Abstract
Purpose
Prior research has identified unrealistic optimism as a bias that might impair informed consent among patient-subjects in early phase oncology trials. Optimism, however, is not a unitary construct – it can also be defined as a general disposition, or what is called dispositional optimism. We assessed whether dispositional optimism would be related to high expectations for personal therapeutic benefit reported by patient-subjects in these trials but not to the therapeutic misconception. We also assessed how dispositional optimism related to unrealistic optimism.
Methods
Patient-subjects completed questionnaires designed to measure expectations for therapeutic benefit, dispositional optimism, unrealistic optimism, and the therapeutic misconception.
Results
Dispositional optimism was significantly associated with higher expectations for personal therapeutic benefit (Spearman r=0.333, p<0.0001), but was not associated with the therapeutic misconception. (Spearman r=−0.075, p=0.329). Dispositional optimism was weakly associated with unrealistic optimism (Spearman r=0.215, p=0.005). In multivariate analysis, both dispositional optimism (p=0.02) and unrealistic optimism (p<0.0001) were independently associated with high expectations for personal therapeutic benefit. Unrealistic optimism (p=.0001), but not dispositional optimism, was independently associated with the therapeutic misconception.
Conclusion
High expectations for therapeutic benefit among patient-subjects in early phase oncology trials should not be assumed to result from misunderstanding of specific information about the trials. Our data reveal that these expectations are associated with either a dispositionally positive outlook on life or biased expectations about specific aspects of trial participation. Not all manifestations of optimism are the same, and different types of optimism likely have different consequences for informed consent in early phase oncology research.
Keywords: Dispositional Optimism, Therapeutic Misconception, Therapeutic Optimism, Informed Consent, Cancer Research
Patient-subjects in early phase oncology trials often report high expectations for personal therapeutic benefit. Although it is true that some participants may benefit, these trials are not designed to provide participants with therapeutic benefit.1 The ethical significance of this optimism therefore has been an on-going concern among researchers and ethicists.1–7 Although some have claimed that expressions of optimism alone are never problematic in clinical research3, prior studies have documented the potential for optimism to impair informed consent.5,6 This apparent contradiction may be explained by the fact that optimism is not a unitary psychological construct.8 Failure to distinguish the different types of optimism can lead researchers and ethicists to disagree when debating its ethical significance for informed consent.
One type of optimism, unrealistic optimism, is an event-specific bias that has been associated with distortions in risk/benefit assessment in a range of health-related contexts including early phase oncology trials.5,9–11 People possessing this bias tend to engage in defensive processing of information, overestimating their prospects for benefit and/or underestimating their susceptibility to risks associated with the event in question. However, a different type of optimism is an enduring personality characteristic referred to as dispositional optimism. People high in dispositional optimism tend to expect positive future outcomes, in the aggregate. Dispositional optimism has not been associated with defensive processing of information, and instead has been found to be positively related to how people attend to and process risk/benefit information, as well as effective coping and well-being.11–14 No research has attempted to determine the extent to which expectations for personal therapeutic benefit reported by patient-subjects in early phase oncology trials are associated with the generally adaptive dispositional optimism rather than the problematic unrealistic optimism. It is also not known how dispositional optimism relates to other factors commonly associated with these high expectations, such as the therapeutic misconception (i.e., the failure to grasp the differences between clinical research and beneficent medical care).15
We conducted a multicenter study to examine these issues. We investigated the relationship between dispositional optimism and expectations of personal therapeutic benefit reported by patient-subjects enrolled in early phase oncology trials. Given the prevalence of dispositional optimism in the general population, we predicted that it would be present among the participants in our study. We hypothesized that dispositional optimism would predict high expectations for personal therapeutic benefit expressed by patient-subjects enrolled in early phase oncology trials, but that it would not be positively correlated with either the therapeutic misconception or unrealistic optimism. Past research has found that dispositional optimists “are more flexible and adaptive in their consideration of information about potential problems and stressors.”13 Improved information processing, we conjectured, could make dispositionally optimistic patient-subjects less susceptible to the therapeutic misconception. Further, past research has found that dispositional optimism is not generally correlated with unrealistic optimism, although it may have independent (and possibly interactive) effects with it.10,16
METHODS
Participants
Participants were patient-subjects enrolled in early phase oncology trials (phase I, I/II or II) at two major cancer centers in the United States. They were 18 years of age or older, and able to speak and read English. The Institutional Review Boards at both sites approved the study.
Definitions, Procedures and Measures
Participants gave written informed consent and participated in a structured face-to-face interview with a research associate who had been trained to administer the questionnaires.
