Abstract
Human immunodeficiency virus-1 (HIV) promotes synaptic simplification and neuronal apoptosis, and causes neurological impairments termed HIV-associated neurological disorders (HAND). HIV-associated neurotoxicity may be brought about by acute and chronic mechanisms that still remain to be fully characterized. The HIV envelope glycoprotein gp120 causes neuronal degeneration similar to that observed in HAND subjects. The present study was undertaken to discover novel mechanisms of gp120 neurotoxicity that could explain how the envelope protein promotes neurite pruning. Gp120 has been shown to associate with various intracellular organelles as well as microtubules in neurons. We then analyzed lysates of neurons exposed to gp120 with liquid chromatography mass spectrometry for potential protein interactors. We found that one of the proteins interacting with gp120 is tubulin β-3 (TUBB3), a major component of neuronal microtubules. We then tested the hypothesis that gp120 binds to neuronal microtubules. Using surface plasmon resonance we confirmed that gp120 binds with high affinity to neuronal specific TUBB3. We have also identified the binding site of gp120 to TUBB3. We then designed a small peptide (Helix-A) that displaced gp120 from binding to TUBB3. To determine whether this peptide could prevent gp120-mediated neurotoxicity, we crosslinked Helix-A to mesoporous silica nanoparticles (Helix-A nano) to enhance the intracellular delivery of the peptide. We then tested the neuroprotective property of Helix-A nano against three strains of gp120 in rat cortical neurons. Helix-A nano prevented gp120-mediated neurite simplification as well as neuronal loss. These data propose that gp120 binding to TUBB3 could be another mechanism of gp120 neurotoxicity.
Keywords: HAND, Nanoparticles, Neurite pruning, Neuronal loss, Tat, Tubulin β-3
INTRODUCTION
Human immunodeficiency virus-1 (HIV) infiltrates the central nervous system and promotes neurophathological features such as neurite injury (Ellis et al. 2007), culminating in HIV-associated neurocognitive disorders (HAND). Although inflammation and microglia activation have been proposed to play a major role in causing the neurophathology of HAND (Everall et al. 2009), the persistence of neurological abnormalities among virally suppressed subjects in the era of antiretroviral therapy has raised new questions about the causes of neuronal dysfunction (Gelman 2015). Microglia are the primary target cells for HIV in the brain; nevertheless, their activation alone may not be sufficient to promote the neurological impairment seen in HAND subjects. Thus, more studies are needed to identify additional mechanisms whereby HIV is neurotoxic despite the use of antiretroviral therapy.
The lack of evidence of HIV infection of neurons has prompted investigations to better explain mechanisms of HIV-mediated neurotoxicity. Viral proteins such as gp120 (Hesselgesser et al. 1998), Tat (Maragos et al. 2003), Nef (van Marle et al. 2004), and others (Mattson et al. 2005) have emerged as the potential neurotoxic agents to explain HIV-mediated neuronal degeneration. Among these proteins, gp120 appears to exhibit cytotoxic activities in the picomolar range (Meucci et al. 1998, Dreyer et al. 1990, Kaul et al. 2007, Bansal et al. 2000). This protein activates apoptotic pathways that lead to neuronal dysfunction and loss (Kaul et al. 2001). Such pathways include dysregulation of calcium homeostasis (Haughey & Mattson 2002), activation of oxidative stress (Mattson et al. 2005), and induction of the proapoptotic transcription factor p53 (Garden et al. 2004). Moreover, neurons exposed to recombinant gp120 or transgenic mice overexpressing gp120 exhibit apoptosis and dendritic simplification (Toggas et al. 1994, Bachis et al. 2012), all of which are pathological features seen in HAND subjects. However, the cellular mechanisms whereby gp120 causes synaptic pruning are still under investigation. Targeting such mechanisms may lead to an effective prevention of the HIV-mediated neuronal injury.
Gp120, which can be secreted by HIV-infected cells, was first described to induce neuronal apoptosis through direct binding to chemokine receptors CXCR4 and CCR5 (Hesselgesser et al. 1998, Meucci et al. 1998, Kaul et al. 2007). Moreover, independent investigations have demonstrated that T-tropic CXCR4 preferring gp120IIIB is endocytosed by neurons in vitro (Berth et al. 2015, Bachis et al. 2003) and in vivo (Ahmed et al. 2009). Importantly, internalized gp120IIIB associates with microtubules (MTs) (Bachis et al. 2006), most likely after binding to mannose binding lectin (Teodorof et al. 2014). MTs are the major avenue for intracellular transport of many organelles, including mitochondria (Sheng & Cai 2012), and synaptic vesicles to distal axons and dendrites. Changes in the integrity of MTs are sufficient to alter proper energy supply within neurites and synapses leading to acute or chronic axonal and dendritic fragmentation (Wang et al. 2012). Thus, gp120 accumulation inside neurons may decrease neuronal survival by binding to MTs and disrupting their function. In this study, we examined a novel neuropathological mechanism of axonal and dendritic degeneration caused by gp120 by investigating the direct interaction of the viral protein with MTs. We provide evidence that gp120 binds to the carboxy terminal tail (CTT) of neuronal specific tubulin through a conserved α-helix region. When such binding is blocked, gp120 neurotoxic effects are prevented.
Materials and Methods
Reagents
All gp120s and Tat as well as mouse monoclonal gp120 antibody were purchased from Immunodiagnostics Inc, Woburn, MA. Human recombinant tubulin β-3 (TUBB3) was obtained from MyBioSource, San Diego, CA. Porcine tubulin dimer and assembled MTs (containing ~25% of TUBB3) were purchased from Cytoskeleton, Inc, Denver, CO. Helix-A and -B and tubulin CTTs (<98% pure) were synthesized by and purchased from Genscript, Piscataway, NJ.
Primary cortical neurons
Animals studies were done in strict accordance with the Laboratory Animal Welfare Act, with National Institutes of Health Guide for the Care and Use of Laboratory Animals, and after approval from the Georgetown University Animal Care and Use Committee.
Neuronal cultures were prepared from the cortex of embryonic (E17–18) Sprague Dawley rats (Charles River Lab, Wilmington, MA) following an established protocol (Avdoshina et al. 2010). Cells were seeded onto poly-L-lysine pre-coated plates in Neurobasal Medium containing 2% B27 supplement, 25 nM glutamate, 0.5 mM L-glutamine, and 1% antibiotic-antimycotic solution (Invitrogen, Carlsbad, CA). Cultures were grown at 37°C in 5% CO2/95% air for 7 days on glass coverslips. Cultures contained ~5% non-neuronal cells.
