Abstract
Objective
The present study examined predictors and moderators of dropout among 165 adults meeting DSM-IV criteria for posttraumatic stress disorder (PTSD) and alcohol dependence (AD). Participants were randomized to 24 weeks of naltrexone (NAL), NAL and prolonged exposure (PE), pill placebo, or pill placebo and PE. All participants received supportive AD counseling (the BRENDA manualized model).
Method
Logistic regression using the Fournier approach was conducted to investigate baseline predictors of dropout across the entire study sample. Rates of PTSD and AD symptom improvement were included to evaluate the impact of symptom change on dropout.
Results
Trauma type and rates of PTSD and AD improvement significantly predicted dropout, accounting for 76% of the variance in dropout. Accidents and “other” trauma were associated with the highest dropout, and physical assault was associated with the lowest dropout. For participants with low baseline PTSD severity, faster PTSD improvement predicted higher dropout. For those with high baseline severity, both very fast and very slow rates of PTSD improvement were associated with higher dropout. Faster rates of drinking improvement predicted higher dropout among participants who received PE.
Conclusions
The current study highlights the influence of symptom trajectory on dropout risk. Clinicians may improve retention in PTSD-AD treatments by monitoring symptom change at regular intervals, and eliciting patient feedback on these changes.
Keywords: dropout, attrition, predictors, prolonged exposure, post-traumatic stress disorder, alcohol dependence
Alcohol dependence (AD) is frequently comorbid with posttraumatic stress disorder (PTSD; Kessler, Chiu, Demler & Walters, 2005), and the presence of one of these disorders carries a significantly higher risk of being diagnosed with the other (Breslau, Davis, & Schultz, 2003). Compared to individuals with PTSD or AD alone, those with PTSD-AD exhibit greater PTSD and AD symptom severity, higher rates of comorbid disorders, higher rates of suicide attempt, and greater functional impairment (Blanco, et al., 2013). PTSD treatment research has predominantly excluded patients with comorbid alcohol dependence, due to concerns that addressing trauma may increase alcohol use, or that ongoing alcohol use may impede PTSD treatment effects. Research published in the last five years, however, has begun to evaluate the efficacy of various pharmacological and psychological treatment combinations that address PTSD and AD symptoms concurrently (Foa et al., 2013; Hien et al., 2015; Sannibale et al., 2013). While the outcomes from these studies have been promising, many patients discontinue treatment prematurely or are lost for follow-up (e.g., 32% in Foa et al., 2013; 42% in Hien et al., 2015).
No previous research has identified patient characteristics associated with dropout during concurrent treatment of PTSD and AD. In the broader literature on treatment of PTSD and substance use disorders (SUD), a small number of studies have investigated factors associated with dropout, with mixed results. Importantly, none of these studies used multiple predictor analyses, which examine many predictors simultaneously, thereby controlling for the effects of related constructs. Instead, these studies examined group differences between treatment completers and dropouts on various pre-treatment variables. Compared to completers, some studies have found lower levels of education (Brady, Dansky, Back, Foa, & Carroll, 2001) and greater pretreatment SUD and PTSD severity (Najavits, Weiss, Shaw, & Muenz, 1998) among treatment dropouts. In contrast, other studies have found no relationships between pre-treatment variables and treatment dropout, including demographic characteristics (Hien et al., 2004; McGovern et al., 2009; Najavits, Weiss, Shaw, & Muenz, 1998; Triffleman, 2000), baseline SUD or PTSD severity (McGovern et al., 2009; Mills et al., 2012), trauma type (Mills et al., 2012), or age at the time of the trauma (Mills et al., 2012).
Studies of AD treatment alone have also found few consistent predictors of treatment dropout. Demographic characteristics have predicted dropout in some studies (e.g., younger age: Vuoristo-Myllys, Lahti, Alho, & Julkunen, 2013; male gender: Schilling and Sachs, 1993), whereas other studies have failed to identify significant demographic predictors (e.g., age: Ray, Hutchison, & Bryan, 2006; gender: Elbreder, de Souza e Silva, Pillon, & Laranjeira, 2011; Vuoristo-Myllys, Lahti, Alho, & Julkunen, 2013). Similarly, comorbid depression has been identified as a predictor of AD treatment dropout in some studies (e.g., Filho & Baltieri, 2012) and found unrelated to dropout in other studies (e.g., Kavanagh et al., 2006). Perhaps most illustrative of all, some studies have found that baseline drinking severity is associated with higher dropout (e.g., Graff et al., 2009), other studies have found it to predict lower dropout (e.g., Ray, Hutchinson, & Bryan, 2006), and still others have found it to be unrelated to dropout from AD treatment (e.g., Filho & Baltieri, 2012).
Predictor findings are similarly mixed in PTSD treatment studies, which typically include AD as an exclusion criterion. Female gender was associated with dropout in one study (e.g., Eftekhari, Ruzek, Crowley, Rosen, Greenbaum, & Karlin, 2013) and unrelated to dropout in other studies (e.g., Hagenaars, van Minnen & Hoogduin, 2010). Younger age has predicted dropout in some studies (e.g., Rizvi, Vogt, & Resick, 2009) but not others (e.g., van Minnen, Arntz, & Keijsers, 2002). Inconsistent findings have been reported for employment status (e.g., Foa et al., 1999; but see Taylor et al., 2003), education (e.g., Rizvi, Vogt, & Resick, 2009; but see Hagenaars, van Minnen, & Hoogduin, 2010), depressive symptoms (e.g., Garcia, Kelley, Rentz, & Lee, 2011; but see Hagenaars, van Minnen, & Hoogduin, 2010), experience of childhood abuse (e.g., van Minnen, Arntz, & Keijsers, 2002; but see Zayfert et al., 2005), and greater PTSD severity (e.g., Marks, Lovell, Noshirvani, Livanou, & Thrasher, 1998; but see Eftekhari et al., 2013). An exception to these mixed findings is that comorbid SUDs have been predictive of dropout from PTSD treatment with some consistency (e.g., Najavits, 2015; Szafranski, Gros, Menefee, Wanner, & Norton, 2014; van Minnen et al., 2002).
To date, the majority of predictor studies have looked exclusively at pre-treatment patient characteristics. However, patterns of symptom change during treatment may have important implications for treatment retention. The hypothesis here is that patients' decisions about continuing versus leaving treatment are impacted by the amount of symptom improvement they have experienced. One study has investigated the relationship between dropout and drinking improvement during AD treatment (Vuoristo-Myllys, Lahti, Alho, & Julkunen, 2013), and found no significant association. In contrast, a study examining dropout during cognitive behavioral therapy for anxiety (Krishnamurthy, Khare, Klenck, & Norton, 2015) showed that during any given week, patients with the highest anxiety symptom severity were most likely to drop out of treatment. Interestingly, rapid symptom improvement was associated with a higher rate of dropout, but only among patients with low baseline levels of anxiety. Patients who drop out of treatment are often considered treatment failures and are assumed to have poor outcomes. The findings of Krishnamurthy et al. suggest that some patients may discontinue treatment because they have improved sufficiently and perceive additional treatment as unnecessary.