Dispositional Optimism
Dispositional optimism refers to “the generalized positive expectancy that one will experience good outcomes.”11 People who score high in dispositional optimism tend generally to accentuate the positive and downplay the negative. Consistent with established practice, we used the Revised Life Orientation Test (LOT-R) to measure dispositional optimism. The LOT-R was developed by Carver and Scheier and has demonstrated discriminant validity and reliability in numerous studies.17 This instrument consists of ten self-report items, four of which are filler items. The self-report items ask patient-subjects to respond to statements such as “In uncertain times, I usually expect the best” and “Overall, I expect more good things to happen to me than bad.” Each item is rated on a 5-point scale ranging from 0 (strongly disagree) to 4 (strongly agree). We analyzed the six dispositional optimism questions individually, as well as the mean score on all items combined. To calculate LOT-R scores, we dropped the four filler questions, reverse-coded the negatively worded questions (e.g., “I hardly ever expect things to go my way”), and added them to the positively worded questions (e.g., “I am always optimistic about my future”). The total LOT-R score was used as a measure of dispositional optimism (DO score). In addition, the optimism and pessimism sub-scales (obtained from the 3 items each with positive and negative wording respectively) were analyzed separately.
Expectations for Personal Therapeutic Benefit
Expectations for personal therapeutic benefit refer to patient-subjects’ non-comparative expressions of hope/concern regarding their own participation in the trial in which they are enrolled. Unlike dispositional optimism, expectations for personal therapeutic benefit are event-specific. We used a Personal Therapeutic Benefit Questionnaire to measure these expressions of hope/concern. Patient-subjects rated their expectations of experiencing 7 research-related benefits and their personal concern about experiencing 2 research-related risks (the ratings of the latter questions were reversed). Sample questions include “indicate your own personal hope about having your cancer controlled by the drugs you get in the trial” and “indicate your own personal concern about experiencing a health problem caused by the drugs you get in the trial.” Responses were given on a four-point Likert scale ranging from “I don’t feel at all optimistic/concerned about this happening to me” to (4) “I feel quite optimistic/concerned about this happening to me.” A Personal Therapeutic Benefit score was determined by calculating the mean score of the nine questions. The Personal Therapeutic Benefit Questionnaire was developed by this research team, pilot tested among cancer patients, and demonstrated ample face validity. Cronbach Coefficient Alpha was 0.86, which is considered a good internal consistency.
Therapeutic Misconception
The Therapeutic Misconception occurs when patient-subjects conflate the contexts of research and therapy, thereby inaccurately attributing therapeutic intent to research procedures.2 The TM scale was used to determine the presence and magnitude of the therapeutic misconception. The TM scale asks patient-subjects to rate their level of agreement with respect to ten research-related statements.18–19 A sample statement is “a researcher’s most important task is to make sure that the research will help the people who participate.” Each item was rated on a 5-point scale ranging from 1 (agree) to 5 (disagree). The ratings were reversed to ease interpretation of results. The scale includes three dimensions associated with TM: perceptions of the degree of individualization of the intervention, benefit from participation, and the purpose of the trial. The TM scale was developed by Appelbaum and Lidz and has demonstrated reliability in several previous studies.18–19 A total TM score, as well as a total score per dimension, were determined by calculating the mean score of the ten questions and the mean score of each dimension respectively.
Unrealistic Optimism
Unrealistic optimism is a bias in which a person mistakenly believes that she is more likely to experience positive outcomes with respect to a specific event compared to similar others facing the same event. Unlike dispositional optimism, unrealistic optimism is event-specific; unlike expectations for personal therapeutic benefit it is comparative. We used the Comparative Risk/Benefit Assessment Questionnaire (CRBA) modeled after an instrument developed by Weinstein to measure unrealistic optimism.5,20 (The CRBA questionnaire is the standard method for measuring perceived comparative risk.9–11) This questionnaire asks respondents to compare their chances of experiencing 9 research-related events with the chances of similar others experiencing these same events. A sample question is “Compared with other patients invited to participate in the same cancer research trial you are invited to participate in, what are the chances that your life expectancy will be increased by the drugs you get in the trial?” Respondents then answer the question by choosing one response on a seven-point interval scale, with values from −3 (much below average) to +3 (much above average). Comparative risk/benefit judgments are considered unbiased when the mean judgment of the group is zero. Each item score, as well as the mean score of all items, was used in the statistical analyses.
Statistical Considerations
Data were entered in a REDCap (http://project-redcap.org/) database using a double entry procedure. Discrepancies in data entry were identified and corrected by a third operator. Descriptive statistics (e.g., percent for categorical variables, and mean and standard deviation for continuous variables) were used to summarize demographic and clinical characteristics. Spearman’s correlation coefficient was used to assess the association of dispositional optimism and its two subscales with the other measures and the association of therapeutic misconception with unrealistic optimism and expectations of personal therapeutic benefit. Kruskal-Wallis test or Wilcoxon rank sum test were used to assess the association between the dispositional optimism score and demographic characteristics.