Neuronal processes
To determine the length of neuronal processes, neurons were fixed in 4% paraformaldehyde/phosphate buffer with 4% sucrose for 20 min at room temperature. Fixed cells were blocked and permeabilized in 5% non-fat milk in TBS-T (150nM NaCl, 20mM Tris-base, pH 7.5, 0.1% Triton X100) for 1 hr at room temperature. Cells were incubated overnight at 4°C with mouse anti-microtubule associated protein 2 (MAP2) antibody (1:5000; Sigma-Aldrich, MO). Coverslips were washed with PBS-T and corresponding fluorescence-conjugated secondary antibody (1:2000; Invitrogen, CA) were applied for 1 hr at room temperature. Coverslips were washed with TBS-T and mounted with Fluoro-Gel with TES buffer (Electron Microscopy Science, PA). Cells were imaged using an FV300 laser confocal scanning system attached to an Olympus IX-70 (Tokyo, Japan) upright microscope. Image scale was calibrated and length of MAP2 positive processes was measured in three randomly selected fields (10 neurons per field) using ImageJ.
Cell viability
The viability of primary cortical neurons was estimated by Hoechst 33258 and propidium iodide (Hoechst/PI; Sigma-Adrich) co-staining and visualized using a fluorescence microscope Olympus IX71 as previously described (Rozzi et al. 2014). Hoechst/PI-positive cells were then counted using ImageJ and expressed as a percentage of the total number of neurons.
Gp120 toxicity was evaluated by measuring lactate dehydrogenase (LDH) release using CytoTox 96® NonRadioactive Cytotoxicity Assay, according to manufacturer’s instructions (Promega Corp, Madison, WI). LDH quantitation was done by measuring wavelength absorbance at 490 nm. Data are normalized to the amount of LDH released from vehicle-treated cells.
Liquid chomatography–mass spectrometry
Neurons exposed to gp120 were lysated in RIPA buffer with protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA) at 4°C and then incubated with mouse anti-gp120 antibody. Gp120 complex of interacting proteins was eluted from immobilized protein A beads, reduced, alkylated and digested with trypsin. The peptide mixtures were separated and analyzed by liquid chromatography mass spectrometry (LCMS), using a LTQ Orbitrap XL™ Hybrid FTMS (Fourier Transform Mas Spectometer) (Thermo Fisher Scientific). Peptide separation was performed with solvent A (0.1% HCOOH in H2O) and solvent B (0.1% formic acid in acetonitrile), using the following gradient: from 5% B for 1 min to 60% B in 70 min. Peptides were analyzed using a precursor mass range of 300–5000 Da. SEQUEST (Eng et al. 1994) was employed using the following parameters: trypsin as proteolytic enzyme, 2 max missed cleavage, S-carbamidomethylation of cystein, a precursor mass tolerance of 30 ppm and a fragment mass tolerance of 0.1 Da, and rat as taxonomic origin of the samples.
Surface plasmon resonance
Biacore™ T200 (GE Health care Bio-Science, Piscataway, NJ), was used to determine the kinetic parameters for the binding of recombinant TUBB3, tubulin dimer, and assembled MTs (ligands) to the various strains of gp120 or related peptides (analytes). In brief, recombinant TUBB3, tubulin dimer, and assembled MTs were covalently attached to different flow cells of a carboxymethyldextran 5 sensor chip by amine coupling. Chip surface was activated for 720 sec at 10 µl/min with a 1:1 mixture of 0.1 M N-Hydroxysuccinimide and 0.5 M 1-Ethyl-3-(-3-dimethylaminopropyl)-carbodiimide hydrochloride. Each ligand was diluted in 10 mM sodium acetate, pH 4.0 (final concentrations were 0.67 µg/ml for TUBB3, 33.3 µg/mL for tubulin dimer, and 50 µg/mL for MTs) and injected 280 sec for TUBB3, 150 sec for tubulin dimer, and 1400 sec for MTs at 10 µl/min flow rate. After ligand capture, the surfaces were deactivated by injecting 1M ethanolamine for 720 sec at 10 µl/min. HBS-P (10 mM HEPES, pH7.4, 150 mM NaCl, 0.05% P-20) with 2 mM MgCl2 used as the running buffer. Flow cell 1 on each chip was left empty and used as a reference surface. TUBB3 (1040 RU), tubulin dimer (2086 RU), and assembled MTs (990 RU) were captured on the remaining three flow cells. Kinetic studies were performed by injecting different concentrations of gp120ADA, IIIB or MN, in triplicate. Chip surface was regenerated with 10 mM glycine pH 1.5 injected for 30 sec at a flow rate of 100 µl/min. Each sample was injected for 60 sec (contact time) followed by a dissociation time of 300 sec at a flow rate of 100 µl/min. Binding studies with peptides Helix-A and Helix-B peptides were done in the same way as described above by injecting increasing concentrations of Helix-A and Helix-B peptides. Competition experiments with Helix A and Helix B peptides were performed by mixing 100 nM of various gp120 strains with 10 µM of each peptide and injecting over the sensorchip surface. Data were analyzed with Biacore T200 Evaluation Software (version 1) to determine the equilibrium dissociation constant (KD) from a 1:1 binding model.
Dot blot analysis
CTTs of tubulin (2 µg each) were spotted on a nitrocellulose membrane and allowed to dry. The membrane was then incubated in blocking buffer (5% BSA in TBST [150nM NaCl, 20mM Tris-base, pH 7.5, 0.05% Tween 20]) for 2 hr followed by incubation with a solution composed of 5 µg recombinant gp120 (for binding to CTTs). The membranes were washed and then incubated with a mouse anti-gp120 antibody (1:1000; Immunodiagnostics) in TBST for 2h. Membranes were washed and incubated in goat anti-mouse HP-conjugated secondary antibody (1:20,000; Jackson ImmunoResearch, PA). Visualization of the bands was then accomplished by the addition of Super Signal West Pico-Stable Peroxidase Solution and Luminol/Enhancer Solution (Pierce, Rockford, IL). The detection of binding was considered positive when it was above the background noise (no anti-gp120 antibody or no CTT).
Synthesis and characterization of mesoporous silica nanoparticles
The materials were purchased from the following sources: tetraethyl orthosilicate (TEOS), 3-(aminopropyl) triethoxysilane (APTES), hexadecyltrimethylammonium bromide (CTAB), 2-propanol (IPA), ethanol, HCl, 2-mercaptoethanol (BME), and N-(3-diethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) from Sigma-Aldrich (St. Louis, MO); 2-ethylsulfonic acid (MES), and NaCl from Acros Organics (Fairlawn, NJ); NH4F and N-hydroxysulfosuccinimide (Sulfo-NHS) from ThermoFisher Scientific (Waltham, MA).