The present study utilized data from a randomized controlled trial (RCT; Foa et al., 2013) to examine predictors of dropout among patients with comorbid PTSD-AD. In this RCT, all participants received supportive counseling for AD (the BRENDA manualized model; Starosta, Leeman, & Volipcelli, 2006) and were randomized to one of four concurrent treatment conditions: 1) Prolonged Exposure (PE) + placebo, 2) PE + naltrexone (NAL), 3) NAL, or 4) placebo. At post-treatment, all groups showed large reductions in PTSD and AD symptoms, with no differences observed between the PE (with BRENDA) and no PE (BRENDA alone) arms on reduction of PTSD symptoms. Individuals who received NAL achieved better drinking outcomes than those who received placebo.
To our knowledge, no prior study has examined predictors of dropout during concurrent PTSD-AD treatment. Moreover, no study has examined the relationship between symptom change and treatment dropout among patients with comorbid PTSD-AD or PTSD-SUD. Examining predictors of treatment dropout is critical in order to identify patients who may benefit from additional monitoring and interventions to maintain treatment engagement. While the previous findings on dropout are largely conflicting, newer statistical approaches increase power to detect predictor effects where they might exist, and reduce the possibility of identifying predictors that are better accounted for by related constructs.
In the current study, we employ an approach developed by Fournier (Fournier, 2009) that maintains sufficient power to test a wide range of potential predictors across construct domains, while minimizing the likelihood of identifying predictors that are in fact proxies for third variables. Further, using the Fournier method, we are able to examine the influence of symptom improvement by evaluating baseline characteristics and rates of symptom change in a concurrent model. Seven predictor domains were evaluated: 1) demographics, 2) socio-economic factors, 3) comorbid disorders, 4) trauma features, 5) PTSD features, 6) alcohol features, and 7) slopes of improvement during treatment. We hypothesized that dropout would be more likely to occur among patients who experience either very slow improvement (e.g., they may perceive that treatment is not helping them), or very rapid improvement (e.g., they have benefited and thus may not perceive a need for more treatment). Consistent with Krishnamurthy et al.'s (2015) findings, we hypothesized that dropout would be highest among those who made fast improvements but started with lower symptom severity (i.e., individuals who might have achieved expected treatment gains prior to the prescribed number of sessions).
Methods
Participants
Participants were 165 adults meeting DSM-IV criteria for AD and PTSD who were enrolled in a randomized, single-blind treatment study taking place at the University of Pennsylvania's Center for the Treatment and Study of Anxiety and the Philadelphia Veterans' Affairs Hospital. Participants had a mean age of 42.8 (SD = 9.8), and the majority were men (65.5%) and Black/African-American (63.6%). The mean score on the PSS-I at baseline was 28.1 (SD = 7.9), indicating a moderately severe level of PTSD severity, and participants drank alcohol on an average of 74.8 (SD = 25.2) of the 90 days prior to study enrollment. Participants were excluded for: 1) current substance dependence other than nicotine or cannabis, 2) current psychotic or bipolar disorder, 3) active suicidal or homicidal ideation with intent, 4) opiate use in the month prior to enrollment, 5) medical illness that could interfere with treatment (e.g., active hepatitis, AIDS), or 6) pregnancy or nursing. Baseline participant characteristics can be found in Table 1.
Table 1.
Baseline participant characteristics (n = 165).
| Variable | Number (%) |
|---|---|
| Gender | |
| Female | 57 (34.5%) |
| Male | 108 (65.5%) |
| Race/Ethnicity | |
| Black/African-American | 105 (63.6%) |
| White/Caucasian | 50 (30.3%) |
| Hispanic/Latino | 7 (4.2%) |
| Other | 3 (1.8%) |
| Living Alone | 36 (21.8%) |
| Employed | 57 (34.5%) |
| Median Income Range | $15,000-20,000 |
| Education | |
| Some College or More | 84 (50.9%) |
| High School or Less | 81 (49.1%) |
| Types of Trauma | |
| Sexual Assault | 42 (25.5%) |
| Physical Assault | 58 (35.8%) |
| Combat | 19 (11.5%) |
| Accident | 15 (9.1%) |
| Other | 30 (18.2%) |
| Number of additional Axis I diagnoses | |
| 0 | 95 (57.6%) |
| 1 | 44 (26.7%) |
| 2 | 19 (11.5%) |
| 3 | 3 (1.8%) |
| 4 | 3 (1.8%) |
| 5 | 1 (.6%) |
| Other Substance Use Disorder | 35 (21.2%) |
| Current Personality Disorder | 41 (24.8%) |
|
| |
| Variable | M (SD) |
|
| |
| Age | 42.78 (9.76) |
| Alcohol Dependence Duration (years) | 13.36 (11.04) |
| PTSD Duration (years) | 14.55 (15.26) |
| PSS-I | 28.14 (7.86) |
| Percent Days Drinking | 74.82 (25.26) |
| Alcohol Craving | 18.38 (6.91) |
| ASI | 27.44 (13.95) |
| BDI | 26.31 (11.54) |
Note: The precise n per variable differed due to missing data on some variables. ASI, Anxiety Sensitivity Index. BDI, Beck Depression Inventory. PSS-I, PTSD Symptom Scale Interview.
Procedure
For a detailed description of the trial procedure, see Foa et al., 2013. At intake, potential participants completed a psychiatric evaluation, a physical examination, and laboratory assessments to determine study eligibility. They then completed outpatient detoxification requiring three or more consecutive days of alcohol abstinence, measured by self-report and breathalyzer testing. For participants presenting with elevated withdrawal symptoms during detoxification that required medical management, or for those deemed high risk for poor response to detoxification based on prior history, Oxazepam was administered as needed. All eligible participants were supported as needed to complete this brief detoxification (e.g., some participants completed repeat detoxifications, or were monitored over a longer period of time) to prevent life threatening withdrawal symptoms. Following informed consent, participants were randomly assigned to one of four treatment conditions: naltrexone (NAL) with or without PE (NAL + PE, NAL + No PE), or pill placebo (PLA) with or without PE (PLA + PE, PLA + No PE). All patients received concurrent supportive counseling focused on alcohol use and medication management (BRENDA). Assessments by evaluators blind to study condition and self-report questionnaires were completed every four weeks during treatment (week 0 through week 24). All study procedures were approved by the University of Pennsylvania institutional review board.
Measures
Structured Clinical Interview for DSM-IV (SCID-IV)
The SCID (First & Gibbon, 2004) is a semi-structured interview that assesses lifetime and current DSM-IV Axis I diagnoses. The SCID was used to confirm diagnoses of alcohol dependence and PTSD, as well as to evaluate the presence of other psychological disorders at baseline and post-treatment. The SCID is a widely used, reliable measure, with joint inter-rater reliability coefficients for different disorders ranging from 0.60 to 0.83 (Lobbestael, Leurgans, & Arntz, 2010).
