of polymer chain relaxation due to elevated temperature and increased osmotic pressure resulting from accumulation of dissolved drug and degradation species [15]. Unfortunately, the water uptake into these microspheres was not reported in these studies.
The microsphere internal structure may significantly affect drug release. In protein loaded microspheres prepared using the double emulsion-solvent evaporation method, pore closing and opening events were observed to affect drug release [10, 16]. In a multi-layer reservoir type microsphere formulation, rupture of the outer layer caused by inner layer swelling was observed and shown to govern drug release [17]. Microsphere structure collapse and particle agglomeration have been reported during the later stages of drug release [18, 19]. While pore formation is generally believed to happen internally, detailed pore morphology has yet to be revealed. Microsphere morphology change during drug release is usually determined via scanning electron microscopy (SEM). To evaluate the internal structure of microspheres, samples are usually incubated for a period of time, then collected, dried and cut/crushed/fractured before SEM imaging [10, 16, 19, 20]. This sample preparation process may create defects which may alter the internal structure of the particles. Accordingly, low temperature scanning electron microscopy (cryo-SEM) may be a better technique to investigate microsphere internal structure since the samples are flash-frozen in liquid nitrogen and then freeze-fractured to maintain the structural characteristics for imaging. This technique has been successfully used for biological samples [21]. To the best of our knowledge, cryo-SEM has never been used to investigate PLGA microsphere internal structure changes.
Dexamethasone containing PLGA microspheres embedded in poly vinyl alcohol (PVA) hydrogels have been developed as composite coatings for subcutaneous implants to inhibit the foreign body reaction [22–27]. The efficacy of these coatings was shown to be dependent upon the drug release profile from the PLGA microspheres [26, 28]. Dexamethasone release from PLGA microspheres can typically be divided into three phases, a burst release phase, a lag phase and a second drug release phase following a bulk degradation mechanism. When embedded in PVA hydrogels, the burst release phase has been shown to be slightly extended due to the diffusional resistance caused by the hydrogel [29]. The lag phase and second drug release phase are mainly controlled by drug release from the microspheres [30]. The PVA hydrogels maintain a neutral pH and are permeable to water and other small molecules. The PVA hydrogel also provides a protective layer to maintain microsphere structure during drug release [20]. Therefore, investigating the composite coating will provide valuable information regarding drug release from PLGA microspheres.
In this study, two different PLGA microsphere formulations with different drug release profiles were prepared. PLGA microsphere/PVA hydrogel composite coatings were evaluated from three aspects: 1) the swelling properties (water uptake) determined as the swelling ratio, 2) internal structure change evaluated using cryo-SEM, and 3) glucose diffusion through the coating investigated using microdialysis. The swelling and internal structure change of the coatings may facilitate understanding of the physicochemical properties of the composites for glucose sensors coating design.
2. Material and Methods
2.1. Materials
Dexamethasone was purchased from Cayman Chemical (Ann Arbor, MI), poly (vinyl alcohol) (PVA, Mw 30–70 KD), sodium chloride (NaCl, ACS grade), sodium azide (NaN3), sodium phosphate dibasic dihydrate (Na2HPO4·2H2O), sodium phosphate monobasic (NaH2PO4) and dimethyl sulfoxide (DMSO) were purchased from Sigma–Aldrich (St. Louis, MO). PVA (99% hydrolyzed, Mw 133 KD) was purchased from Polysciences, Inc. (Warrington, PA). PLGA Resomer® RG503H 5050 (RG503H, with molecular weight approximately 25 kDa) was a gift from Boehringer-Ingelheim. PLGA 5050 DLG1A (DLG1A, with molecular weight approximately 7 kDa) was purchased from Lakeshore Biomaterials (Birmingham, AL). Methylene chloride (DCM), acetonitrile (ACN, HPLC grade), and tetrahydrofuran (THF, HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA). NanopureTM quality water (Barnstead, Dubuque, IA) was used for all studies.
2.2. Methods
2.2.1. Preparation of PLGA microspheres
Dexamethasone loaded microspheres were prepared using an oil-in-water (o/w) emulsion solvent extraction/evaporation technique. Two microsphere formulations were prepared using either RG503H or DLG1A PLGA. 500 mg of PLGA were dissolved in 2 ml of methylene chloride and 50 mg of dexamethasone were dispersed in this solution and these dispersions were sonicated using a bath sonicator for 20 minutes. The dispersions were then further mixed using a bench top homogenizer (T25, IKA Works, Inc., Wilmington, NC) at 10,000 rpm for 1 min. The organic phase was then slowly added to 10 ml of PVA solution (1% (w/v), average Mw 30–70 KDa) and homogenized at 10,000 rpm for 2.5 min. The emulsions were then transferred to 125 ml of an aqueous PVA solution (0.1% (w/v), Mw 30–70 KDa) and stirred at 600 rpm. A vacuum was applied to the aqueous phase for 2.5 hours to evaporate the methylene chloride and harden the microspheres. The hardened microspheres were transferred to 50 mL centrifuge tubes and collected via centrifugation at 1500 rpm for 2 minutes. The microspheres were then washed thrice with deionized water (10 mL each time), collected using the same centrifugation procedure as before and dried using a freeze dryer. The prepared microspheres were stored at 4°C until further use.
2.2.2. Characterization of PLGA microspheres
The microspheres were characterized for drug loading, glass transition temperatures and morphology. The drug loading was evaluated by dissolving approximately 5 mg of microspheres in 10 mL THF and analyzed using HPLC. Briefly, the solution was filtered (Millex® HV, PTFE 0.45 μm syringe filter) and 5 μl samples were injected into the HPLC column. A Perkin Elmer series 200 HPLC system (Shelton, CT) equipped with a UV absorbance detector (240 nm wave length for dexamethasone analysis) was used. Acetonitrile/water/phosphoric acid (35/70/0.5, v/v/v) was used as the mobile phase. A Zobax C18 (4.6 mm × 15 cm, Agilent, Santa Clara, CA) analytical column was used with a flow rate of 1 ml/min. The chromatographs were analyzed by PeakSimple™ Chromatography System (SRI instruments, Torrance, CA). A TA Q1000 differential scanning calorimeter (DSC) (TA, New Castle, DE) was used to determine the glass transition temperature (Tg) of the prepared microspheres. Modulated DSC was performed with the following cycle: the samples were heated at a rate of 3 °C/min from 4°C to 80 °C at a modulating oscillatory frequency of 1°C/min. The thermograms were analyzed using Universal Analysis software (TA Instruments) to determine the glass transition temperature. The morphology of the microspheres was determined using a scanning electron microscopy (a FEI Nova NanoSEM 450 unit). Samples were mounted on carbon taped aluminum stubs and sputter coated with gold for 1.5 min at 6 mA before imaging.