A modified Stöber reaction was used to synthesize mesoporous silica nanoparticles (MSNs). In detail, 162.909 mg ammonium fluoride and 144.87 mg CTAB were dissolved in 48.24 ml water (80°C for 1 hr). 0.988 ml of TEOS was then added drop-wise to the solution and the reaction proceeded for 2 hr at 80°C. After two ethanol washes MSNs were left overnight in a 2% HCl in ethanol solution to remove surfactants. The particles were washed with 50% ethanol and stored in IPA. MSNs were modified with APTES in a solution of 2% APTES and 5% Millipore water by volume in 2-propanol at a concentration of 1 mg nanoparticle/ml APTES solution. The modification took place at 35°C for 2 hr under constant and vigorous agitation. MSNs-APTES were conjugated to Helix-A peptide via EDC/sulfo-NHS coupling reaction. The fluorescein isothiocyanate (FITC)-conjugated Helix-A peptide was activated in a solution of 2mM EDC/5mM sulfo-NHS in 0.1 M MES and 0.5 M NaCl for 15 min at 1 mg/ml. After peptide activation, BME was added at a concentration of 20 mM. 1 mg of MSN-APTES was dispersed in the reaction solution. The conjugation took place at room temperature for 2 hr under constant and vigorous agitation. Hydroxylamine HCl was added to the solution at a concentration of 10 mM to quench the reaction.
Dynamic Light Scattering and Zeta (ζ)-potential characterization were performed using a Zetasizer ZEN3600 (Malvern, Worcestershire, UK). For DLS, scattered light detection was measure at 90° to the incident beam (a 25mW laser at 660 nm wavelength). For ζ-potential analysis the same parameters were used but scattered light was detected at 15°C. The ζ-potential of MSN-APTES was positive (39.5 mV), and after the crosslink with the peptide (MSN-APTES-Helix-α) decreased to 12.06 mV. Fourier Transformed Infrared Spectroscopy was performed by creating a pellet of 5% sample and 95% KBr (Sigma-Aldrich) by volume and analyzing absorbance of the pellet on a Nicolet 6700 FT-IR Spectrometer (Thermo Fisher Scientific). The spectra were reported after background subtraction, baseline correction and binomial smoothing (11 points). The second derivatives were obtained by the Savitsky–Golay method (third grade polynomial, 5 smoothing points) using the OMNIC software (ThermoFisher).
Transmission electron microscopy of nanoparticles
Nanoparticles localization inside neurons was performed by transmission electron microscope (TEM). In brief, after exposure to nanoparticles, primary rat cortical neurons were fixed in 3% glutaraldehyde, 2% paraformaldehyde in 0.1M cacodylate buffer, pH 7.3 and were washed in 0.1 M cacodylate buffer and treated with 0.1% Millipore-filtered buffered tannic acid, post-fixed with 1% buffered osmium tetroxide for 30 min, and stained with 1% Millipore-filtered uranyl acetate. The samples were washed several times in water, then dehydrated in increasing concentrations of ethanol, infiltrated, and embedded in LX-112 medium. The samples were polymerized in a 60°C oven for 2 days. Ultrathin sections were cut in a Leica Ultracut microtome (Leica, Deerfield, IL), stained with uranyl acetate and lead citrate in a Leica electron microscopy stainer, and examined in a JEM-1010 TEM (JEOL, LTD, Skishima, Tokio, Japan) at an accelerating voltage of 80 kV. Digital images were obtained using Advanced Microscopy Techniques (AMT Corp, Woburn, MA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc.). Results are depicted as mean ± standard error of mean. For a comparison of more than two groups, an ANOVA test, followed by a proper post-hoc test for multiple comparisons, was applied. P values of <0.05 indicate statistical significance.
Results
HIV protein gp120 binds to neuronal specific tubulin
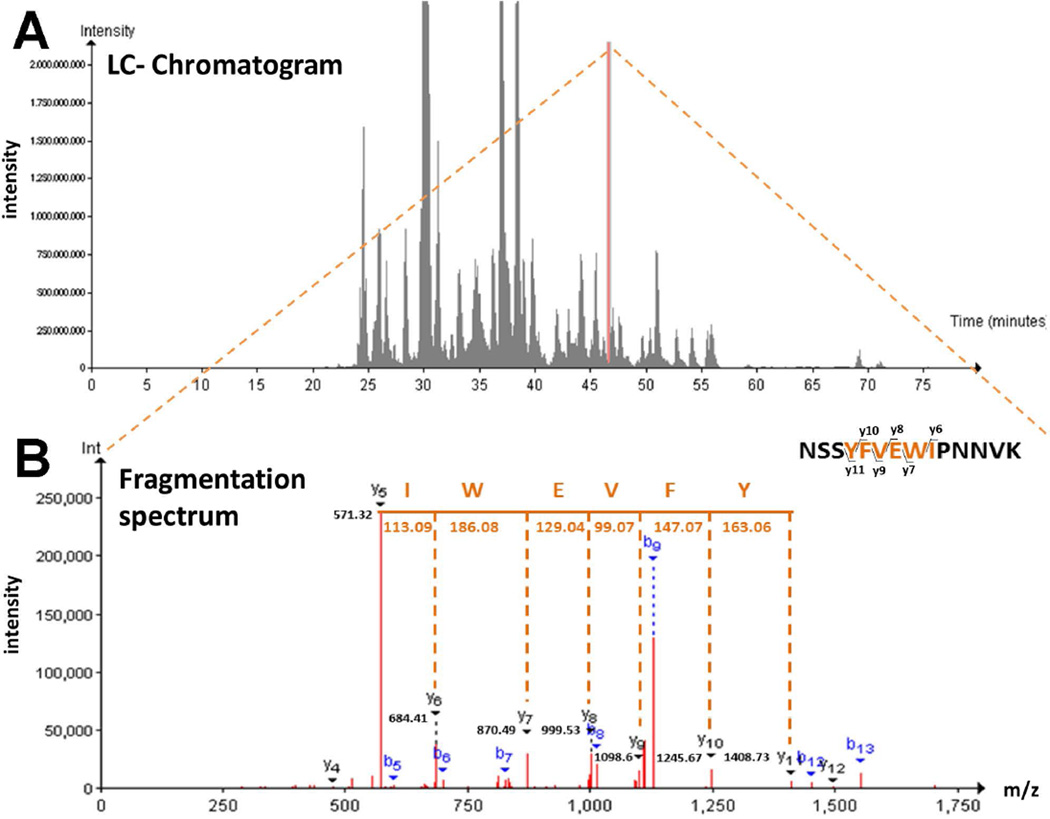
We have previously shown that gp120IIIB is endocytosed into neurons where it localizes around MTs (Bachis et al. 2006). To verify gp120 interaction with MTs, we exposed rat cortical neurons to gp120IIIB (5 nM) for 24 hr. Lysates were prepared and immunoprecipitated with a gp120 antibody. We then used LCMS to separate and analyze gp120 interacting proteins. We were able to detect various peaks of interacting proteins. One of these peaks (with a retention time of 46.45 min) is associated with TUBB3 (Fig. 1A), a major component of neuronal MTs, as confirmed by the analysis of the fragmentation spectrum of the ion with mass/charge ration 848.93 associated with this peptide [337–350] of TUBB3 (Fig. 1B).