Psychiatric Research Interview for Substance and Mental Disorders (PRISM
The PRISM (Hasin et al., 1996) is a semi-structured interview that assesses disorders commonly comorbid with SUDs. In this trial, the PRISM was used to evaluate the presence of antisocial personality disorder and borderline personality disorder. The PRISM shows good diagnostic validity for Axis II personality disorders as well as excellent inter-rater reliability (Torrens, Serrano, Astals, Pérez-Domínguez, & Martín-Santos, 2004).
PTSD Symptom Scale – Interview Version (PSS-I)
The PSS-I (Foa, Riggs, Dancu, & Rothbaum, 1993) is a 17-item clinician-administered interview assessing PTSD symptom severity according to DSM-IV criteria over the prior two weeks. The PSS-I identifies an index trauma (i.e., the traumatic memory that is most bothersome to the patient at present), which was coded into one of five categories: sexual assault, combat, physical assault, accident, or “other” trauma. The PSS-I yields a total score ranging from 0-51, with a higher score indicating greater severity. In the current sample, this measure demonstrated excellent internal consistency (α = .90), very good one-month test-retest reliability (r = .80), good inter-rater reliability (ICC = .073), and good convergent validity with the SCID-IV PTSD diagnosis (κ = .75) (Powers, Gillihan, Rosenfield, Jerud, & Foa, 2012).
Timeline Follow-Back Interview (TLFB)
The TLFB (Sobell & Sobell, 1992) utilizes a calendar method to assess the amount of alcohol consumed each day, and in the current study it was used to assess percentage of days drinking (PDD) over the past month. The TLFB demonstrates good test-retest reliability (α = .79-.94) and concurrent validity (r = .84-.95 with collateral reports of drinking; Maisto, Sobell, & Sobell, 1982; Sobell, Maisto, Sobell, & Cooper, 1979).
The Penn Alcohol Craving Scale (PACS)
The PACS (Flannery, Volpicelli, & Pettinati, 1999) is a 5-item self-report measure assessing alcohol craving during the prior week. Total scores range from 0-30, with higher scores indicating greater craving. The PACS demonstrates excellent internal consistency (α = .92), high item-total correlations (r = .80-.92), and good concurrent validity with the Obsessive Compulsive Drinking Scale (r = .55; Modell, Glaser, Mountz, Schmaltz, & Cyr, 1992), another validated measure of alcohol craving. In the current sample, Cronbach's alpha = .91.
Anxiety Sensitivity Index (ASI)
The ASI (Reiss, Peterson, Gursky, & McNally, 1986) is a 16-item self-report questionnaire assessing fear of anxiety-related sensations and beliefs about the negative consequences of anxiety. Total scores range from 0-64, with higher cores indicating greater anxiety sensitivity. The measure has shown good discriminant and predictive validity (Taylor, Koch, & Crockett, 1991), adequate test-retest reliability, and good internal consistency (Reiss et al., 1986). In the current sample, Cronbach's alpha = .92.
Beck Depression Inventory II (BDI-II)
The BDI-II (Beck, Steer, & Brown, 1996) is a widely used, well-validated tool for assessing of depressive symptoms. The scale includes 21 items that yield a total score ranging from 0-63. Higher scores correspond to greater depressive symptoms in the past week. In the current sample, Cronbach's alpha = .92.
Treatments
Supportive Counseling (BRENDA)
All participants were offered 18 sessions of supportive AD counseling over the course of 24 weeks following the BRENDA model (Starosta, Leeman, & Volpicelli, 2006). These sessions were conducted by the study nurse weekly for the first 12 weeks and biweekly in the next 12 weeks. The counseling focused on medication management and enhancing compliance through motivational interviewing (Miller & Rollnick, 1991). Each 30-40 minute session included medication dispensing, compliance monitoring, provision of education around alcoholism, and support and advice around drinking. The specific components of the model, from which the name BRENDA is derived, are as follows: Biopsychosocial evaluation, Report to the patient on assessment, Empathic understanding of the patient's situation, Needs collaboratively identified by the patient and treatment provider, Direct advice to the patient on how to meet those needs, and Assess reaction of the patient to advice and adjust as necessary for best care (Starosta, Leeman, & Volpicelli, 2006). The mean number of BRENDA sessions completed was 12.09 (SD = 4.84).
Prolonged Exposure (PE)
PE consisted of 18, 90-minute sessions provided on the same schedule as BRENDA sessions (i.e., weekly for the first 12 weeks and biweekly for the next 12 weeks). The main components of PE are in-vivo exposure (confronting trauma-related situations in real life) and imaginal exposure (repeated recounting of the trauma memory) followed by processing (discussion of thoughts and feelings evoked by recounting the trauma memory). PE was provided by doctoral-level clinical psychologists. Based on a randomly selected sample (15%) of video-recorded PE sessions, overall treatment adherence was 96%. The average number of PE sessions completed in the NAL + PE group was 6.18 sessions (SD = 3.86), and in the PLA + PE group was 6.48 sessions (SD = 3.49), with no significant difference between groups (p = .73).
Naltrexone
Naltrexone is an opiate antagonist used to treat alcohol dependence. It is approved by the U.S. Food and Drug administration. Participants started on a daily dose of 50 mg/day for at least three days before titrating up within one week to the target dose of 100 mg/day. Most participants tolerated this dose, while a small number (n = 3) experienced side effects and were titrated back down to 50mg/day. Medication compliance was assessed through weekly pill counts in the first three months and biweekly pill counts during the following three months. Eighty five percent of participants met criteria for adherence to medication and supportive counseling, defined as at least 80% medication compliance and attendance to supportive counseling.
Treatment Dropout
Fifty-three (32.1%) participants dropped out of treatment. Dropout did not differ significantly across treatment groups (χ23=1.55; p=.67). Eighteen participants (10.9%) dropped out before week 4; eleven (6.7%) dropped between weeks 4 and 8, six participants (3.6%) dropped between weeks 8 and 12; and eighteen (10.9%) dropped out between weeks 12 and 24.
Data Analysis
The Fournier approach (see Amir et al., 2011; Fournier et al., 2009; Powers et al., 2014; Smits et al., 2013; Wheaton et al., 2015) was employed to identify significant predictors and moderators of dropout during treatment (weeks 0-24). Using this approach, potential predictors/moderators are grouped into domains of related variables. Significant predictors/moderators are identified within each domain, and then the significant predictors/moderators from each domain are entered concurrently into a final model. This procedure permits examination of a large number of predictors without substantially increasing the risk of either Type I or Type II error. Type I error is minimized because variables are identified that are predictive over and above others variables within their domain, and over and above significant predictors from the other domains. Type II error is minimized because the analysis does not include all potential predictors/moderators in a single, very large model. Analyses were conducted using logistic regression.
Putative predictors and moderators were grouped into seven domains: 1) demographics (age, gender, white vs. minority race), 2) socio-economic factors (co-habitation status, employment status, education level, income), 3) comorbid disorders (number of comorbid Axis I disorders, presence vs. absence of additional substance use disorders, presence vs. absence of a personality disorder, depressive symptom severity), 4) trauma features (index trauma type [sexual assault, combat, physical assault, accident, other trauma], number of traumatic events), 5) PTSD features (baseline PSS-I, age of trauma onset, PTSD duration, anxiety sensitivity), 6) alcohol features (baseline percent days drinking, craving, age of AD onset, duration of AD), and 7) slopes of improvement during treatment (average slope of improvement in PSS-I and in PDD, the square of the slopes of improvement, plus the interaction of initial PSS-I severity and slopes of improvement).