2.2.3. PVA hydrogel composite coatings
The PLGA microsphere/PVA hydrogel composites were prepared using a previously developed freeze-thaw method [28]. Different amounts of PLGA microspheres (0, 50, 75, and 100 mg) were dispersed in 1 mL 5% (w/w) PVA solutions (133 KDa) and the dispersions were filled into 1-mL syringes (BD precision glide). Three freeze–thaw cycles (2 hour at − 20 °C and 1 hour at ambient temperature for each cycle) were applied to the suspension to form the crosslinked PVA hydrogels with PLGA microspheres embedded. The crosslinked composites were then removed from the syringes, air dried and stored in 4 °C for further use.
2.2.4. Coating of microdialysis probes
CMA20 microdialysis probes (Harvard Apparatus, Holliston, MA) with 20,000 molecular weight cutoff, 10 mm polyethersulfone (PES) membranes, were used for microdialysis testing. For coating the probes, the microspheres were dispersed in 5% (w/w) PVA solution (133 KDa) and one freeze-thaw cycle was applied to the dispersion to thicken the gel before coating the microdialysis probes. Teflon tubing (0.047 inch inner diameter) was used to provide a cylindrical mold for coating. Using a syringe, the thickened gel solution was quickly dispensed into the tubing. The microdialysis probe tips (0.5 mm diameter) were inserted in the tubing and 2 additional freeze-thaw cycles were applied. The tubing was removed from the hydrogel-coated microdialysis probes and the coatings were allowed to be air dried and stored in 4 °C for further use. Microdialysis probes coated with PVA hydrogel only were prepared using the same method except that microspheres were not added into the PVA solution.
2.2.5. In vitro release testing
In vitro release testing was performed for the PLGA microsphere/PVA hydrogel (99% hydrolyzed, Mw 133 KD) composite coatings. Approximately 2 mg of each formulation was immersed in a 2 ml Eppendorf vial containing 1.8 ml of 10 mM PBS (pH 7.4) with 0.1% NaN3 and incubated at 37 °C under constant agitation. At pre-determined time points, all the release media was removed and replenished with fresh media. Sink conditions were maintained throughout. The samples were filtered through 0.45 μm syringe filters and the concentration of dexamethasone in each sample was determined using the HPLC method as described above.
2.2.6. Swelling of composite coatings
The swelling characteristics of the composite coatings were obtained by measuring their initial and swollen weights in phosphate buffered solution (10 mM, pH 7.4, PBS). Approximately 5 mg of completely dried samples were weighed (Wd) and immersed into 1.8 mL PBS solutions incubated at 37 °C. At predetermined time points, the samples were then weighed again to obtain the swollen weight (Ws (t)) after being removed from the solutions and carefully dried using kimwipes to absorb any surface water. The degree of swelling was calculated as the swelling ratio using the equation below.
where Wd is the initial weight of dried coating and Ws(t) is the weight of swollen coating measured at the specified incubation time interval (t).
2.2.7. Glucose diffusion through composite coatings
All the microdialysis probes (without coating, with PVA coatings, with PVA/PLGA composite coatings) were incubated in the Franz cell apparatus with 5 mL PBS maintained at 37 °C for 3 hours prior to the experiments to ensure complete hydration of the coatings. The probes were then connected to a syringe pump equipped with a 3-mL syringe filled with PBS. The pumping rate was set at 5 μL/min. After a 30 min equilibration period, 2 mL of 6 mg/mL glucose solution was added into the Franz cells and the perfusion fluid was collected every 6 mins for 30 min. The same test was repeated at pre-determined time points and the microdialysis probes were incubated in PBS maintained at 37 °C between each test. The glucose concentration in the outlet dialysate (Cd) and medium external to the dialysis probe (Ce) were measured using a YSI 2300 Stat Plus Glucose & Lactate analyzer (YSI Inc., Yellow Spring, Ohio). The permeability was determined as relative recovery (RR), which was calculated using the following equation:
where Cd(t) is the glucose concentration in the dialysate and Ce(t) is the glucose concentration in the medium external to the dialysis probe at the specified time points (t).
2.2.8. Low temperature scanning electron microscopy (cryo-SEM)
In order to investigate the internal structure change of composite coatings, cryo-SEM was performed to evaluate the samples after incubation in PBS at 37 °C after specified time points. Samples of ~ 1 mm3 size were mounted onto standard specimen carriers (for the EM VCT100 16BU012098-T holder, Leica Microsystems) surrounded by buffer, and plunge-frozen in liquid nitrogen slush. The samples were freeze-fractured at −140 °C, etched for 2 min at −95°C, and sputter coated with 7 nm thickness of platinum in the cryo-SEM sample prep station (EM MED 020, Leica Microsystems). Samples were then transferred under vacuum to the FEG-SEM (Nova NanoSEM 450, FEI) and imaged at −140 °C (EM VCT100 cryo shuttle and cryo stage, Leica Microsystems).
3. Results
3.1. Characterization of PLGA microspheres
Two types of microspheres were prepared using different polymers as shown in Table 1. DLG1A polymer has lower intrinsic viscosity and is more hydrophilic. The Tg of DLG1A microspheres was approximately 43.3 °C and had a molecular weight of approximately 7 kDa. RG503H has higher intrinsic viscosity and is more hydrophobic. The molecular weight of this polymer is approximately 25 kDa which is higher than that of DLG1A, leading to a higher Tg of approximately 48.2 °C. The Similar drug loading was obtained for both microsphere formulations, around 7.6% (w/w).
Table 1.