Figure 1. LC-MS/MS analysis identifies TUBB3 as an interactor of gp120.
(A). Total ion chromatogram of the whole data set of gp120 interactors. Indicated in orange is the peak associated to peptide [337–350] of TUBB3, with a retention time of 46.45 min. (B) The fragmentation spectrum of peptide [337–350] of TUBB3 is shown to demonstrate TUBB3 identification among gp120 interactors. The spectrum shows the predicted peptide sequence and the identified peptide fragment (y-ion). Peptide information: sequence= NSSYFVEWIPNNVK, m/z= 848.93; charge=2.
Next we validated the direct interaction of gp120 with TUBB3 using surface plasmon resonance (SPR). To confirm the specificity of binding, SPR was also conducted with tubulin dimer and assembled MTs. At least three strains of gp120 (Freedman et al. 2003) have been characterized for their ability to bind to either CCR5 (M-tropic), CXCR4 (T-tropic) or both receptors (dual-tropic). Thus, to establish their relative affinity we have used gp120ADA (CCR5), gp120IIIB (CXCR4) and gp120MN (dual tropic). All strains of gp120 showed binding in the nM range to TUBB3, tubulin dimer and assembled MTs (Table 1). Gp120ADA and IIIB exhibited higher affinity for TUBB3, tubulin dimer and assembled MTs than gp120MN (Table 1). To examine specificity of this binding, we have used Tat, another viral protein that is endocytosed by neurons (Liu et al. 2000) and causes synaptodendritic injury (Nath & Steiner 2013). SPR experiments showed that Tat had no specific binding to TUBB3, tubulin dimer or MTs (Table 1).
Table 1.
Binding affinity of gp120s to TUBB3, tubulin dimer and assembled MTs.
| Ligand Analyte |
TUBB3 | Tubulin dimer | Assembled MTs | |||
|---|---|---|---|---|---|---|
| U-value | KD (nM) | U-value | KD (nM) | U-value | KD (nM) | |
| gp120ADA | 3 | 10.3 | 4 | 11.2 | 5 | 12.0 |
| 2 | 2 | 3 | ||||
| gp120IIIB | 2 | 7.5 | 2 | 12.3 | 7 | 10.0 |
| 2 | 4 | 4 | ||||
| gp120MN | 12 | 740 | 26 | 79.2 | 7 | 62.7 |
| 15 | 5 | 7 | ||||
| Tat | no specific binding | |||||
Recombinant TUBB3, bovine tubulin dimer, and assembled MTs were immobilized on a Biacore® CM5 chip by amine coupling. Different concentrations of gp120s (from 3.13 to 100 nM) or Tat (from 1.6 nM to 100 nM) were injected over the surface in triplicates in each experiment. The kinetics of interaction was analyzed using Biacore T200 instrument as described in Materials and Methods. KD was calculated from two independent experiments, in triplicates.
To screen for gp120 binding to additional tubulin isoforms, we performed dot blot analysis with CTT of various tubulins (Table 2). Gp120 binding was also observed with tubulin α1A/1B, another tubulin isoform expressed exclusively in neurons, but not to other tubulin isoforms that are expressed in a variety of cells (Table 2).
Table 2.
Sequence and characteristics of tubulin isoforms used for dot blot analysis.
| Tubulin isoform |
Sequence | Expression | Distinctive feature |
Binding to gp120 |
|---|---|---|---|---|
| α1/β1 | 441EGEGEEEGEEY451 | widely expressed | tyrosinated isoform | No |
| α1/β1 | 441EGEGEEEGEE450 | widely expressed | detyrosinated isoform | No |
| α1/β1 | 441EGEGEEEGE449 | expressed exclusively in neurons | Δ2 isoform1 | Yes |
| α4 | 441EDEDEGEEY449 | widely expressed | tyrosinated isoform | No |
| α4 | 441EDEDEGEE448 | widely expressed | detyrosinated isoform | No |
| α4 | 441EDEDEGE447 | widely expressed | Δ2 isoform1 | No |
| β2A/2B | 437EEEEGEDEA455 | widely expressed; highly abundant in neurons | No | |
| β3 | 438EDDEEESEA446 | expressed exclusively in neurons | Yes |
irreversible derivative of detyrozination
Binding of gp120 (IIIB or ADA) to the indicated sequences of CTT tubulin isoforms was performed by dot blot as described in Materials and Methods.
Helix-A of gp120 binds to CTTs
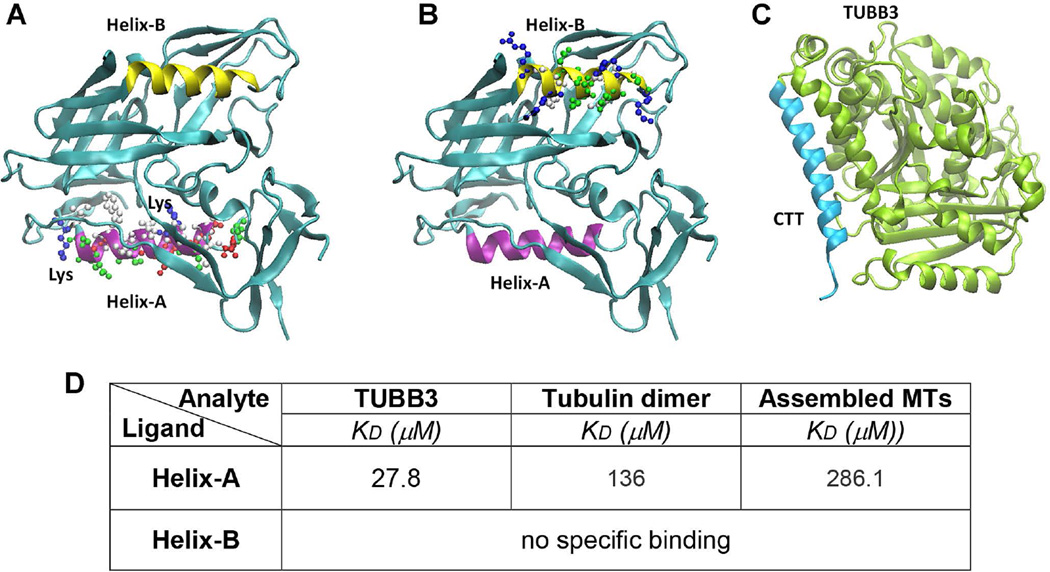
MTs are copolymers assembled from tubulin heterodimers, comprised of α- and β-tubulin heterodimer repeats. The carboxy-terminal tail (CTT) of both α- and β-tubulin, which are found on the outer surface of the MTs, plays critical roles in regulating MT assembly and function, and in determining tubulin conformation (Luchko et al. 2008). Gp120 does not contain a canonical basic groove for tubulin CTTs and therefore cannot dock CTT through this motif. However, gp120 includes multiple α-helix motifs (Kwong et al. 1998); therefore, we hypothesized that gp120 could bind to CTT though helix-helix interaction (Fig. 2). Such interaction has been suggested by a recent report (Bullock et al. 2011) showing that 15% of the entries in the Protein Data Bank are multiprotein complexes, and 62% of these complexes involve a helix at the protein interface. To test whether a helix motif of gp120 could bind to the CTT of TUBB3 (Fig. 2C), we synthetized two short peptides NDMVEQMHEDIISLWDQSLK (Helix-A) and RAKWNNTLKQIASK (Helix-B) that correspond to two α-helix regions of gp120 (Figs. 2A and B). We tested their ability to bind to TUBB3, tubulin dimer and assembled MTs by SPR. Fig. 2D shows that Helix-A but not Helix-B peptide binds to TUBB3 in the lower micromolar range. Binding of Helix-A peptide to tubulin dimer and assembled MTs was in the higher micromole range, suggesting low affinity binding. These results might be consistent with a rapid dissociation of Helix-A from TUBB3 due to its mass (~1,696Da) being smaller than that of gp120 (~110,000Da).