We calculated the linear slopes of improvement per week for each participant for the PSS-I (PTSD symptoms) and PDD (percentage days drinking), both of which were measured every 4 weeks during treatment. Slopes were calculated using multilevel modeling (MLM), in which the outcome (PSS-I or PDD) was predicted by a random intercept and a random slope (time measured in weeks since baseline). The OLS estimates for the slopes of improvement over time for each participant were used as predictor/moderators of dropout. MLM calculates the slopes for the period of time the participant participated in the study, regardless of missing data or dropout. More negative slopes indicated faster improvement.
The stepwise Fournier procedure was performed for each domain as follows: In Step 1, all potential moderator variables within the domain are included in the logistic regression analysis predicting dropout (yes/no). Step 2 includes only the variables with a significance level p<.20 in Step 1. Step 3 includes all terms from Step 2 that were p<.10. The analysis in Step 4 is then comprised of the variables from Step 3 that were significant at p<.05. This stepwise procedure using these a priori criteria is performed for each domain of predictors, and identifies significant predictors/moderators of dropout from each domain. However, Step 4 cannot be interpreted alone as this step only identifies significant predictors/moderators of dropout from within a given domain, without accounting for predictors/moderators from the other domains. Thus, each variable that is significant in Step 4 from each domain is then included in a final model. Variables coding treatment condition (the main effect for PE, the main effect for NAL, and the PE × NAL interaction) were included in all logistic regression models regardless of their significance level. For each domain, each predictor/moderator was interacted with all three treatment condition variables to evaluate moderation of treatment effects.
To examine the significant moderator interactions, we followed the approach developed by Aiken and West (1991), calculating the effect of the treatments at high and low levels of the moderator (defined as 1 SD above and 1 SD below the mean, respectively). This technique allows one to understand how the relationship between treatment and outcome varies for high and low values of the moderator.
Variables were converted to Z-scores to facilitate comparisons and to center them at their means for the interactions. Five of our variables of interest were missing greater than 5% of their data: income (7%), alcohol craving (13%), depressive symptoms (15%), anxiety sensitivity (18%), and presence of a personality disorder (28%). To avoid dropping cases, multiple imputation was employed to address missing data. Twenty datasets were imputed, and the results were “pooled” across the 20 datasets according to the appropriate algorithm in SPSS 21.0.
Power analyses using the program G*Power 3.1.9.2 indicated that we had a power of .83 to detect a medium effect size (OR = 1.8) for a continuous predictor that had a small correlation with other predictors, and a power of .796 to detect a medium effect size for a predictor that had a medium correlation with the other predictors in the model.
Results
We first calculated the slopes of improvement in PSS-I and PDD for use as predictors/moderators of dropout rates. For the PSS-I, the mean slope was -.60 points per week. For PDD, the mean slope was -2.19% per week. Because slopes were negatively skewed (PSS-I slope skewness=-1.49; PDD slope skewness=2.49), slopes were transformed using the log of the negative of the slope, as recommended by Tabachnick and Fidell (2013).1
Predictor/Moderator Analyses
Below we report the results for all significant predictors identified in Step 4 of each domain, followed by results for the variables that remained significant in Step 4 of the final model. Some interactions were significant in the analyses of separate domains but were no longer significant when combined with other variables in the final model. Since these interactions were non-significant when fully controlling for the other significant variables from other domains, we present the statistics for these interactions for each domain (below) but do not discuss the details of these effects until they are verified as significant in the final model.
Predictors of Dropout within Each Domain
Demographics
Step 4 of the analysis indicated that the only predictor/moderator of dropout in the demographic domain was a race × NAL interaction, b=.50, p=.006. While dropout did not vary by medication condition for white participants (p=.75), minority participants dropped out more frequently in PLA than in NAL.
Socio-economic factors
Step 4 of the socio-economic factors domain showed that the PE × Cohabitation interaction was significant, b=.33, p=.039. For participants who were cohabitating, dropout was higher for participants receiving PE than for those not receiving PE, whereas dropout did not vary by PE condition for participants who were not cohabitating (p=.30). No other socio-economic variable was significantly related to dropout.
Comorbid disorders
Step 4 of the comorbid disorders domain indicated that participants with more comorbid substance use disorders were more likely to drop out, regardless of condition, b=.33, p=.049.
Trauma features
Step 4 of the trauma features domain indicated that dropout rates differed by trauma type, χ2(4)=10.34, p=.035. The details of these differences are discussed below in the results for the final model.
PTSD features
None of the other PTSD features were significantly related to dropout.
Alcohol features
Step 4 of the alcohol features domain showed a significant PE × baseline alcohol craving interaction, b=-.41, p=.043. For those in the PE conditions, higher alcohol craving was significantly related to higher odds of dropout (p<.05). This relationship was not found among participants who did not receive PE.
Slopes of improvement
Slopes of improvement in both PSS-I and PDD (and their squares) were significantly related to dropout (ps<.001). In addition, there was a significant PE × slope of improvement in PDD interaction (p=.001) and a significant baseline PTSD severity × slope of improvement in PSS-I (p=.006). The direction of effects is discussed below in the results for the final model.
Predictors of Dropout in the Final Model
Results from the final model are presented in Table 2 and in Figures 1 and 2. The final model accounted for a significant amount of the variation in dropout, Nagelkerke's R2=.758, and correctly predicted dropout status for 88.5% of the sample (95.5% of completers and 73.6% of dropouts).
Table 2.
Predictors and moderators of dropout in Step 4 of the Fournier analysis.