Physicochemical properties of the PLGA microsphere formulations
| Formulation | Polymer type | Polymer intrinsic viscosity (dl/g)* | Tg (°C) | Drug loading (w/w) |
|---|---|---|---|---|
| I | 5050DLG1A | ~0.08 | 43.3 | 7.72 ± 0.33% |
| II | 5050RG503H | ~0.4 | 48.2 | 7.63 ± 0.28 % |
Information provided in Analytical Report from Lakeshore Biomaterials
Microsphere morphology was evaluated using SEM (Figure 1). Both formulations presented spherical shaped particles. Some irregular shaped particles were observed on the surface of the DLG1A (low MW) formulations while the surface of microspheres prepared using the RG503H (high MW) was smooth. These particles are considered to be crystalline dexamethasone, which was not encapsulated inside the microspheres. The crystalline nature of these particles was confirmed using polarized light microscopy (data not shown). Surface dexamethasone may lead to higher drug burst release from this formulation.
Figure 1.
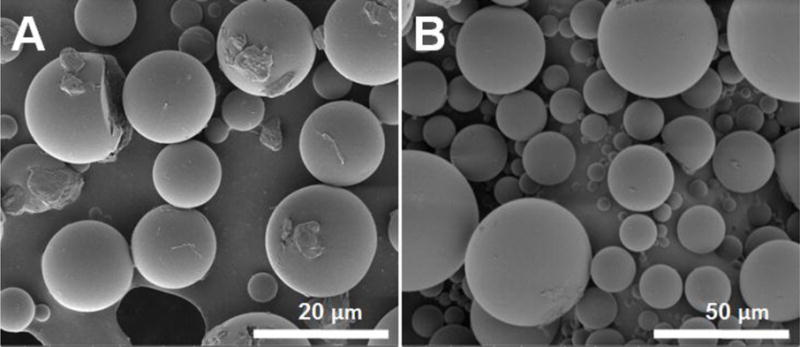
SEM images of PLGA microspheres prepared using DLG1A (A) and RG503H (B)
3.2. In vitro drug release from composite coatings
Release profiles were obtained for composite coatings prepared using the two microsphere formulations (Figure 2). DLG1A based composite coatings exhibited a burst release of approximately 50% at 3 hours, more than 70% of the dexamethasone was released within 24 hours, and drug release was complete within 10 days. For composite coatings prepared using RG503H based microspheres, three release phases were observed including an initial burst release phase, followed by a lag phase with minimal drug release and a secondary zero-order release phase. Approximately 35% of the drug was released during the burst phase within 24 hours. The lag phase lasted for approximately 9 days and the drug release plateaued at approximately 1 month with more than 90% of the loaded drug released.
Figure 2.
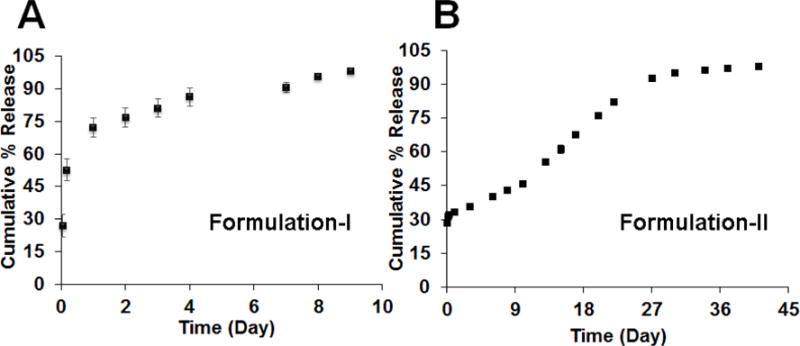
In vitro release profiles of composite coatings prepared using DLG1A (A) and RG503H (B) based microspheres
3.3. Swelling of composite coatings
Swelling properties of the composite coatings prepared with RG503H (high MW) microspheres are shown in Figure 3. The weight change of the samples is plotted in Figure 3-A. There was significant swelling within first few hours. After the initial swelling on the first day, the coatings without microspheres retained similar weight throughout the 45-day testing period. For the coatings with embedded microspheres, following the initial 1-day swelling period the coating weight was maintained for approximately 6 days following which the weight increased significantly until approximately day 24 and then decreased. From the swelling ratio plot shown in Figure 3-B, the composite coatings gained approximately 60% to 100% of their initial weight while the PVA hydrogel alone (no microspheres) gained more than 170% of the initial weight during the first 24 hours. A negative correlation with the amount of microspheres embedded in the hydrogel was observed during the first 24 hours. While the swelling ratio of the PVA hydrogel alone was maintained at around 170% for 45 days, the swelling ratio of composite coatings increased starting from day 9 and reached a maximum after approximately 24 days. The maximum swelling ratio for the composite coatings ranged from 220% to 280% with a positive correlation with the amount of microspheres embedded.
Figure 3.
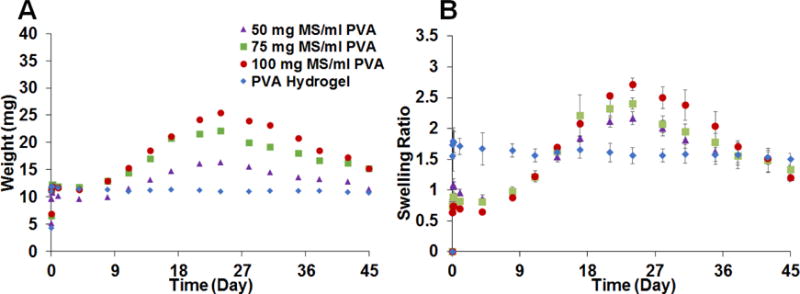
Swelling of PLGA microsphere/PVA hydrogel composite coatings prepared using RG503H based microspheres. The weight change of the samples is shown in A (n=3) and the swelling ratio change is shown in B (n=3). The data is presented as average ± SD for the swelling ratio.
The swelling properties of the composite coatings prepared with DLG1A microspheres (low MW) are shown in Figure 4. The coatings loaded with the DLG1A microspheres continued to gain weight after incubation and reached a maximum at around 13 days. After day 13, the weight started to decrease during the testing period. As was the case for the coatings loaded with RG503H microspheres, these composite coatings gained approximately 60% to 100% of their initial weight within the first 24 hours. The maximum swelling ratio of these formulations occurred at day 13 and ranged from approximately 200% to 250% for the various microsphere concentrations. A positive correlation between the maximum swelling ratio and the concentration of microspheres embedded in the coating was observed.
Figure 4.