Figure 2. 3D structure of gp120 and TUBB3.
The tridimensional structures of gp120 (A and B) show that two helices in gp120 (Helix-A in purple and Helix-B in yellow) occupied two opposite sides and both could be contributing to the effective binding to the CTT (C, light blue) of TUBB3. Furthermore, we indicated the lysine residues present in Helix-A that could be responsible in the stabilization of helix-helix interaction between Helix-A and CTT. D. BiacoreT200 was used to determine the kinetic parameters for the binding of recombinant TUBB3, tubulin dimer, and assembled MTs Helix-A and HeliX-B peptides. Peptides were injected at different concentrations (10 nM – 500 µM). Data are from three independent experiments.
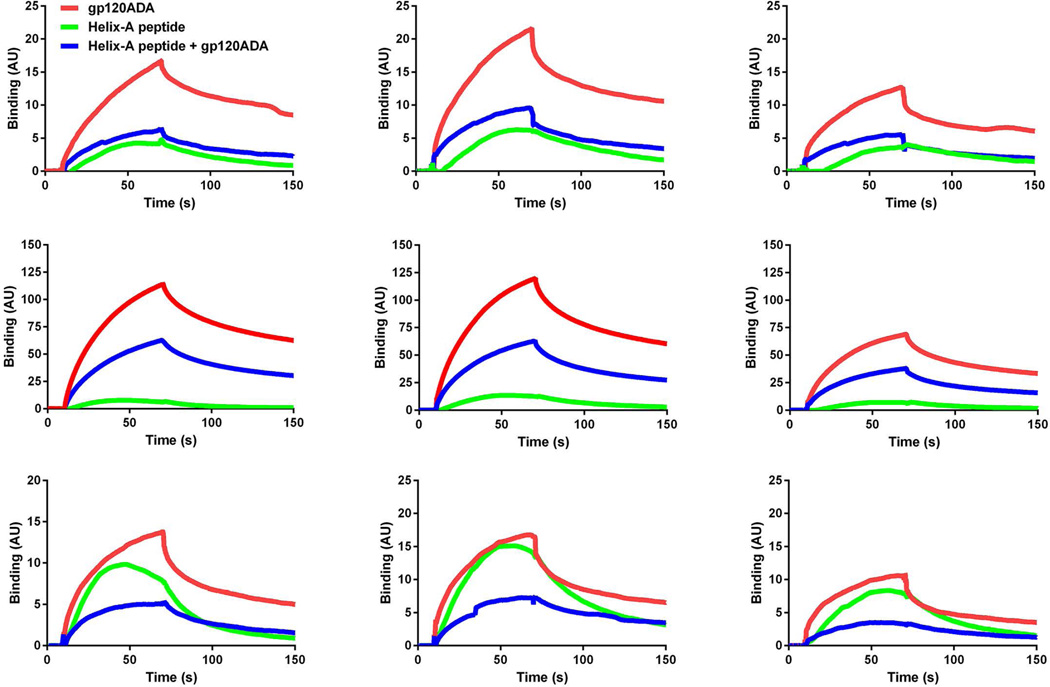
To further establish whether gp120 and Helix-A peptide compete for the same binding site, TUBB3, tubulin dimer and assembled MTs were incubated with gp120 and Helix-A. Fig. 3 shows that Helix-A peptide displaces all gp120 strains from binding to recombinant tubulins and MTs. Thus, our findings suggest that gp120 could bind to a neuron-specific isoform of tubulin through the twenty amino acid domain of its conserved α-helix region.
Figure 3. Helix-A peptide competes for gp120s binding to MTs.
BiacoreT200 was used to determine the kinetic parameters for the binding of recombinant TUBB3, tubulin dimer, and assembled MTs to gp120s in the absence and presence of Helix-A peptide. Gp120s (100 nM) or Helix-A peptide (10 µM) were injected over the surface alone or as a premix. Representative data are from one of three independent, highly reproducible experiments.