Univariate and multivariate linear regression analyses were carried out to further evaluate factors that were independently associated with expectations of personal therapeutic benefit and therapeutic misconception (dependent variables). Factors tested (independent variables) included total unrealistic optimism score, dispositional optimism score, age, gender, study site, ethnicity, education, religion, cancer type, participation in previous research, domestic status, marital status and, interchangeably, therapeutic misconception and expectations for personal therapeutic benefit. Stepwise procedure was performed for variable selection. Standard model diagnostics were performed, testing first for non-linearity of independent variables vs. dependent variable. Additionally, we examined outliers and influential points looking at studentized residuals, Cook’s D and DFITTS. We then tested for normality of residuals using kernel density plot, quantile-quantile plot and the Shapiro-Wilk test. Multicollinearity was inspected using variance inflation factor (VIF). The model diagnostics did not show any significant deviations from the model assumptions. The scatterplots of response and factors variables showed linearity, and there were no detectable influential points or outliers. The residuals were approximately normal, and there was no indication of significant multicollinearity.
A p-value of < .05 was considered statistically significant. Data management and analysis were conducted using Statistical Analysis System (SAS) version 9.4.21
RESULTS
Patient Characteristics
We approached 233 patient-subjects who were enrolled in early phase oncology trials. Of these, 171 (73%) agreed to be interviewed for our study. Demographic and clinical characteristics of the participants are presented in Table 1. The average age was 59 years old. We interviewed slightly more women than men (51% vs. 49%). The largest race/ethnic group was white (non-Hispanic) (85%). A substantial majority of the participants had completed college (68%); were married (67%); and had not participated in a prior clinical research study (69%).
Table 1.
Demographic Characteristics
| Characteristics | No. of patients (N=171) | % | Mean | Min | Max | SD | |
|---|---|---|---|---|---|---|---|
| Age | 58.64 | 18 | 85 | 12.78 | |||
|
| |||||||
| Age Group | (18–49) | 31 | 18.13 | ||||
| (50–64) | 73 | 42.69 | |||||
| (65–74) | 57 | 33.33 | |||||
| (75 +) | 10 | 5.85 | |||||
|
| |||||||
| Study Site | Site 1 | 85 | 49.71 | ||||
| Site 2 | 86 | 50.29 | |||||
|
| |||||||
| Gender | Male | 84 | 49.12 | ||||
| Female | 87 | 50.88 | |||||
|
| |||||||
| Race | White(non-Hispanic) | 146 | 85.38 | ||||
| African American/or Black of US origin | 17 | 9.94 | |||||
| Other | 8 | 4.68 | |||||
|
| |||||||
| Education | Grade school/High school | 54 | 31.58 | ||||
| College | 81 | 47.37 | |||||
| Graduate/Prof. School | 36 | 21.05 | |||||
|
| |||||||
| Religion | Protestant | 65 | 38.01 | ||||
| Catholic | 26 | 15.2 | |||||
| Agnostic | 12 | 7.02 | |||||
| Other† | 68 | 39.77 | |||||
|
| |||||||
| Cancer Problem | Blood cancer | 54 | 31.58 | ||||
| Lung Cancer | 26 | 15.2 | |||||
| Breast cancer | 23 | 13.45 | |||||
| Other | 68 | 39.77 | |||||
|
| |||||||
| Domestic Status (Do you live alone) | Yes | 31 | 18.13 | ||||
| No | 140 | 81.87 | |||||
|
| |||||||
| Marital Status | Single | 28 | 16.47 | ||||
| Married | 115 | 67.65 | |||||
| Divorced | 17 | 10 | |||||
| Widowed | 10 | 5.88 | |||||
|
| |||||||
| Participation in Previous Research Study | Yes | 53 | 30.99 | ||||
| No | 118 | 69.01 | |||||
|
| |||||||
| Phase of Clinical Trial | Phase I | 89 | 52.05 | ||||
| Phase II | 63 | 36.84 | |||||
| Phase I/II | 19 | 11.11 | |||||
Includes Baptist, Christian, Methodist, Buddhist, Spiritual, none, no response etc.
Magnitude of Dispositional Optimism, Therapeutic Misconception, Expectations for Personal Therapeutic Benefit and Unrealistic Optimism
Descriptive statistics on the LOT-R questionnaire (dispositional optimism), the therapeutic misconception scale, the personal therapeutic benefit questionnaire and CRBA questionnaire are reported in Table 2. Neither dispositional optimism nor unrealistic optimism was significantly associated with study site, age, gender, race/ethnicity, education, religion or type of cancer (p>0.05). See Table 3.
Table 2.