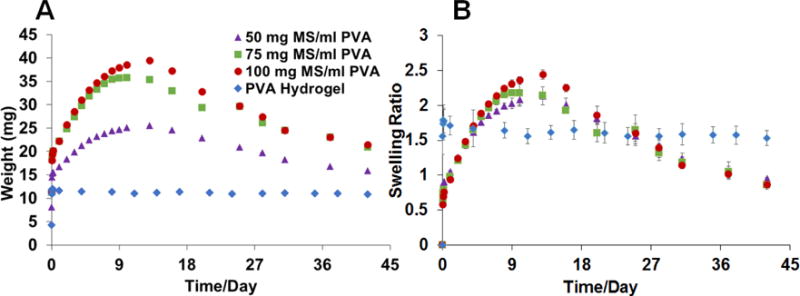
Swelling of PLGA microsphere/PVA hydrogel composite coatings prepared using DLG1A based microspheres. The weight change of the samples is shown in 4 (n=3) and swelling ratio change is shown in 4 (n=3). The data is presented as average ± SD for the swelling ratio.
3.4. Glucose diffusion through composite coatings
RR of glucose from the microdialysis probes is an indication of glucose permeability through various coatings (as shown in Figure 5). The RR of glucose through uncoated microdialysis probes and PVA hydrogel coated probes were maintained at approximately 37% and 21%, respectively over the testing period. When coatings containing embedded microspheres (prepared with the RG503H polymer) were applied to the probes, the RR decreased with increase in the microsphere concentration. For those coatings loaded with PLGA microspheres, the RR decreased initially following incubation and reached a minimum at approximately 24 days and then started to increase. The lowest RR (~5%) was observed for the 100 mg MS/ml PVA formulation at day 24.
Figure 5.
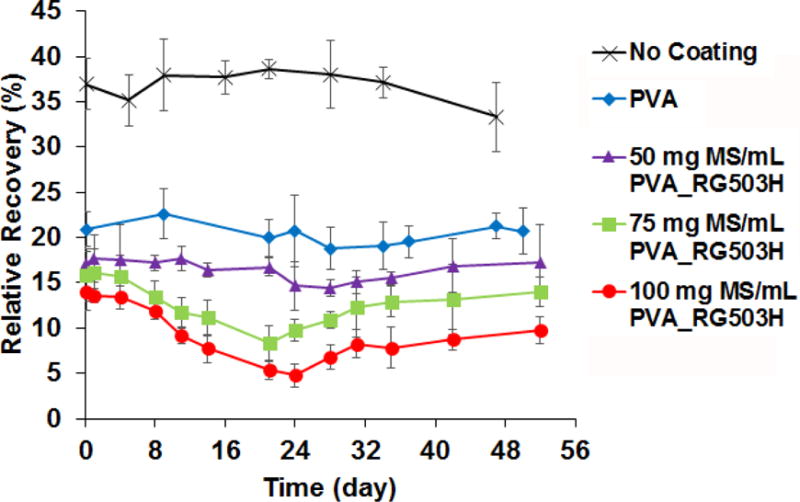
Effect of different types of coating and incubation time on glucose relative recovery obtained using microdialysis probes (n=3 for each time point). Microspheres used were prepared using the RG503H polymer.
In order to further investigate the effect of microsphere swelling and degradation on glucose diffusion through the composite coating, glucose RR was also tested for coatings prepared using DLG1A microspheres (MS) following different incubation periods. Similar results to those for the coatings embedded with the RG503H microspheres were obtained, except that the time scale was faster, as shown in Figure 6. The RR decreased initially after incubation and reached the lowest point (~5%) at day 16 before it started to increase. When compared to the RR obtained for the composite coatings prepared using the RG503H microspheres, the initial RR decrease for the composites prepared with the DLG1A formulation was more abrupt and the minimum RR was reached earlier (approximately 16 days compared to 24 days).
Figure 6.
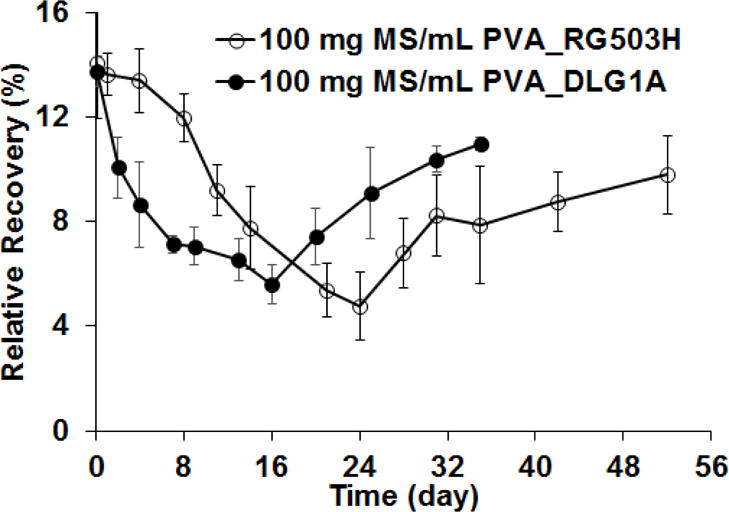
Glucose relative recovery from composite coatings embedded with microspheres (100 mg MS/ml PVA) prepared using RG503H and DLG1A polymers
3.5. Internal pore formation
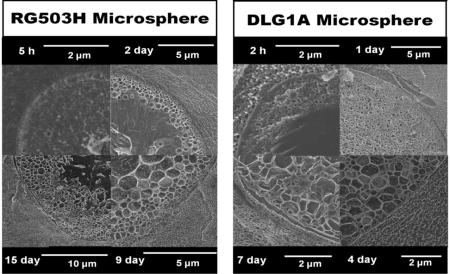
Composite swelling and glucose diffusion characteristic changes are associated with internal structural change in the coatings. Cryo-SEM was performed on DLG1A microsphere based composite coatings after incubation for different time periods. Figure 7 shows the internal structure of the composite coatings following 2-h, 1-day, 3 days and 7 days. Two distinct layers, an external layer with small pores and an internal layer without pores, were observed in the microspheres following 2 hours of incubation (Figure 7-A2). From the highest magnification (~26000X) image (Figure 7-A3), it was determined qualitatively that the pores formed at 2 hours are similar in size to the pores of the PVA hydrogel matrix. Interestingly, a transitional phase was observed between the microspheres and the PVA hydrogel indicating some possible interaction between the two phases (Figure 7-A2). Following 24 hours incubation, pores were observed throughout these microspheres (Figure 7-B2) and the pore size increased with time (comparing Figure 7-B3 with Figure 7-A3). After 3 and 7 days of incubation, increase in particle size was observed in the low magnification images (comparing Figure 7-C1, 7-D1 to Figure 7-A1). It can be observed from the high magnification images that the size of the internal pores continued to increase (Figure 7-C3, 8-D3). The transitional phase between the microspheres and the hydrogel disappeared in the later time points (Figure 7-C3, 8-D3). It is also worth noting that these microspheres rapidly lost their spherical shape even after 2 hours of incubation. During the whole incubation period, no significant changes were observed in the PVA hydrogel structure other than the transitional phase.