| Domain/Predictor | b | Wald Statistic | p |
|---|---|---|---|
| Demographic Factors | |||
|
| |||
| Race (white vs. non-white) | .24 | 1.33 | .172 |
| Race × NAL | -.50 | 2.79 | .006 |
|
| |||
| Socio-Economic Factors | |||
|
| |||
| Cohabitation | .00 | 0.00 | .986 |
| Cohabitation × PE | .38 | 2.04 | .039 |
|
| |||
| Comorbid Factors | |||
|
| |||
| Number of Other Subst. Use Disorders | -.33 | 1.97 | .049 |
|
| |||
| Trauma Features | |||
|
| |||
| Sexual Assault | .37 | 1.81 | .071 |
| Combat | .21 | 1.04 | .280 |
| Accident | .32 | 1.68 | .081 |
| Other Trauma | .58 | 3.01 | .003 |
|
| |||
| PTSD Features | |||
|
| |||
| Age of Trauma Onset | -.21 | 1.23 | .219 |
|
| |||
| Alcohol Features | |||
|
| |||
| Craving | .05 | 0.24 | .809 |
| Craving × PE | -.41 | 2.02 | .043 |
|
| |||
| Slopes of Improvement | |||
|
| |||
| Initial PSS-I Severity | -.14 | 0.31 | .759 |
| PDD Slope | 4.89 | 3.60 | .000 |
| PDD Slope × PE | 3.64 | 2.84 | .005 |
| PDD Slope squared | 18.31 | 2.53 | .011 |
| PDD Slope squared × PE | 9.01 | 1.25 | .212 |
| PDD Slope squared × NAL | 7.37 | 1.03 | .303 |
| PDD Slope squared × NAL × PE | 18.05 | 2.38 | .017 |
| PSS-I Slope | .79 | 1.28 | .198 |
| PSS-I Slope squared | 7.27 | 4.24 | .000 |
| PSS-I slope × Initial Severity | -1.71 | 2.63 | .008 |
|
| |||
| Final Model | |||
|
| |||
| Initial PSS-I Severity | .00 | 0.00 | .993 |
| PDD Slope | 3.61 | 3.92 | .000 |
| PDD Slope × PE | 2.77 | 3.02 | .003 |
| PSS-I Slope | 1.02 | 1.79 | .074 |
| PSS-I Slope squared | 7.26 | 4.28 | .000 |
| PSS-I Slope × Initial Severity | -1.79 | 2.81 | .005 |
| Sexual Assault | .49 | 1.11 | .268 |
| Combat | .95 | 2.57 | .010 |
| Accident | .81 | 2.45 | .014 |
| Other | 1.07 | 2.93 | .003 |
Note: All predictors were z-scored. Since Trauma Type was a categorical variable, it was coded by 4 dummy variables. All 4 dummy variables were included even if some of the individual variables were not significant. The significance test for Trauma Type was the test of the 4 dummy variables as a whole. NAL, Naltrexone. PE, Prolonged Exposure. PDD, Percent Days Drinking. PSS-I, PTSD Symptom Scale Interview.
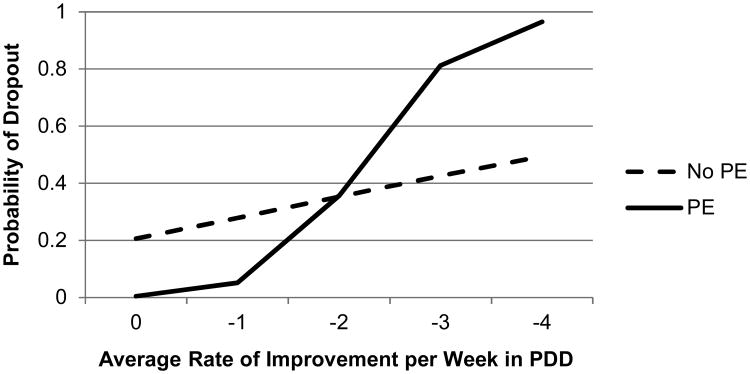
Figure 1.
Effect of drinking improvement on dropout, moderated by treatment condition.
Note: More negative rate indicates faster improvement. PDD, percent days drinking. PE, prolonged exposure.
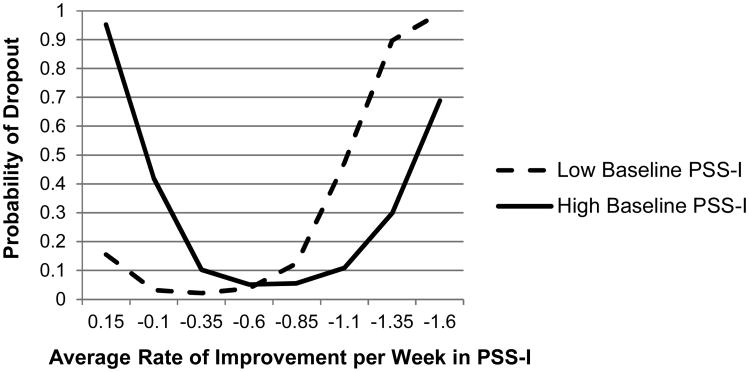
Figure 2.
Effect of PTSD improvement on dropout, moderated by baseline PTSD severity.
Note: More negative rate indicates faster improvement. PSS-I, PTSD Symptom Scale – Interview Version.
Trauma type was a significant predictor of dropout (p<.001). Participants who experienced an accident or “other trauma” had the highest dropout rate (40.0% and 50.0%, respectively), and had significantly higher rates than those who experienced a physical assault (18.6%), b=.81, p=.014 and b=1.07, p=.003. Those with sexual assault trauma and combat trauma had intermediate dropout rates (35.7% and 31.6% respectively), but those rates were not significantly higher than the rate for those with physical assault trauma (ps>.08).
The only other significant predictors of dropout were the slopes of improvement in PDD and PSS-I (see Figures 1 and 2). The relationship between slope of improvement in PDD and dropout was moderated by PE condition, b=7.93, p=.003. Faster slopes of improvement in PDD were significantly related to higher dropout rates for those receiving PE, b=18.59, p<.001. However, for those who did not receive PE, the relationship between PDD improvement and dropout was not significant (see Figure 1), p=.10.
The relationship between slope of improvement in PSS-I and dropout was curvilinear (see Figure 2). Thus, the total effect of slope of PSS-I on dropout was a combination of an overall linear trend plus a significant quadratic trend, b=52.34, p<.001. The linear trend was moderated by baseline PTSD severity, b=-7.15, p=.005. To better understand this moderator effect, we examined the relationship between slope of improvement and dropout for those with high baseline severity (1 SD above the mean, PSS-I=36.0) and for those with low baseline severity (1 SD below the mean, PSS-I=20.3). As depicted in Figure 2, regardless of baseline severity, participants with slopes of improvement near the mean (around -.6 points on the PSS-I per week) showed the lowest dropout rates. Among participants with low baseline severity, dropout rates were low for those who improved slowly and high for those who improved quickly. In contrast, among participants with high baseline severity, dropout rates were high for those who improvement very slowly and for those who improved very quickly.
Exploratory Analyses
We examined whether any of the study variables predicted early dropout (before week 12, n=35) vs. later dropout (after week 12, n=18) using the Fournier analysis. No predictors/moderators emerged that differentiated between these two groups. We also examined whether we could differentiate between very early dropout (before week 4, n=18) and those who dropped out after week 4 (n=35). Again, no significant predictors/moderators emerged. Finally, we examined whether any variable predicted dropout before week 8 (n=29) vs. those who dropped out after week 8 (n=24). No significant predictors or moderators emerged.
Discussion
To our knowledge, the current study is the first to investigate predictors of dropout in a comorbid PTSD-AD sample and used data from a large (n = 165) RCT (Foa et al., 2013). Given generally inconsistent findings from previous studies of dropout, we employed a statistical approach (Fournier et al., 2009) that permits the inclusion of a large number of candidate predictors while controlling for related constructs, but without significant reduction in power. We also investigated slopes of symptom change to evaluate how rates of improvement during treatment impact risk for dropout. Of the wide range of predictors examined, only trauma type and rate of symptom change (PDD and PSS-I) emerged as significant predictors of dropout. These factors correctly predicted dropout status for approximately 89% of the sample, and thus accounted for a very large amount of the observed variance in retention.