Dispositional Optimism, Therapeutic Misconception, Expected Therapeutic Benefit and Unrealistic Optimism Descriptive Statistics
| Variables | Theoretical range | Mean Scores | Std. Dev. | 25th Percentile | 75th Percentile |
|---|---|---|---|---|---|
| Dispositional Optimism | (1.67 – 5) | 4.07 | 0.24 | 3.67 | 4.67 |
| Therapeutic Misconception | (1.30 – 5) | 3.79 | 0.77 | 3.4 | 4.4 |
| Expected Therapeutic Benefit | (1.66 – 4) | 3.16 | 0.59 | 2.67 | 3.67 |
| Unrealistic Optimism | (−1.77 – 3) | 1 | 0.97 | 0.33 | 1.67 |
Table 3.
Association between Demographic Characteristics and Types of Optimism
| Demographic Characteristics | Level | Dispositional Optimism | Unrealistic Optimism | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | P-Value | Mean | SD | P-Value | ||
| Study Site | Site 1 | 85 | 3.959 | 0.711 | 0.054 | 0.928 | 0.779 | 0.195 |
| Site 2 | 86 | 4.184 | 0.573 | 1.074 | 1.127 | |||
|
| ||||||||
| Age Group | (18–49) | 31 | 3.919 | 0.722 | 0.344 | 0.853 | 0.878 | 0.288 |
| (50–64) | 73 | 4.071 | 0.670 | 1.026 | 0.967 | |||
| (65–74) | 57 | 4.114 | 0.611 | 0.981 | 1.057 | |||
| (75 +) | 10 | 4.317 | 0.506 | 1.400 | 0.713 | |||
|
| ||||||||
| Gender | Male | 84 | 4.030 | 0.678 | 0.499 | 1.089 | 0.882 | 0.236 |
| Female | 87 | 4.113 | 0.630 | 0.917 | 1.046 | |||
|
| ||||||||
| Race | White(non-Hispanic) | 146 | 4.078 | 0.653 | 0.821 | 0.994 | 0.912 | 0.684 |
| African American/or Black of US origin | 17 | 4.088 | 0.693 | 0.974 | 1.387 | |||
| Other | 8 | 3.938 | 0.642 | 1.194 | 1.082 | |||
|
| ||||||||
| Education | Grade school/High school | 54 | 3.935 | 0.626 | 0.130 | 0.940 | 1.072 | 0.795 |
| College | 81 | 4.111 | 0.694 | 1.058 | 0.917 | |||
| Graduate/Prof. School | 36 | 4.190 | 0.578 | 0.966 | 0.945 | |||
|
| ||||||||
| Religion | Agnostic | 12 | 4.111 | 0.613 | 0.847 | 0.630 | 0.518 | 0.179 |
| Protestant | 65 | 4.097 | 0.639 | 0.906 | 0.981 | |||
| Catholic | 26 | 4.109 | 0.757 | 1.192 | 0.882 | |||
| Other | 68 | 4.027 | 0.643 | 1.085 | 1.038 | |||
|
| ||||||||
| Cancer Problem | Blood cancer | 54 | 4.065 | 0.607 | 0.342 | 0.961 | 0.880 | 0.636 |
| Breast cancer | 23 | 4.196 | 0.670 | 1.184 | 1.161 | |||
| Lung cancer | 26 | 4.205 | 0.675 | 1.158 | 0.987 | |||
| Other | 68 | 3.985 | 0.674 | 0.912 | 0.968 | |||
Association of Dispositional Optimism with Expectations for Personal Therapeutic Benefit
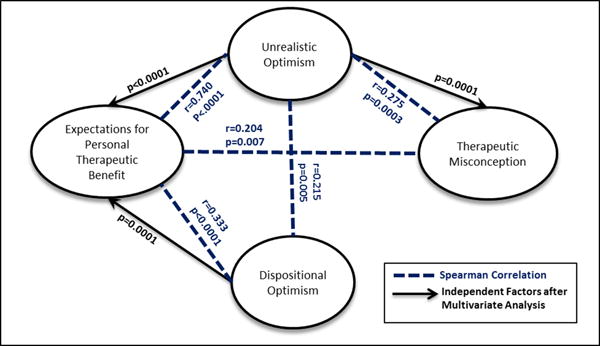
Consistent with our hypotheses, dispositional optimism was significantly associated with higher expectations for personal therapeutic benefit (Spearman r=0.333, p<0.0001). Regarding the subscales of the LOT-R, expectations for personal therapeutic benefit were strongly positively associated with the optimism subscale (Spearman r=0.404, p<0.0001), and weakly negatively associated with the pessimism subscale (Spearman r=−.192, p=0.012).
Association of Dispositional Optimism with Therapeutic Misconception
Consistent with our hypotheses, dispositional optimism was not associated with the therapeutic misconception (Spearman r=−0.075, p=0.329). The optimism and pessimism subscales were also not associated with the total TM score (respectively: Spearman r=0.085, p=0.267; Spearman r=0.136, p=0.077), although the optimism subscale was correlated with perceptions of likelihood of benefit (Spearman r=0.156, p=0.041).