Mesoporous silica nanoparticles
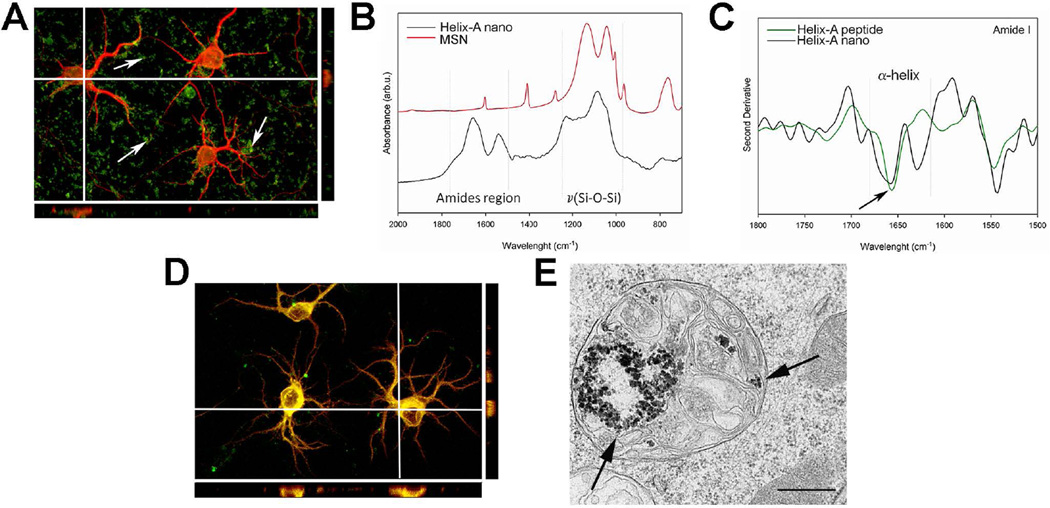
The ability of the Helix-A peptide to prevent gp120 binding to MTs allowed us to speculate that this peptide might be neuroprotective against gp120-mediated synaptic simplification and neurite pruning (Toggas et al. 1994). The Helix-A peptide alone could not penetrate the cell membrane of primary cortical neurons (Fig. 4A). Therefore, we crosslinked it to mesoporous silica nanoparticles (MSNs) (Tang et al. 2012) modified with 3-(aminopropyl) triethoxysilane, to obtain Helix-A nano using a recently developed method (Parodi et al. 2014). The stability and the chemical features of Helix-A nano were determined by infrared spectroscopy (Fig. 4B). The spectrum of Helix-A nano showed intense and broad peaks in the amides region (1700–1500 cm−1) that are a clear indication of a high concentration of the peptide on the MSN’s surface. Moreover, second derivative analysis of the Amide I region (Fig. 4C) indicated a band centered at 1656 cm−1 confirming the stable α-helix secondary structure of the peptide. In the second derivative spectra, the components that make up the amide I band appear as well-resolved peaks in which the main absorption is still centered at 1656 cm−1 (Fig. 4C), demonstrating that the structure of the peptide has not been modified during the synthesis process (Taraballi et al. 2010).
Figure 4. Helix-A nano crosses the neuronal membranes.
A. Representative image (mag 60×) of rat cortical neurons exposed for 24 hr to FITC-labeled Helix-A peptide (5 µM) and co-stained with the neuronal marker MAP (red). Neurons were optically sliced and a Z-stack was created using the Fluo View software. Arrows point at Helix-A peptide (green) outside cells, indicating that Helix-A peptide is not cell-membrane-penetrable. B. Infrared spectroscopy of Helix-A nano. The gray line highlights the Amide region typical of peptide components. C. The second derivative of the Amide region shows the secondary structure of the Helix-A peptide that is mainly α-helix (arrow). D. Shown is a representative confocal image (mag 60×) of cortical neurons exposed to Helix-A nano (5 µM) for 24 hours. Please note that the majority of green fluorescence is inside MAP2 positive cells (green+red=yellow), indicating that Helix-A nano penetrates neuronal membranes. E. Representative TEM image showing Helix-A nano in lysosomes. Bar=500 nm.
Cortical neurons were then exposed to Helix-A nano and endocytosis was determined by fluorescence imaging using MAP2 as a neuronal marker. Unlike Helix-A peptide alone, Helix-A nano was evenly distributed inside the cytosol (Fig. 4D). To further determine endocytosis of Helix-A, we analyzed the inclusion of Helix-A nano into lysosomes by TEM. Several lysosomes showed accumulation of Helix-A nano (Fig. 4E), supporting the immunostaining data that MSNs is a viable delivery method for this peptide.
Helix-A nano is neuroprotective against gp120
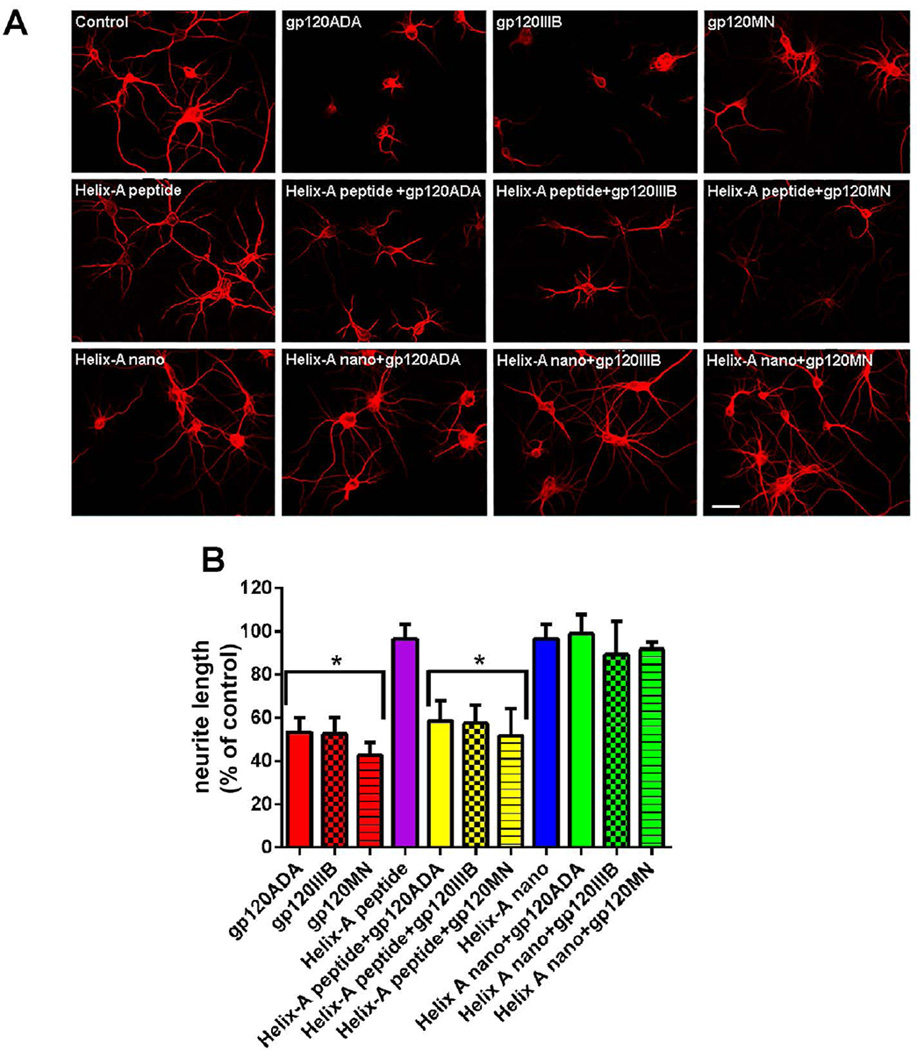
To further establish whether binding to MTs plays a role in gp120 neurotoxicity, we examined whether Helix-A nano could reverse both gp120-mediated synaptic simplification and neuronal cell death. Cortical neurons were exposed to the three different strains of gp120 alone or in combination with Helix-A peptide or Helix-A nano, and neuronal processes were visualized by MAP2 immunostaining 24 hr later (Fig. 5A). Gp120 treatment reduced neuronal processes (Fig. 5), confirming that this viral protein promotes neurite pruning (Toggas et al. 1994). Helix-A nano or Helix-A peptide alone did not affect the lengh of neuritis (Fig. 5); however, Helix-A nano but not Helix-A peptide prevented the gp120-mediated neurite pruning (Fig. 5).