Figure 7.
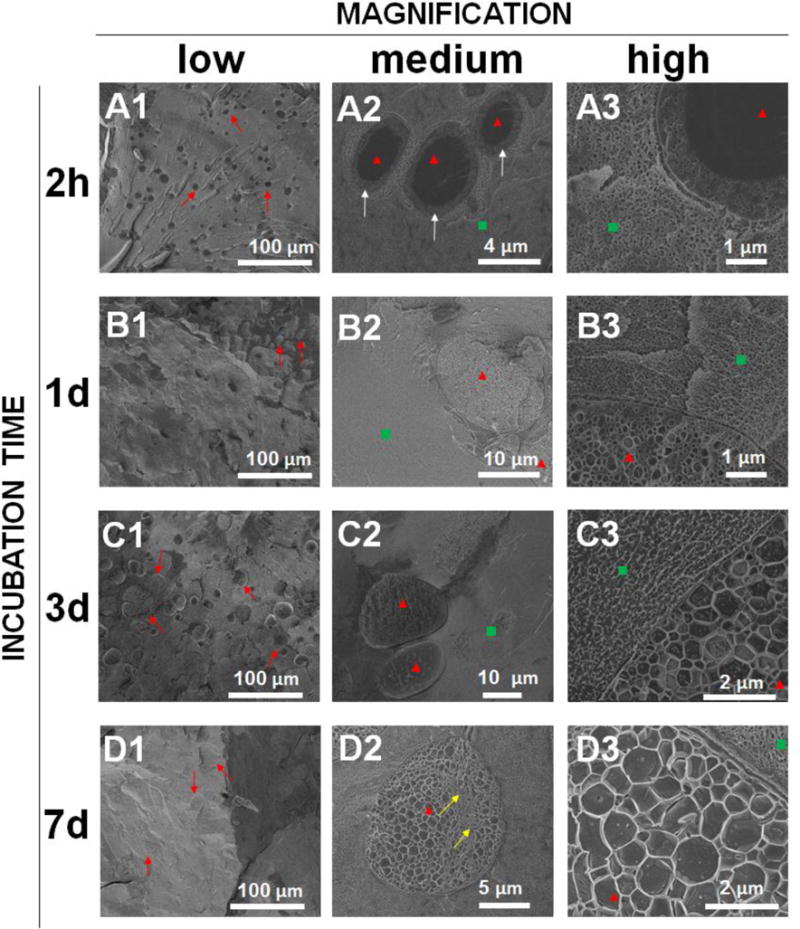
Cryo-SEM images showing the internal structure of the PLGA microsphere/PVA hydrogel composites (prepared using DLG1A polymer) after incubation in phosphate buffer for 2 hours (A1, A2, A3), 1 day (B1, B2, B3), 3 days (C1, C2, C3) and 7 days (D1, D2, D3). Images are provided at low magnification (A1, B1, C1, D1), medium magnification (A2, B2, C2, D2) and high magnification (A3, B3, C3, D3). Red arrows point at the microspheres at low magnification, red triangles point at the microspheres at medium/high magnification, green squares point at the hydrogel at medium/high magnification and white arrows point at the interphase between the microsphere and the PVA hydrogel.
Figure 8.
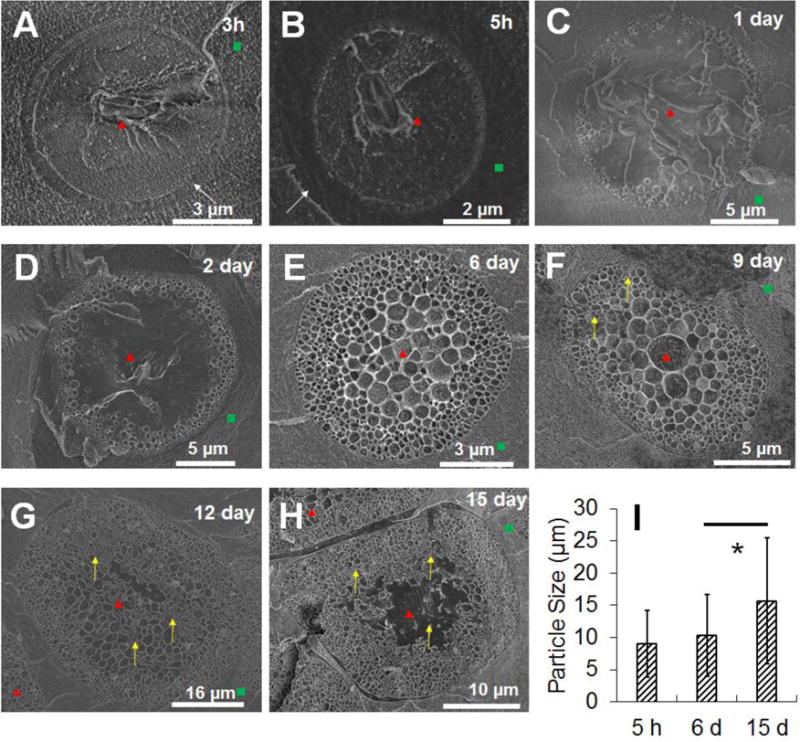
Cryo-SEM images showing the internal structure of the PLGA microsphere/PVA hydrogel composites (prepared using the RG503H polymer) following incubation in phosphate buffer for 3h, 5h, 1 day, 2 days, 4 days, 9 days, 12 days and 15 days (corresponding to A, B, C, D, E, F, G, H, respectively). The red triangles point at the microspheres, the green squares point at the hydrogel, the white arrows point at the interphase between microsphere and PVA hydrogel, and the yellow arrows point at the deformation/collapse of the porous structure. I shows particle size change over time obtained by analyzing low magnification images (approximately 1000× data not shown) following 5h, 6 day and 15 day incubation. Approximately 100 particles were analyzed for each image.* indicates statistical significance analyzed using paired student t-test (p<0.001)
Cryo-SEM was also performed on PLGA microsphere/PVA hydrogel composites prepared using the RG503H polymer (Figure 8). Pores started to present close to the surface of the microspheres following 5 hours incubation. The pores grew in a pattern from the outside to the inside with small pores located close to the exterior and larger pores in the center of the microspheres. By day 6, the interior of the microspheres was filled with pores. The pore size increased from day 6 to day 9 (from approximately 240±138 nm to approximately 367±197 nm). Minor internal structure deformation was observed at day 9 (Figure 8-F). Starting from day 12, internal structure collapse was observed from the interior to the exterior as shown in Figures 8-G and 8-H. Microsphere particle size was analyzed and the particle size increased significantly from day 6 to day 15 (Figure 8-I), which coincides with the swelling ratio change shown in Figure 3. The transitional layer between the microspheres and the PVA hydrogel was also observed for this formulation as shown in Figures 8-A and 8-B. It is worth noting that these microspheres maintained their spherical shape until day 6 following which shape changes were observed.