Association of Dispositional Optimism and Unrealistic Optimism
Dispositional optimism was weakly associated with unrealistic optimism (Spearman r=0.215, p=0.005). The optimism subscale of the LOT-R held a stronger association with unrealistic optimism (Spearman r=0.279, p=0.0002); the pessimism subscale was not associated with unrealistic optimism (Spearman r=−0.127, p=0.097).
Factors Associated with Therapeutic Misconception
In univariate regression analyses, five factors were significantly associated with therapeutic misconception: Male gender (p=0.004), an educational level of HS or less (p<0.0001), participation in previous research (p=0.007), total unrealistic optimism score (p=0.0003) and expectation for personal therapeutic benefit (p=0.007). In multivariate regression analysis (Table 4), however, expectation for personal benefit and previous research participation dropped out of the model and the factors that were independently associated with therapeutic misconception were male gender (p=0.004), lower education level (p<0.0001), and unrealistic optimism (p=0.0001). Thus, whereas dispositional optimism was not independently associated with therapeutic misconception, unrealistic optimism was.
Table 4.
Univariate Linear Regression Analysis to evaluate the Association with Expectations for Personal Therapeutic Benefit and Therapeutic Misconception (significant association was indicated in bold)
| \Expectations for Personal Therapeutic Benefit
|
Therapeutic Misconception
|
||||||
|---|---|---|---|---|---|---|---|
| Variable | Comparison | Estimate | SE | Overall P-value | Estimate | SE | Overall P-value |
| Age | −0.002 | 0.004 | 0.639 | 0.006 | 0.005 | 0.222 | |
|
| |||||||
| Study Site | Site 1 vs. Site 2 | −0.081 | 0.091 | 0.376 | −0.170 | 0.117 | 0.149 |
|
| |||||||
| Gender | Male vs. Female | 0.013 | 0.091 | 0.889 | 0.338 | 0.115 | 0.004 |
|
| |||||||
| Ethnic group | White vs. Other | −0.057 | 0.217 | 0.852 | −0.538 | 0.276 | 0.073 |
| African American/or Black of US origin vs. Other | −0.131 | 0.256 | 0.326 | 0.326 | |||
|
| |||||||
| Education | Grade School/High School vs. Graduate/Prof. School | 0.053 | 0.128 | 0.655 | 0.572 | 0.157 | <.0001 |
| College vs. Graduate/Prof. School | 0.107 | 0.119 | 0.029 | 0.146 | |||
|
| |||||||
| Religion | Agnostic vs. Other | −0.135 | 0.187 | 0.887 | −0.448 | 0.237 | 0.062 |
| Protestant vs. Other | −0.050 | 0.104 | −0.317 | 0.132 | |||
| Catholic vs. Other | −0.011 | 0.138 | −0.157 | 0.175 | |||
|
| |||||||
| Cancer Type | Blood Cancer vs. Other | −0.004 | 0.108 | 0.373 | −0.070 | 0.140 | 0.273 |
| Breast Cancer vs. Other | 0.213 | 0.143 | 0.067 | 0.185 | |||
| Lung Cancer vs. Other | 0.131 | 0.137 | 0.283 | 0.177 | |||
|
| |||||||
| Previous Research | Yes vs. No | −0.027 | 0.098 | 0.784 | −0.342 | 0.125 | 0.007 |
|
| |||||||
| Domestic Status (Do you live alone) | Yes vs. No | −0.117 | 0.118 | 0.323 | −0.077 | 0.153 | 0.615 |
|
| |||||||
| Marital Status | Single vs. Widowed | 0.115 | 0.219 | 0.243 | −0.035 | 0.285 | 0.816 |
| Married vs. Widowed | 0.227 | 0.196 | 0.110 | 0.255 | |||
| Divorced vs. Widowed | 0.422 | 0.236 | 0.119 | 0.309 | |||
|
| |||||||
| Total DO | 0.274 | 0.067 | <.0001 | −0.054 | 0.090 | 0.548 | |
|
| |||||||
| Total UO | 0.453 | 0.032 | <.0001 | 0.217 | 0.059 | 0.0003 | |
|
| |||||||
| Total TM/Total Expected Therapeutic Benefit | 0.158 | 0.058 | 0.007 | 0.264 | 0.097 | 0.007 | |
Factors Associated with Expectations for Personal Therapeutic Benefit
In univariate analyses, high expectations for personal therapeutic benefit were significantly associated with unrealistic optimism (p<0.0001), total therapeutic misconception score (p=0.007) and dispositional optimism (p<0.0001) (Figure 1). In multiple linear regression analysis, however, only dispositional optimism (p=0.02) and unrealistic optimism (p<0.0001) were found to be independent predictors of high expectations for personal therapeutic benefit (Table 5).