Participants who experienced accidents or “other trauma” exhibited the highest dropout rates. Specifically, 50% of patients with “other trauma” and 40% of patients who experienced an accident dropped out of treatment. In contrast, participants who experienced a physical assault had the lowest rate of dropout (18.6%). To our knowledge, this is the first study to find links between trauma type and dropout. “Other trauma” referred to various traumas that did not fit into any of the specific trauma categories. Examples include witnessing violence against others, or finding a loved one unexpectedly deceased. It is possible that because accidents and “other trauma” types are not as widely recognized as potential precursors to PTSD as sexual assault, physical assault, or combat, patients who have experienced these traumas may view themselves differently in terms of their PTSD status or need for ongoing PTSD treatment. Interestingly, findings from the same RCT demonstrated a predictive relationship between trauma type and treatment outcome, such that sexual assault was associated with worse PTSD outcomes, and combat trauma was associated with worse PTSD and drinking outcomes (Zandberg et al., 2016). This suggests that the types of trauma associated with dropout risk are different than those associated with attenuated treatment response. Together, these findings indicate that trauma type may be a particularly important factor to examine further in concurrent PTSD-AD treatment research.
Most of the existing studies on dropout risk have focused exclusively on baseline patient characteristics. In the current study, we examined rates of change in both drinking and PTSD and found that slopes of improvement significantly predicted dropout status. Overall, dropout rates were not significantly different in the groups that included PE, compared to the groups that did not include PE. However, a moderator finding emerged: With respect to drinking behavior, faster rates of improvement in drinking predicted higher risk for dropout, but only among participants receiving PE in addition to BRENDA. This was not the case among participants receiving BRENDA without PE. This finding may reflect the primary motivations of participants in this study, and the differences between the conditions that did and not did include PE. Many participants in the current trial reported to study staff that they were primarily seeking treatment for AD. They were then randomized to treatment that either focused on drinking, or that included concurrent PE. PE involves confrontation of distressing trauma memories and completion of between-session exercises, whereas BRENDA without PE did not place these demands on patients. Participants who were primarily motivated to reduce their drinking and experienced fast reductions in this area (i.e., the symptoms they cared most about) may have perceived the effort required in PE to be unnecessary. Alternatively, it is possible that patients receiving BRENDA and PE concurrently may have perceived the extra effort and distress involved in PE to be a threat to their alcohol recovery. These interpretations require additional study to evaluate empirically.
On measures of PTSD symptom change, rate of improvement predicted dropout for participants in all treatment conditions. Participants who experienced an average rate of PTSD improvement had the lowest dropout rates (less than 10%). Consistent with Krishnamurthy et al. (2015), the impact of improvement on dropout was moderated by baseline severity level. Our study adds to the findings of Krishnamurthy et al. by investigating the curvilinear effects of rate of improvement. This allowed us to detect that, among participants with low baseline PTSD severity, faster rates of improvement predicted higher dropout than slower rates of improvement. In contrast, for those with high initial severity, higher dropout was exhibited for those with both fast and slow rates of improvement.
When PTSD symptoms were lower at baseline, fast improvement predicted much higher dropout, such that over 80% of participants who improved more than 1.35 points on the PSS-I per week dropped out. This may be because these individuals achieved a low level of symptoms relatively early on, and therefore decided that they would not benefit from continuing treatment. Indeed, exploratory analyses indicated that of the 7 participants with low baseline severity and fast improvement, 6 dropped out once their PSS-I score dropped below a 12, which is considered a minimal level of PTSD symptoms. This suggests that, contrary to common assumption, dropout is not necessarily indicative of treatment non-response. In fact, treatment termination may result from accelerated treatment response if baseline PTSD severity is low.
On the other hand, for participants with high baseline PTSD severity, dropout rates were elevated for those with slow or minimal improvement (40% of individuals with -.1 slope dropped out, n=5), and especially for those who showed a trend toward symptom increase (>90% of individuals who showed positive slopes dropped out, n=5). Slow improvement in PTSD symptoms may increase dropout risk by reducing motivation and the perceived credibility of treatment among patients with high PTSD severity. Alternatively, it is possible that continued high PTSD symptoms interfere with treatment participation in a manner that motivates dropout (e.g., interfering with travel to therapy). The current findings suggest that psycho-education preparing patients for the possibility of slow PTSD improvement may be particularly crucial to present and re-visit among patients with higher baseline PTSD severity in order to mitigate dropout risk.
The results of the current study are at odds with the findings of Foa, Zoellner, Feeny, Hembree, & Alvarez-Conrad (2002), who found that PTSD symptom exacerbation did not predict subsequent dropout from PE. However, the current study differs from Foa et al. (2002) in at least four important ways: First, in the current study, positive slopes may have resulted from minor and un-meaningful fluctuations in PTSD scores, whereas Foa et al. operationalized symptom exacerbation to ensure it was a reliable increase in PTSD using session-by-session measures. Second, the current study examined the moderating effects of baseline PTSD severity, and found the effect of positive slope only among participants with high baseline severity (where Foa et al. did not investigate moderators). Third, the effect of positive slope seen here was evident across all treatment groups, and not limited to conditions that included PE. Finally, the current study by definition included individuals with comorbid AD, and the relationship between symptom exacerbation and dropout may be different in this population. Additional research is needed to evaluate the impact of symptom exacerbation among comorbid PTSD/AD patients.
Limitations
Several limitations should be noted. Assessments of PTSD and drinking symptoms took place every four weeks throughout treatment. While these assessments have the advantage of being administered by blind independent evaluators, rather than patient self-report, slope of change calculations would have been more precise with session-by-session data, and future studies investigating the influence of symptom trajectories on dropout should include more frequent assessments. This study did not include individuals with bipolar disorder or additional substance use disorders (with the exception of nicotine and cannabis). Given that both of these comorbidities are common in alcohol dependent populations, it is important to note that the current results may not generalize to these patient presentations. Similarly, this study looked specifically at concurrent treatment of AD and PTSD; thus, the findings may not extend to PTSD-SUD populations. Lastly, all participants in this RCT received supportive counseling (BRENDA) for AD, and were randomized to receive medication or placebo for alcohol symptoms, and/or PE for PTSD. Therefore, the findings may not apply to other forms of combined PTSD-AD treatments (e.g., other behavioral treatments for PTSD), and any findings pertaining to PE reflect the combination of PE and BRENDA.
Conclusions and Future Directions
The current findings suggest that trauma type and rates of within-treatment symptom change are important predictors of dropout among PTSD-AD patients. Patients presenting to care with traumas that are less commonly associated with PTSD (specifically, accidents and events that did not involve sexual abuse, physical assault, or combat) may require additional psychoeducation or other engagement strategies to promote treatment retention. The current results indicate that, across all treatment groups, faster PTSD improvement predicted poor retention in treatment. This finding points to the importance of individualizing treatment length to match patient response. In research settings, this can be accomplished by employing variable length treatment designs rather than requiring a prescribed number of sessions. Further, when baseline PTSD symptoms are moderate or severe, the current study indicates that slow PTSD improvement also increases dropout risk. Given the strong relationship between rates of change and dropout observed in this study, clinicians may improve retention in PTSD-AD treatments by monitoring symptom levels on a regular basis, and discussing these patterns openly with patients to assess their impressions and commitment to ongoing treatment.