4. Discussion
4.1. Amount of microsphere water uptake during swelling
PVA hydrogels have been widely used as biocompatible materials[31]. The short term swelling property of these hydrogels has been thoroughly investigated [32, 33]. They usually reach swelling equilibrium within a few hours which is consistent with the results reported here for the hydrogels alone and those loaded with the RG503H microspheres (Figure 3). In the case of the hydrogels containing the DLG1A microspheres, a fast hydrogel equilibration period was not observed and this is probably due to masking by the rapid swelling of these microspheres. The long-term swelling properties of the PLGA microsphere/PVA hydrogel composites has not been previously reported. A positively correlated microsphere concentration dependent long-term swelling pattern indicates that the microspheres were absorbing significant amounts of water. The onset of microsphere swelling coincides with the onset of the second drug release phase of RG503H microspheres and persisted during the remainder of the drug release period. Although a significant particle size change has been reported for PLGA microspheres at the onset of the second rapid drug release phase, the water uptake was not quantified in this study [14]. In the current study, we were able to quantify the amount of water uptake by the microspheres as they were embedded in the PVA hydrogels and the hydrogels only absorbed water during the initial fast equilibrium phase. The approximate maximum amount of water taken up by the microspheres was calculated using the equation below:
where Wd is the initial weight of the dry coating and Ws(tmax) is the weight of the swollen coating measured at the maximum swelling time (t), Ws(3) is the weight of the swollen coating following 3 hours incubation, CMS is the amount (mg) of microspheres in one mL of 5% (w/w) PVA solution.
For both microspheres, the maximum amount of water uptake ranges from 2 to 3 times their weight (Table 2).
Table 2.
Calculated maximum swelling ratio of microspheres embedded in the composite coatings according to Figures 3 and 4
| Microsphere Concentration | 50 mg/mL PVA | 75 mg/mL PVA | 100 mg/mL PVA |
|---|---|---|---|
| DLG1A MS | 2.49 ± 0.03 | 2.33 ± 0.27 | 2.62 ± 0.12 |
| RG503H MS | 2.15 ± 0.18 | 2.55 ± 0.11 | 2.97 ± 0.12 |
4.2. Drug release mechanism from microspheres
Different release profiles were observed for the composites prepared with the two different microsphere formulations (Figure 2). The high burst release observed for the DLG1A microsphere/PVA hydrogel composites is consistent with the low PLGA molecular weight, the hydrophilicity of these microspheres and the observation of surface associated dexamethasone (SEM images in Figure 1). In contrast, dexamethasone release from the RG503H formulation followed the typical tri-phasic PLGA microsphere release profile. The burst release, duration of the lag phase and the period for complete drug release is very similar to previously reported results obtained for RG503H PLGA microsphere/PVA hydrogel composites [20]. The tri-phasic release profile indicates that the RG503H microspheres follow a bulk erosion mechanism.
The burst release of drug from microspheres is usually considered to be a result of surface drug diffusion. From our cryo-SEM results, the formation of transitional layers in the PVA hydrogel at early time points (Figures 7-A2, 8-A and 8-B) may be due to large amounts of drug diffusing out of the microspheres and disturbing the intrinsic gel structure during the burst release phase. The coincidence between the drug release profile and water uptake for both microsphere formulations indicates swelling as a possible contributing factor to drug release from both formulations. Increased osmotic pressure due to accumulation of degradation products as well as polymer matrix plasticization have been suggested as contributing factors to microsphere swelling [14]. From the current investigation, the internal structure changes (i.e. development of a pore structure) also play a significant role affecting microsphere swelling. The onset of microsphere swelling was shown to correlate with the development of an internal porous structure for both types microspheres investigated (day 1 and day 6 for DLG1A and RG503H, respectively). During the lag phase, the following sequence of events occurs: 1) pores build up internally until the entire microsphere structure is porous, 2) the microspheres swell following pore formation, and 3) the significant influx of water leads to internal structure deformation followed by the onset of the second drug release phase. For hydrophobic drugs, the microspheres form a natural “osmotic pump” to maintain osmotic pressure from two aspects: 1) the internal microsphere space is filled with saturated drug solution providing consistent osmotic pressure; 2) osmotic pressure contributed by degraded oligomers and monomers is compromised from the microsphere swelling, and accordingly the second drug release phase is pseudo zero order. The surface layer of the microspheres remained intact during the drug release phase. This may be the reason that the microspheres continued to swell during the entire drug release phase.