Figure 1.
Factors Associated with Expectations for Personal Therapeutic Benefit
Table 5.
Multivariate Regression Analysis of Factors that are Independently Associated with Personal Therapeutic Benefit and Therapeutic Misconception
| Variable | Comparison | Estimate | SE | P-value | |
|---|---|---|---|---|---|
| Independent Factors of Therapeutic Misconception* | Gender | Male vs. Female | 0.310 | 0.105 | 0.004 |
|
| |||||
| Education | Grade School/High School vs. Graduate/Prof. School | 0.601 | 0.146 | <.0001 | |
| College vs. Graduate/Prof. School | 0.036 | 0.136 | |||
|
| |||||
| Total UO Score | 0.215 | 0.054 | 0.0001 | ||
|
| |||||
| Independent Factors of Expectations for Personal Therapeutic Benefit* | Total DO | 0.112 | 0.048 | 0.020 | |
|
| |||||
| Total UO | 0.434 | 0.032 | <.0001 | ||
DISCUSSION
Discussions of optimism in clinical research elicit mixed opinions. Some contend that optimism among trial participants is never ethically problematic.3 Others express concern that optimism in this context reveals a failure of informed consent.5–6 Our study addresses part of this disagreement by exploring two distinct phenomena represented by the term “optimism,” each of which may have different ethical implications.
We investigated whether dispositional optimism could help to explain, in part, the expectations for personal therapeutic benefit expressed by patient-subjects in early phase oncology trials, without entailing commonly studied problems in informed consent. We confirmed our expectation that dispositional optimism among the patient-subjects we studied would be associated with high expectations for personal therapeutic benefit. In fact, dispositional optimism predicted high expectations for personal therapeutic benefit independently of other variables. These findings are not unexpected, as dispositional optimism is commonly defined in terms of generalized positive outcome expectancies.22 Given that dispositional optimism is regarded as a trait and not a product of misunderstanding or irrationality, it should not provoke ethical concern among researchers unless it is associated with other factors that have been found to impair informed decision making. Indeed, expectations for therapeutic benefit that result from a dispositionally optimistic orientation and are not associated with either misunderstanding or bias may reflect hopeful feelings rather than any failure to appreciate relevant information.
Importantly, we found that dispositional optimism was not significantly associated with the therapeutic misconception. Although a substantial number of the patient-subjects we interviewed manifested the therapeutic misconception, dispositional optimism did not appear to be a factor that accounted for it. Research on dispositional optimism in other contexts has found that dispositionally optimistic people, when confronted with a stressful life event, are more likely than people not dispositionally optimistic to process and retain the full range of information provided to them, including negative or unwelcome information.12–14, 22 On this basis, we conjectured that dispositional optimism would not be associated with the therapeutic misconception. In contrast to some recent suggestions in the literature,23–24 the fact that the dispositionally optimistic participants in our study were neither more or less likely to suffer from the therapeutic misconception suggests that therapeutic misconception does not merely reflect participants’ hopeful expressions about the results of their participation in a study.
We found an association between dispositional optimism and unrealistic optimism, but the association was weak. This finding is consistent with past work that shows that dispositional optimism and unrealistic optimism are not strongly associated and represent different types of optimism.11
Past research has found a link between unrealistic optimism and deficits in informed consent.5,6 In our study, whereas dispositional optimism and unrealistic optimism both predicted high expectations for personal therapeutic benefit, only unrealistic optimism predicted the therapeutic misconception. Thus, dispositional optimism appeared to account for some of the high expectations for personal benefit reported by the patient-subjects we studied without being strongly associated with either unrealistic optimism or the therapeutic misconception.
Our study provides clinicians and researchers with a more complete and balanced understanding of how optimism relates to the therapeutic expectations of patient-subjects enrolled in early phase cancer trials. Its findings reveal the ethical complexity of optimism for informed consent in early phase oncology trials. Although one type of optimism can be an ethically benign, and possibly adaptive, dispositional orientation to stressful events, another type of optimism appears to be a bias that has the potential to compromise informed consent. Without carefully distinguishing dispositional optimism from unrealistic optimism, no single conclusion can be drawn about the ethical significance of optimistic expectations for therapeutic benefit in this context.
Our study was subject to some limitations. First, the sample was predominantly White (non-Hispanic); it remains unclear whether our findings are generalizable to other demographic groups. Our study included only adult patient-subjects, mostly of middle-age, enrolled in early phase oncology trials. We do not know whether our findings are generalizable to other research populations, for example patient-subjects of other ages, including children and adolescents, or patient-subjects enrolled in later phase cancer trials or in other clinical research. Second, our study did not investigate other potentially adaptive consequences of dispositional optimism, such as its association with psychological adjustment or improved coping, or potential negative outcomes. A complete picture of the ethical significance of dispositional optimism for patient-subjects enrolled in early phase oncology trials would need to take into account these further potential benefits and harms.