Figure 5. Helix-A nano blocks gp120-mediated neurite pruning.
A. Cortical neurons were exposed to boiled gp120 (control) or to the indicated gp120s (all 5 nM) alone or in combination with Helix-A peptide or Helix-A nano (5 µM each) for 24 hr. Neurons were then fixed and stained for MAP2 as described in Materials and Methods. A. Representative confocal images of MAP2 positive (red) processes (Bar=20µm). B. Quantitative analysis of MAP2 positive processes after various conditions. Data are the mean ± SEM of three independent experiments (n=60 neurons per group per experiment) *p<0.01 vs control. No effect was observed with MSNs alone.
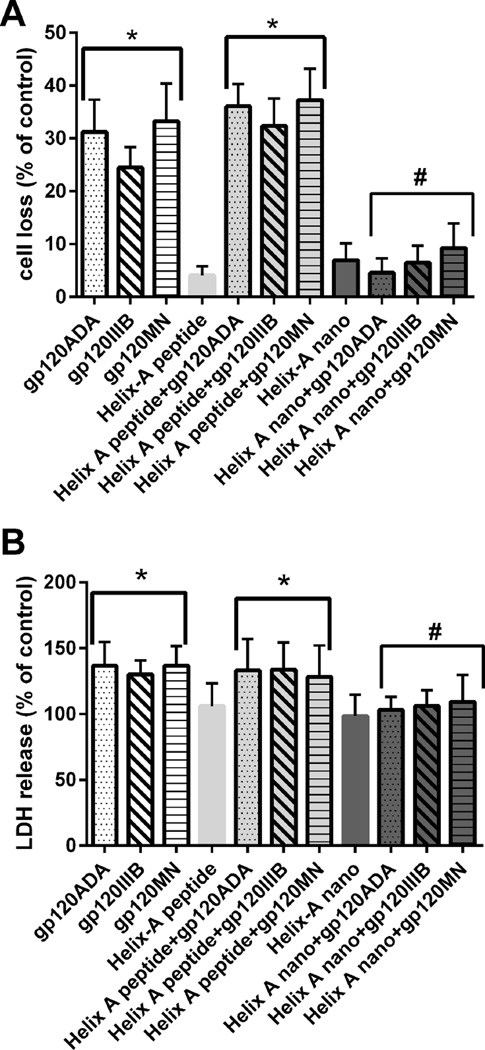
To examine whether Helix-A nano prevents gp120-mediated neuronal loss, cortical neurons were exposed to gp120s alone or in combination to Helix-A nano or Helix-A peptide for 24 hr. Neuronal survival was then measured by Hoetch/PI staining (Fig. 6A) as well as LDH release (Fig. 6B). Helix-A nano blocked gp120-induced neuronal loss. These data provide a strong correlation between the ability of Helix-A nano to displace gp120 from TUBB3 and its neuroprotective activity.
Figure 6. Helix-A nano prevents gp120-induced neuronal cell death.
Cortical neurons were exposed to the indicated stimuli. Cell death was determined by (A) Hoechst/PI staining and (B) LDH 24 hr after gp120s. Data are the mean ± SEM of three separate experiments (n=200 neurons each group per experiment). *p<0.001 vs control; **p<0.01 vs gp120. #p<0.01 vs gp120. Helix-A nano blocked gp120-mediated cell death up to 96 hr whereas MSNs alone did not (data not shown).
Discussion
In this study we have used two sensitive and reliable approaches, LCMS and SPR to provide evidence that gp120 exhibits a specific interaction with TUBB3, a major component of neuronal MTs. We confirmed by dot blot analysis that gp120 binds to two neuronal specific isoforms of tubulin but not to isoforms that are not restricted to neurons. These data are important for an overall understanding of HAND pathology if we consider that TUBB3 and neuronal MTs not only act as “railways” for cargo transport, but in addition, they play a role in neurite elongation and axonal guidance. In fact, altered TUBB3 dynamics or TUBB3 mutations (Tischfield et al. 2010, Poirier et al. 2010, Qu et al. 2013) produce impaired axonal transport of proteins necessary for neuronal survival, as well as inhibit axon outgrowth. Thus, our data suggest that gp120 binding to TUBB3 may be one of the mechanisms to explain reduced synaptic density and axonal degeneration in HAND brains (Ellis et al. 2007) as well as in sensory fibers (Cornblath & McArthur 1988).
The tridimensional structure of gp120 is organized in three α–helix motifs that can determine associations and binding with different proteins (Bullock et al. 2011). Neuronal specific TUBB3 also contains multiple α–helix motifs. Here, we report that an α-helix motif (Helix-A), near the V3 loop domain of gp120, could bind an α-helix region of TUBB3 located in its CTT through helix-helix interactions. Other α–helix structures failed to bind to TUBB3, suggesting that the binding could be sequence-specific possibly stabilized by electrostatic interactions. However, Helix-A showed a lower affinity to TUBB3 than the whole gp120, suggesting than the overall binding between the two proteins is probably reinforced by further interactions with other gp120 regions. Nevertheless, we demonstrated that the Helix-A peptide, designed based on the potential helix-helix interaction of gp120 with the CTT of TUBB3, is able to displace gp120 binding to TUBB3. These findings further supported our hypothesis that gp120 interaction with TUBB3 occurs through this α-helix motif. Future studies will determine whether the hydrophilic or hydrophobic amino acid in the α helix motif of gp120 are crucial for protein-protein interaction.