For both microsphere formulations, small pores formed early close to the exterior and large pores formed later within the microspheres. This pattern can be explained by a local pH gradient from the interior (high acidity) to the surface (low acidity) and the water diffusion kinetics into the microspheres. As the microspheres are in contact with the aqueous phase, water diffusion into the microspheres can result in a concentration gradient of drug and acidic degradation species. The surface layer of the microspheres is exposed to water earlier and therefore starts to degrade faster. However, the acidic degradation products do not build up close to the surface and therefore large pores do not form in this region. In contrast, the microsphere cores are exposed to water later compared to the surface and consequently pore formation occurs slower. However, due to the pH effect from the accumulated acidic degradation products, those pores tend to grow large quickly. The pore morphology is interesting in that the larger pores are spherical in shape indicating that they are not formed as a result of connecting with neighboring pores. The uniform wall thickness between neighboring pores may originate from less viscous acid terminated PLGA oligomers that prefer to segregate their carboxyl ends toward pore surface.
4.3. Effect of swelling on glucose diffusion
Glucose diffusion through the composite coatings is another important characteristic with respect to their application as a coating for glucose biosensors. Glucose diffusion through the composite coatings is affected by the hydrophilicity and porosity of the coatings. When coated with the composites, microdialysis probes can provide insight into coating permeability to a specific analyte (i.e. glucose) in the form of RR. Employing the steady-state mass balance theory, a model has been proposed by Bungay et al. attributing RR to the perfusate flow rate (Qd) and a series of mass transport resistances [34]. The correlation is presented using the equation below:
where Rd, Rz, and Re are transport resistances of the dialysate, dialysis membrane, and external medium, respectively. Under well stirred conditions, the external medium resistance (Re) can be considered as zero. Norton, et al further separated Rz into the resistance contributed by the dialysis membrane (Rm), and that contributed by the hydrogel coating (Rh) using the equation below [35].
Rh can be further defined using the following equation:
where ro is the outer radius of dialysis probe, rh is the outer radius of the coating, L is the length of the microdialysis membrane, Dh is the glucose diffusion coefficient in the coating and φh is the fraction of glucose in the coating. Therefore, the apparent RR should be negatively related to the thickness of the coating (affecting rh) and positively related to the glucose diffusion coefficient (Dh) in the coating.
In the current study, a positive correlation was observed using a plot between the RR and swelling ratio at 3 hours while a negative correlation was observed at maximum swelling date (day 24) for coatings prepared with RG503H microspheres (Figure 9). For coatings prepared using DLG1A microspheres, although the positive correlation at 3 hours was not observed due to the masking effect from microsphere swelling, at maximum swelling date (day 13), such pattern of negative correlation was also observed. The positive correlation at 3 hours can be attributed to a negative dependence of water uptake into the PVA hydrogel on the microsphere concentration (prepared using the RG503H polymer). During this initial phase, more glucose mobility (increase of Dh) is expected with increase in the hydrophilicity of the coating. Further swelling of the coating was observed and water uptake reached a maximum at day 24. As the majority of the coating is composed of water by this time point, the effect of the coating thickness change (change of rh) dominates the contribution to RR leading to a negative correlation between swelling and RR. Glucose diffusion through the microsphere is limited as the microsphere shell was observed throughout the drug release phase (shown in Figure 8) in addition to the high internal osmotic pressure. However, the increase of RR post the second drug release phase can possibly be explained by the disappearance of the microsphere shell and therefore glucose is able to freely pass through the holes that were earlier occupied by the microspheres. In addition, decrease in water uptake was observed post the second drug release phase indicating that the coating was shrinking (decreasing in thickness) with the disappearance of the microsphere shells.
Figure 9.
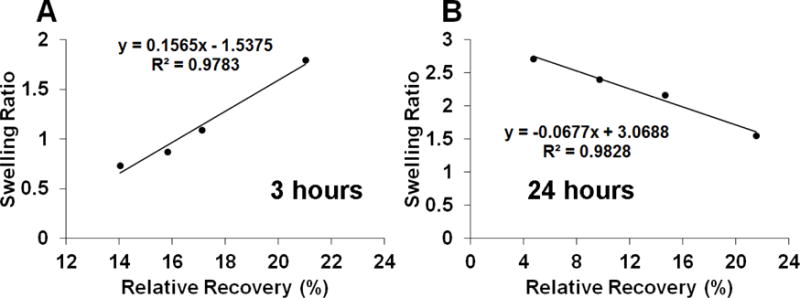
Correlation between coating swelling ratio and RR of glucose through the coatings prepared with RG503H microspheres following 3 hours (A) and 24 days (B) incubation.
5. Conclusion
The present study demonstrated new insights into PGLA microsphere drug release mechanisms through investigation of swelling, internal pore formation and glucose diffusion for two types of PLGA microspheres (with and without lag phase) embedded in PVA hydrogels. For the first time, detailed internal structure of PLGA microspheres during drug release was revealed with the assistance of cryo-SEM. The results suggest that both types of microspheres undergo heterogeneous erosion, and swelling. The outside-in pattern of porosity progression in the microspheres explains the lag phase observed in some PLGA microsphere products. The length of the lag phase is determined by the time required for the entire microsphere to become porous which is controlled by the molecular weight and hydrophobicity of the polymer. The onset of drug release post the lag phase appears to be a consequence of microsphere swelling following pore formation. Continuous microsphere swelling during the second drug release phase may also affect the drug release kinetics. In addition, the timing and amount of water absorption measured during the swelling process will be useful for researchers who are interested in building mechanistic mathematical models depicting drug release from microspheres. This information can be used by researchers to develop microspheres with specific release patterns for different applications. The information generated in this study on the dynamic internal changes in the microspheres will also be useful in understanding drug stability during the release process. Furthermore, the correlation between microsphere swelling and glucose permeation through the coatings will facilitate coating design for glucose sensors and other similar implantable devices.