CONCLUSION
High expectations for therapeutic benefit among patient-subjects in early phase oncology trials should not be assumed to result only from misunderstanding of specific information about the trials. Instead our data reveal that these expectations may be associated with either a general, dispositionally positive outlook on life or biased expectations about specific aspects of trial participation.
Although unrealistic optimism may impair informed consent, dispositional optimism likely does not. As dispositional optimism is a stable personality characteristic, there is little reason to think that it would be dampened by interventions to combat misunderstanding and bias about clinical trials. Thus our findings suggest that those who have claimed that optimism is not a problem for informed consent in early phase oncology trials are partly correct and partly incorrect. Investigators need to know that all forms of optimism are not the same, and that different types of optimism likely have different consequences for informed consent.
Acknowledgments
We thank the patients who participated in this study as well as Kathy Schwend, Noe Coopersmith and Andrea Kuchler for assistance in data collection.
Funding: NIH/NCI grant RO1CA166556. Biostatistics support (MM, RD) was provided by the Biostatistics Shared Resource of the Knight Cancer Institute (NCI 5P30CA069533-16).
Research was performed at Oregon Health & Science University/Knight Cancer Institute and the West Clinic in Tennessee.
Footnotes
Conflict of Interest: Authors have no conflicts of interest to report.
Author Contributions: Lynn A. Jansen: Conceptualization, methodology, software, investigation, resources, writing – original draft, writing – review and editing, visualization, supervision, project administration, and funding acquisition. Daruka Mahadevan: Conceptualization, formal analysis, investigation, writing – original draft, writing – review and editing, supervision, project administration, and funding acquisition. Paul S. Appelbaum: Methodology and writing – review and editing. William MP Klein: Conceptualization, methodology, writing – original draft, writing – review and editing, and supervision. Neil D. Weinstein: Conceptualization, methodology, validation, resources, writing – original draft, and writing – review and editing. Motomi Mori: Methodology, formal analysis, writing – review and editing, visualization, and supervision. Racky Daffé: Methodology, software, validation, formal analysis, investigation, data curation, writing – original draft, writing – review and editing, and visualization. Daniel P. Sulmasy: Conceptualization, methodology, validation, resources, writing – original draft, and writing – review and editing.
Contributor Information
Lynn A. Jansen, Email: jansen@ohsu.edu, Madeline Brill Nelson Chair in Ethics Education-Oregon Health & Science University.
Daruka Mahadevan, Email: dmahadevan@WESTCLINIC.com, Director, New Therapeutics Program and Associate Director of Research – University of Tennessee and West Clinic.
Paul S. Appelbaum, Email: psa21@columbia.edu, Dollard Professor of Psychiatry, Medicine, & Law/Director, Division of Law, Ethics, and Psychiatry/Columbia University College of Physicians & Surgeons.
William MP Klein, Email: kleinwm@mail.nih.gov, Associate Director: Behavioral Research Program-National Institutes of Health/National Cancer Institute.
Neil D. Weinstein, Email: neilw@AESOP.Rutgers.edu, Distinguished Professor Emeritus-Department of Human Ecology-Rutgers University.
Motomi Mori, Email: morim@ohsu.edu, Walter & Clora Brownfield Professor of Cancer Biostatistics, School of Public Health and Medicine, Oregon Health & Science University/Knight Cancer Institute.
Racky Daffé, Email: daffe@ohsu.edu, Senior Research Assistant-Oregon Health & Science University.
Daniel P. Sulmasy, Email: dsulmasy@uchicago.edu, Kilbride-Clinton Professor Professor of Medicine and Ethics in the Department of Medicine and the Divinity School, University of Chicago.