We have hypothesized that the effect of improvement rate on dropout may be explained by the match between patient goals and progress in treatment. Future studies would benefit from including process measures that assess patient's primary goals in combined treatment (i.e., drinking reduction vs. PTSD symptom reduction, or both), their treatment preferences, and their satisfaction with their progress at regular intervals during treatment. Session-by-session measures of motivation and the perceived credibility of treatment would likewise provide additional information that could be used to better understand the links between symptom improvement and the decision to drop out of treatment. Finally, future research should further examine the effects of symptom change on dropout when other treatment models for PTSD-AD and PTSD-SUD are used.
Acknowledgments
This study was funded by National Institute on Alcohol Abuse and Alcoholism grant R01 AA 012428 (PI: Dr. Foa).
Footnotes
This transformation reduces skewness (post transformation skewness for PSS-I and PDD were .02 and -.18, respectively), and reverses the direction of the scale. Transformed slopes of improvement are positive, and higher numbers indicate faster improvement. Log transformed scores were used in all statistical analyses and were reconverted to raw scores in the figures and results section.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications, Inc; 1991. [Google Scholar]
- Amir N, Taylor CT, Donohue MC. Predictors of response to an attention modification program in generalized social phobia. Journal of Consulting and Clinical Psychology. 2011;79(4):533–541. doi: 10.1037/a0023808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: Results from National Epidemiological Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2013;132(3):630–638. doi: 10.1016/j.drugalcdep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Dansky BS, Back SE, Foa EB, Carroll KM. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Preliminary\findings. Journal of Substance Abuse Treatment. 2001;21(1):47–54. doi: 10.1016/s0740-5472(01)00182-9. doi: http://dx.doi.org/10.1016/S0740-5472(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry. 2003;60(3):289–294. doi: 10.1001/archpsyc.60.3.289. doi: http://dx.doi.org/10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Eftekhari A, Ruzek JI, Crowley JJ, Rosen CS, Greenbaum MA, Karlin BE. Effectiveness of national implementation of prolonged exposure therapy in veterans affairs care. JAMA Psychiatry. 2013;70(9):949–955. doi: 10.1001/jamapsychiatry.2013.36. doi: http://dx.doi.org/10.1001/jamapsychiatry.2013.36. [DOI] [PubMed] [Google Scholar]
- Elbreder M, de Souza eS, Pillon SC, Laranjeira R. Alcohol dependence: Analysis of factors associated with retention of patients in outpatient treatment. Alcohol and Alcoholism. 2011;46(1):74–76. doi: 10.1093/alcalc/agq078. doi: http://dx.doi.org/10.1093/alcalc/agq078. [DOI] [PubMed] [Google Scholar]
- Filho JMC, Baltieri DA. Psychosocial and clinical predictors of retention in outpatient alcoholism treatment. Revista Brasileira De Psiquiatria. 2012;34(4):413–421. doi: 10.1016/j.rbp.2012.03.003. doi: http://dx.doi.org/10.1016/j.rbp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II) Hoboken, NJ: John Wiley & Sons, Inc; 2004. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcoholism: Clinical and Experimental Research. 1999;23(8):1289–1295. [PubMed] [Google Scholar]
- Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. Journal of Consulting and Clinical Psychology. 1999;67(2):194–200. doi: 10.1037//0022-006x.67.2.194. doi: http://dx.doi.org/10.1037/0022-006X.67.2.194. [DOI] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6(4):459–473. [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Jr, Oslin D, et al. Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. JAMA: Journal of the American Medical Association. 2013;310(5):488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zoellner LA, Feeny NC, Hembree EA, Alvarez-Conrad J. Does imaginal exposure exacerbate PTSD symptoms? Journal of Consulting and Clinical Psychology. 2002;70(4):1022–1028. doi: 10.1037//0022-006x.70.4.1022. doi: http://dx.doi.org/10.1037/0022-006X.70.4.1022. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. Journal of Consulting and Clinical Psychology. 2009;77(4):775–787. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HA, Kelley LP, Rentz TO, Lee S. Pretreatment predictors of dropout from cognitive behavioral therapy for PTSD in Iraq and Afghanistan war veterans. Psychological Services. 2011;8(1):1–11. doi: http://dx.doi.org/10.1037/a0022705. [Google Scholar]
- Graff FS, Morgan TJ, Epstein EE, McCrady BS, Cook SM, Jensen NK, Kelly S. Engagement and retention in outpatient alcoholism treatment for women. The American Journal on Addictions. 2009;18(4):277–288. doi: 10.1080/10550490902925540. doi: http://dx.doi.org/10.1080/10550490902925540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars MA, van Minnen A, Hoogduin KAL. The impact of dissociation and depression on the efficacy of prolonged exposure treatment for PTSD. Behaviour Research and Therapy. 2010;48(1):19–27. doi: 10.1016/j.brat.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric research interview for substance and mental disorders (PRISM): Reliability for substance abusers. The American Journal of Psychiatry. 1996;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Hien DA, Cohen LR, Miele GM, Litt LC, Capstick C. Promising treatments for women with comorbid PTSD and substance use disorders. The American Journal of Psychiatry. 2004;161(8):1426–1432. doi: 10.1176/appi.ajp.161.8.1426. doi: http://dx.doi.org/10.1176/appi.ajp.161.8.1426. [DOI] [PubMed] [Google Scholar]
- Hien DA, Levin FR, Ruglass LM, López-Castro T, Papini S, Hu M, et al. Herron A. Combining seeking safety with sertraline for PTSD and alcohol use disorders: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2015;83(2):359–369. doi: 10.1037/a0038719. doi: http://dx.doi.org/10.1037/a0038719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh DJ, Sitharthan G, Young RM, Sitharthan T, Saunders JB, Shockley N, Giannopoulos V. Addition of cue exposure to cognitive-behaviour therapy for alcohol misuse: A randomized trial with dysphoric drinkers. Addiction. 2006;101(8):1106–1116. doi: 10.1111/j.1360-0443.2006.01488.x. doi: http://dx.doi.org/10.1111/j.1360-0443.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Corrections: Errors in byline, author affiliations, and acknowledgment in: Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62(7):709. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy P, Khare A, Klenck SC, Norton PJ. Survival modeling of discontinuation from psychotherapy: A consumer decision-making perspective. Journal of Clinical Psychology. 2015;71(3):199–207. doi: 10.1002/jclp.22122. doi: http://dx.doi.org/10.1002/jclp.22122. [DOI] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II) Clinical Psychology & Psychotherapy. 2010;18(1):75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Sobell MB, Sobell LC. Reliability of self-reports of low ethanol consumption by problem drinkers over 18 months of follow-up. Drug and Alcohol Dependence. 1982;9(4):273–278. doi: 10.1016/0376-8716(82)90066-7. [DOI] [PubMed] [Google Scholar]