Acknowledgments
The s thank US Army Medical Research (W81XWH0710688, W81XWH-15-C-0069), NIH (1R21HL09045801, R43EB011886, 9R01EB014586) and NSF/SBIR (1046902, 1230148, and 126100) for funding. This work was performed in part at the Biosciences Electron Microscopy Facility of the University of Connecticut under assistance of Dr. Marie Cantino.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ma G. Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. J Control Release. 2014;193:324–340. doi: 10.1016/j.jconrel.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhou S, Liao X, Li X, Deng X, Li H. Poly-D,L-lactide-co-poly(ethylene glycol) microspheres as potential vaccine delivery systems. J Control Release. 2003;86:195–205. doi: 10.1016/s0168-3659(02)00423-6. [DOI] [PubMed] [Google Scholar]
- 3.Ford Versypt AN, Pack DW, Braatz RD. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres–a review. J Control Release. 2013;165:29–37. doi: 10.1016/j.jconrel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batycky RP, Hanes J, Langer R, Edwards DA. A theoretical model of erosion and macromolecular drug release from biodegrading microspheres. Journal of Pharmaceutical Sciences. 1997;86:1464–1477. doi: 10.1021/js9604117. [DOI] [PubMed] [Google Scholar]
- 5.Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. International Journal of Pharmaceutics. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 6.Siepmann J, Siepmann F. Mathematical modeling of drug delivery. International Journal of Pharmaceutics. 2008;364:328–343. doi: 10.1016/j.ijpharm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- 8.Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17:100–106. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Ghassemi AH, Hennink WE, Schwendeman SP. The microclimate pH in poly(d,l-lactide-co-hydroxymethyl glycolide) microspheres during biodegradation. Biomaterials. 2012;33:7584–7593. doi: 10.1016/j.biomaterials.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Wang BM, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly(d,l-lactide-co-glycolide) microspheres. Journal of Controlled Release. 2002;82:289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- 11.Messaritaki A, Black SJ, van der Walle CF, Rigby SP. NMR and confocal microscopy studies of the mechanisms of burst drug release from PLGA microspheres. Journal of Controlled Release. 2005;108:271–281. doi: 10.1016/j.jconrel.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Gaignaux A, Réeff J, De Vriese C, Goole J, Amighi K. Evaluation of the degradation of clonidine-loaded PLGA microspheres. Journal of Microencapsulation. 2013;30:681–691. doi: 10.3109/02652048.2013.778905. [DOI] [PubMed] [Google Scholar]
- 13.Gasmi H, Willart JF, Danede F, Hamoudi MC, Siepmann J, Siepmann F. Importance of PLGA microparticle swelling for the control of prilocaine release. Journal of Drug Delivery Science and Technology. 2015;30(Part A):123–132. [Google Scholar]
- 14.Gasmi H, Danede F, Siepmann J, Siepmann F. Does PLGA microparticle swelling control drug release? New insight based on single particle swelling studies. Journal of Controlled Release. 2015;213:120–127. doi: 10.1016/j.jconrel.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Brunner A, Mäder K, Göpferich A. pH and osmotic pressure inside biodegradable microspheres during erosion. Pharm Res. 1999;16:847–853. doi: 10.1023/a:1018822002353. [DOI] [PubMed] [Google Scholar]
- 16.Kang J, Schwendeman SP. Pore Closing and Opening in Biodegradable Polymers and Their Effect on the Controlled Release of Proteins. Molecular Pharmaceutics. 2007;4:104–118. doi: 10.1021/mp060041n. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto A, Matsukawa Y, Horikiri Y, Suzuki T. Rupture and drug release characteristics of multi-reservoir type microspheres with poly(dl-lactide-co-glycolide) and poly(dl-lactide) International Journal of Pharmaceutics. 2006;327:110–116. doi: 10.1016/j.ijpharm.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 18.Friess W, Schlapp M. Release mechanisms from gentamicin loaded poly(lactic-co-glycolic acid) PLGA) microparticles. Journal of Pharmaceutical Sciences. 2002;91:845–855. doi: 10.1002/jps.10012. [DOI] [PubMed] [Google Scholar]
- 19.Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. Journal of Controlled Release. 2007;122:338–344. doi: 10.1016/j.jconrel.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Shen J, Burgess DJ. Accelerated in vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings. International Journal of Pharmaceutics. 2012;422:341–348. doi: 10.1016/j.ijpharm.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoué T, Koike H. High-resolution low-temperature scanning electron microscopy for observing intracellular structures of quick frozen biological specimens. J Microsc. 1989;156:137–147. doi: 10.1111/j.1365-2818.1989.tb02913.x. [DOI] [PubMed] [Google Scholar]
- 22.Kastellorizios M, Papadimitrakopoulos F, Burgess DJ. Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants. Journal of Controlled Release. 2015;214:103–111. doi: 10.1016/j.jconrel.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. Journal of Controlled Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. Journal of Controlled Release. 2013;169:341–347. doi: 10.1016/j.jconrel.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Gu B, Wang Y, Burgess DJ. In vitro and in vivo performance of dexamethasone loaded PLGA microspheres prepared using polymer blends. International Journal of Pharmaceutics. doi: 10.1016/j.ijpharm.2015.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. International Journal of Pharmaceutics. 2010;384:78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 27.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling Acute Inflammation with Fast Releasing Dexamethasone-PLGA Microsphere/PVA Hydrogel Composites for Implantable Devices. Journal of Diabetes Science and Technology. 2007;1:8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu B, Wang Y, Burgess DJ. In vitro and in vivo performance of dexamethasone loaded PLGA microspheres prepared using polymer blends. International Journal of Pharmaceutics. 2015;496:534–540. doi: 10.1016/j.ijpharm.2015.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu B, Burgess DJ. Prediction of dexamethasone release from PLGA microspheres prepared with polymer blends using a design of experiment approach. International Journal of Pharmaceutics. 2015;495:393–403. doi: 10.1016/j.ijpharm.2015.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zolnik BS, Burgess DJ. Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres. Journal of Controlled Release. 2008;127:137–145. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Hassan CM, Peppas NA. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules. 2000;33:2472–2479. [Google Scholar]
- 32.Sirousazar M, Kokabi M, Hassan ZM. Swelling behavior and structural characteristics of polyvinyl alcohol/montmorillonite nanocomposite hydrogels. Journal of Applied Polymer Science. 2012;123:50–58. [Google Scholar]
- 33.Holloway JL, Spiller KL, Lowman AM, Palmese GR. Analysis of the in vitro swelling behavior of poly(vinyl alcohol) hydrogels in osmotic pressure solution for soft tissue replacement. Acta Biomaterialia. 2011;7:2477–2482. doi: 10.1016/j.actbio.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Bungay PM, Morrison PF, Dedrick RL. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46:105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- 35.Norton LW, Yuan F, Reichert WM. Glucose Recovery with Bare and Hydrogel-Coated Microdialysis Probes: Experiment and Simulation of Temporal Effects. Analytical Chemistry. 2007;79:445–452. doi: 10.1021/ac061234p. [DOI] [PubMed] [Google Scholar]