References
- 1.Miller Franklin G, Joffe Steven. Benefit in phase 1 oncology trials: therapeutic misconception or reasonable treatment option? Clinical Trials. 2008;5(6):617–623. doi: 10.1177/1740774508097576. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum PS, Roth H, Lidz CW, et al. False hopes and best data: consent to research and the therapeutic misconception. The Hastings Center Report. 1987;12(2):20–24. [PubMed] [Google Scholar]
- 3.Horng S, Grady C. Misunderstanding in clinical research: distinguishing therapeutic misconception, therapeutic misestimation, & therapeutic optimism. IRB: Ethics and Human Research. 2003;25(2):11–16. [PubMed] [Google Scholar]
- 4.Weinfurt KP, Seils DM, Tzeng JP, Compton KL, Sulmasy DP, Astrow AB, Schulman KA, Solrino NA, Meropol NJ. Expectations of benefit in early-phase clinical trials: implications for assessing the adequacy of informed consent. Med Decis Making. 2008;28(4):575–581. doi: 10.1177/0272989X08315242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen LA, Appelbaum PS, Klein WMP, Weinstein ND, Cook W, Fogel JS, Sulmasy DP. Unrealistic Optimism in Early Phase Oncology Trials. IRB: Ethics & Human Research. 2011;32(2):1–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Pentz Rebecca D, White Margaret, Harvey R Donald, Farmer Zachary Luke, Liu Yuan, Lewis Colleen, Dashevskaya Olga, Owonikoko Taofeek, Khuri Fadlo R. Therapeutic misconception, misestimation, and optimism in participants enrolled in phase 1 trials. Cancer. 2012;118(18):4571–4578. doi: 10.1002/cncr.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kass NE, Sugarman J, Medley AM, Fogarty LA, Taylor HA, Daugherty CK, et al. An intervention to improve cancer patients’ understanding of early-phase clinical trials. IRB: Ethics & Human Research. 2009;31(3):1–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Klein William MP, Cooper Katrina L. On the physical health costs of self-enhancement. In: Chang Edward C., editor. Self-Criticism and Self-Enhancement. American Psychological Association; 2008. [Google Scholar]
- 9.Weinstein ND. Optimistic biases about personal risks. Science. 1989;246:1232–1233. doi: 10.1126/science.2686031. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein ND, Klein WMP. Unrealistic optimism: present and future. Journal of Science and Clinical Psychology. 1996;15:1–8. [Google Scholar]
- 11.Radcliffe NM, Klein WMP. Dispositional, unrealistic, and comparative optimism: differential relations with the knowledge and processing of risk information and beliefs about personal risks. Personality and Social Psychology Bulletin. 2002;28:836–846. [Google Scholar]
- 12.Segerstrom SC. Optimism and attentional bias for negative and positive stimuli. Personality and Social Psychology Bulletin. 2001;27:1334–1343. [Google Scholar]
- 13.Aspinwall LG, Richter L, Hoffman RR., III . Understanding how optimism works: An examination of optimists’ adaptive moderation of belief and behavior. In: Chang Edward C., editor. Optimism & Pessimism: Implications for theory research and practice. American Washington D.C Psychological Association; 2001. p. 225. [Google Scholar]
- 14.Aspinwall Lisa G, Brunhart Susanne M. What I do know won’t hurt me: Optimism, attention to negative information, coping, and health. 2000 [Google Scholar]
- 15.Appelbaum Paul S, Lidz Charles W, Grisso Thomas. Therapeutic misconception in clinical research: frequency and risk factors. IRB: Ethics & Human Research. 2004 [PubMed] [Google Scholar]
- 16.Fowler Stephanie L, Geers Andrew L. Dispositional and comparative optimism interact to predict avoidance of a looming health threat. Psychology & health. 2015;30(4):456–474. doi: 10.1080/08870446.2014.977282. [DOI] [PubMed] [Google Scholar]
- 17.Scheier Michael F, Carver Charles S, Bridges Michael W. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of personality and social psychology. 1994;67(6):1063. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 18.Appelbaum PS, Anatchkova M, Albert K, Dunn LB, Lidz CW. Therapeutic misconception in research subjects: development and validation of a measure. Clinical Trials. 2012;9:748–761. doi: 10.1177/1740774512456455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lidz CW, Appelbaum PS, Grisso T, Renaud M. Therapeutic misconception and the appreciation of risks in clinical research. Soc Sci Med. 2004;58:1689–1697. doi: 10.1016/S0277-9536(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein ND. Unrealistic optimism about susceptibility to health problems. Journal of Behavioral Medicine. 1982;5(4):441–460. doi: 10.1007/BF00845372. [DOI] [PubMed] [Google Scholar]
- 21.Statistical Analysis System (SAS) Version 9.4. SAS Institute Inc; Cary NC, USA: http://www.sas.com/en_us/company-information.html. [Google Scholar]
- 22.Scheier Michael F, Carver Charles S. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health psychology. 1985;4(3):219. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 23.Weinfurt Kevin P, Sulmasy Daniel P, Schulman Kevin A, Meropol Neal J. Patient expectations of benefit from phase I clinical trials: linguistic considerations in diagnosing a therapeutic misconception. Theoretical medicine and bioethics. 2003;24(4):329–344.29. doi: 10.1023/a:1026072409595. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Vries R, Wilson Renee, Parnami Sonali, Frank Samuel, Kieburtz Karl, Holloway Robert G. Research participants’ “irrational” expectations: common or commonly mismeasured? IRB: Ethics & Human Research. 2012;35(1):1–9. [PubMed] [Google Scholar]