- Marks I, Lovell K, Noshirvani H, Livanou M, Thrasher S. Treatment of posttraumatic stress disorder by exposure and/or cognitive restructuring: A controlled study. Archives of General Psychiatry. 1998;55(4):317–325. doi: 10.1001/archpsyc.55.4.317. [DOI] [PubMed] [Google Scholar]
- McGovern MP, Lambert-Harris C, Acquilano S, Xie H, Alterman AI, Weiss RD. A cognitive behavioral therapy for co-occurring substance use and posttraumatic stress disorders. Addictive Behaviors. 2009;34(10):892–897. doi: 10.1016/j.addbeh.2009.03.009. doi: http://dx.doi.org/10.1016/j.addbeh.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York, NY: Guilford Press; 1991. [Google Scholar]
- Mills KL, Teesson M, Back SE, Brady KT, Baker AL, Hopwood S, et al. Ewer PL. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: A randomized controlled trial. JAMA: Journal of the American Medical Association. 2012;308(7):690–699. doi: 10.1001/jama.2012.9071. [DOI] [PubMed] [Google Scholar]
- Modell JG, Glaser FB, Mountz JM, Schmaltz S, Cyr L. Obsessive and compulsive characteristics of alcohol abuse and dependence: Quantification by a newly developed questionnaire. Alcoholism: Clinical and Experimental Research. 1992;16(2):266–271. doi: 10.1111/j.1530-0277.1992.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Reports. 2015;7:43. doi: 10.12703/P7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najavits LM, Weiss RD, Shaw SR, Muenz LR. “Seeking safety”: Outcome of a new cognitive-behavioral psychotherapy for women with posttraumatic stress disorder and substance dependence. Journal of Traumatic Stress. 1998;11(3):437–456. doi: 10.1023/A:1024496427434. [DOI] [PubMed] [Google Scholar]
- Powers MB, Gillihan SJ, Rosenfield D, Jerud AB, Foa EB. Reliability and validity of the PDS and PSS-I among participants with PTSD and alcohol dependence. Journal Of Anxiety Disorders. 2012;26(5):617–623. doi: 10.1016/j.janxdis.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Bryan A. Psychosocial predictors of treatment outcome, dropout, and change processes in a pharmacological clinical trial for alcohol dependence. Addictive Disorders & their Treatment. 2006;5(4):179–190. doi: http://dx.doi.org/10.1097/01.adt.0000210701.63165.5a. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behaviour Research and Therapy. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rizvi SL, Vogt DS, Resick PA. Cognitive and affective predictors of treatment outcome in cognitive processing therapy and prolonged exposure for posttraumatic stress disorder. Behaviour Research and Therapy. 2009;47(9):737–743. doi: 10.1016/j.brat.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannibale C, Teesson M, Creamer M, Sitharthan T, Bryant RA, Sutherland K, et al. O'Leary M. Randomized controlled trial of cognitive behaviour therapy for comorbid posttraumatic stress disorder and alcohol use disorders. Addiction. 2013;108(8):1397–1410. doi: 10.1111/add.12167. [DOI] [PubMed] [Google Scholar]
- Schilling RF, Sachs C. Attrition from an evening alcohol rehabilitation program. The American Journal of Drug and Alcohol Abuse. 1993;19(2):239–248. doi: 10.3109/00952999309002683. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Hofmann SG, Rosenfield D, DeBoer LB, Costa PT, Simon NM, et al. Pollack MH. D-cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: Prognostic and prescriptive variables. Journal of Consulting and Clinical Psychology. 2013;81(6):1100–1112. doi: 10.1037/a0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. Timeline follow-back: A technique for assessing self-reported alcohol consumption; pp. 41–72. [Google Scholar]
- Taylor S, Thordarson DS, Maxfield L, Fedoroff IC, Lovell K, Ogrodniczuk J. Comparative efficacy, speed, and adverse effects of three PTSD treatments: Exposure therapy, EMDR, and relaxation training. Journal of Consulting and Clinical Psychology. 2003;71(2):330–338. doi: 10.1037/0022-006x.71.2.330. doi: http://dx.doi.org/10.1037/0022-006X.71.2.330. [DOI] [PubMed] [Google Scholar]
- Starosta AN, Leeman RF, Volpicelli JR. The BRENDA model: Integrating psychosocial treatment and pharmacotherapy for the treatment of alcohol use disorders. Journal of Psychiatric Practice. 2006;12(2):80–89. doi: 10.1097/00131746-200603000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6th. Boston: Pearson; 2013. [Google Scholar]
- Taylor S, Koch WJ, Crockett DJ. Anxiety sensitivity, trait anxiety, and the anxiety disorders. Journal of Anxiety Disorders. 1991;5(4):293–311. [Google Scholar]
- Torrens M, Serrano D, Astals M, Pérez-Domínquez G, Sr, Martín-Santos R. Diagnosing comorbid psychiatric disorders in substance abusers: Validity of the Spanish versions of the psychiatric research interview for substance and mental disorders and the structured clinical interview for DSM-IV. The American Journal of Psychiatry. 2004;161(7):1231–1237. doi: 10.1176/appi.ajp.161.7.1231. doi: http://dx.doi.org/10.1176/appi.ajp.161.7.1231. [DOI] [PubMed] [Google Scholar]
- Triffleman E. Gender differences in a controlled pilot study of psychosocial treatments in substance dependent patients with post-traumatic stress disorder: Design considerations and outcomes. Alcoholism Treatment Quarterly. 2000;18(3):113–126. [Google Scholar]
- Van Minnen A, Arntz A, Keijsers GPJ. Prolonged exposure in patients with chronic PTSD: Predictors of treatment outcome and dropout. Behaviour Research and Therapy. 2002;40(4):439–457. doi: 10.1016/s0005-7967(01)00024-9. [DOI] [PubMed] [Google Scholar]
- Vuoristo-Myllys S, Lahti J, Alho H, Julkunen J. Predictors of dropout in an outpatient treatment for problem drinkers including cognitive–behavioral therapy and the opioid antagonist naltrexone. Journal of Studies on Alcohol and Drugs. 2013;74(6):894–901. doi: 10.15288/jsad.2013.74.894. [DOI] [PubMed] [Google Scholar]
- Wheaton M, Rosenfield D, Foa EP, Simpson HB. Augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: What moderates improvement? Journal of Consulting and Clinical Psychology. 2015 doi: 10.1037/ccp0000025. Advance online publication. doi: http://dx.doi.org/10.1037/ccp0000025. [DOI] [PMC free article] [PubMed]
- Zandberg LJ, Rosenfield D, McLean CP, Powers MB, Asnaani A, Foa EB. Concurrent treatment of post-traumatic stress disorder and alcohol dependence: Predictors and moderators of outcome. Journal of Consulting and Clinical Psychology. 2016;84(1):43–56. doi: 10.1037/ccp0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayfert C, DeViva JC, Becker CB, Pike JL, Gillock KL, Hayes SA. Exposure utilization and completion of cognitive behavioral therapy for PTSD in a “real world” clinical practice. Journal of Traumatic Stress. 2005;18(6):637–645. doi: 10.1002/jts.20072. doi: http://dx.doi.org/10.1002/jts.20072. [DOI] [PubMed] [Google Scholar]