Binding of gp120 to TUBB3 appears to be crucial for the neurotoxic effect of gp120. However, Helix-A peptide, which displaces gp120 binding to TUBB3 in vitro, does not penetrate neuronal membrane. Thus, to test the hypothesis, we coupled Helix-A peptide to nanoparticles to deliver the peptide inside the cellular compartment. Both immunofluorescence and TEM showed that this method allows for the delivery of Helix-A peptide inside neurons. Helix-A nano, but not Helix-A peptide abolished all neurotoxic effects of gp120, which include neurite pruning, as well as neuronal cell death. These findings support the hypothesis that gp120 binding to TUBB3, which may alter the function of MTs as axonal transporters of organelles and vesicles, could be one of the key mechanisms of gp120 neurotoxicity. Indeed, our study has shown that gp120 impairs mitochondria transport in neurons (Avdoshina et al. in press). Recent studies have highlighted that a defective trafficking in neurons can cause synaptic injury and neurodegeneration (Itoh et al. 2013, Burte et al. 2015). For example, the hallmark of Huntington’s disease, polyQ repeats mutant huntingtin, has been shown to disrupt the association of key components of the motor machinery to MTs, (Gauthier et al. 2004), leading to a reduction of brain-derived neurotrophic factor (BDNF) transport. BDNF is an essential trophic factor that is produced in neurons and it is both retrogradely and anterogradely transported (Altar et al. 1997). Thus, our data suggest a prominent direct intracellular mechanism of neurotoxicity that relies on gp120 interaction with neuronal cytoskeleton, which could impair neuronal trafficking and transport of important pro-survival factor, including BDNF. This mechanism could explain why HAND subjects (Bachis et al. 2012) or neurons exposed to gp120 (Nosheny et al. 2004) exhibit lower levels of BDNF than controls. However, we must exert caution because preliminary LCMS data have shown that gp120 also interacts with other filament proteins including vimentin (data not shown). Thus, more experiments are needed to reveal whether the binding to TUBB3 alone is sufficient for the toxic effect of gp120.
Gp120 binds to chemokine receptors CXCR4 or CCR5 through a specific V3 loop domain (Huang et al. 2005). Activation of these receptors has been shown to increase cytosolic free Ca2+ and extracellular receptor kinase (ERK), which are believed to participate in gp120 neurotoxicity (Meucci et al. 1998). Nevertheless, the CXCR4-mediated increases in ERK and Ca2+ have also been shown to promote neuronal migration and differentiation (Tran et al. 2007). Thus, while ERK, Ca2+ and other signaling molecules associated with CXCR4 are appealing mediators to explain gp120 toxicity, CXCR4 signaling alone may not reflect the entire mechanism underlying the apoptotic effect of gp120. Previous studies have shown that either recombinant gp120 (Bachis et al. 2006, Ahmed et al. 2009, Berth et al. 2015) or viral-derived gp120 (Bachis et al. 2009) is internalized by neurons and it is packed into vesicles after binding to mannose binding lectin for efficient trafficking along MTs (Teodorof et al. 2014). Internalization of gp120 is bloked by the AMD3100 (Bachis et al. 2003), a CXCR4 antagonist that prevents gp120 toxicity (Melli et al. 2006, Meucci et al. 1998), suggesting that the endocytotic process of gp120 could be crucial for the neurotoxic effect. Our data support this notion because the survival rate of neurons that internalize gp120 is greatly increased by Helix-A nano. This peptide has no affinity for chemokine receptors, but competes with the binding of gp120 to TUBB3 though a conserved α-helix domain that is not homologous to the V3 loop domain. Thus, the results presented here indicate that although chemokine receptor activation is required for gp120 neurotoxicity, these receptors may only serve as carriers to deliver gp120 inside neurons.
In addition to a sufficient energy supply, neuronal survival is balanced by retrograde and anterograde protein transport and their clearance by autophagy, an evolutionary conserved pathway that involves sequestration of cytoplasmic material into the lysosomes (Jahreiss et al. 2008). Accumulation of proteins, due to impaired clearance, results in their aggregation and formation of toxic byproducts. Our data show that gp120, once inside, accumulates in neurons. Previous data have shown that neuronal autophagy is activated in the presence of gp120 (Fields et al. 2013); however autophagy may only last for a few hours (Passeri et al. 2014). Thus, it is plausible to suggest a scenario in which gp120 accumulates inside neurons, binds to MTs and alters axonal transport. Such a hypothesis is in line with recent suggestions that changes in axonal transport by toxic proteins, such as α-synuclein, mutated huntingtin or β-amyloid, can cause the progression of neuronal loss seen in Parkinson’s, Huntington’s and Alzheimer’s diseases, respectively (Morfini et al. 2009). Remarkably, the neurotoxic effects observed in our study occur even in the absence of the virus suggesting that gp120 is sufficient to initiate an irreversible neurodegenerative process which may overlap with other endogenous neurotoxins or other patho-physiological insults. Without intervention, gp120 continues to accumulate and predisposes neurons to inflammatory responses, which amplify the progression of the clinical pathology. Our discovery provides new significant data that will help in the design of adjunct therapies against HAND.
Acknowledgments
This work was supported by US National Institute of Health grants NS079172 and NS074916 (to I.M.), P30AI087714 (pilot award from DC D-CFAR to V.A.), Cullen Trust for Health Care Foundation [Project ID: 18130014], the Brown Foundation [Project ID: 18130011], and the Italian Ministry of Health [Project code: GR-2010-2318370] (to E.T.). SPR experiments were done at the Biacore Molecular Interactions Shared Resources supported by NIH-P30 CA51008. We would like to thank Dr. Abraham T. Kallarakal for his excellent technical help in running Biacore instrument, the PHRI Analytical Imaging Facility.
Abbreviations
- APTES
3-(aminopropyl) triethoxysilane
- BDNF
brain-derived neurotrophic factor
- CTT
carboxy terminal tail
- CTAB
hexadecyltrimethylammonium bromide
- EDC
N-(3-diethylaminopropyl)-N’-ethylcarbodiimide hydrochloride
- ERK
extracellular receptor kinase
- FITC
fluorescein isothiocyanate
- HAND
HIV-associated neurocognitive disorders
- HIV
Human immunodeficiency virus-1
- Hoechst/PI
Hoechst 33258 and propidium iodide
- LCMS
liquid chromatography mass spectrometry
- LDH
lactate dehydrogenase
- MAP2
microtubule associated protein 2
- MES
2-ethylsulfonic acid
- MSNs
mesoporous silica nanoparticles
- MTs
microtubules
- SPR
surface plasmon resonance
- Sulfo-NHS
NH4F and N-hydroxysulfosuccinimide
- TEM
transmission electron microscope
- TEOS
tetraethyl orthosilicate
- TUBB3
tubulin β-3
Footnotes
The authors declare that they have no competing interest.
References
- Ahmed F, MacArthur L, De Bernardi MA, Mocchetti I. Retrograde and anterograde transport of HIV protein gp120 in the nervous system. Brain Behav Immun. 2009;23:355–364. doi: 10.1016/j.bbi.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58:1630–1639. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Fields JA, Castellano P, et al. The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox Res. doi: 10.1007/s12640-016-9608-6. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci. 2012;32:9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Biggio F, Major EO, Mocchetti I. M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol. 2009;4:150–160. doi: 10.1007/s11481-008-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Berth S, Caicedo HH, Sarma T, Morfini G, Brady ST. Internalization and axonal transport of the HIV glycoprotein gp120. ASN Neuro. 2015;7 doi: 10.1177/1759091414568186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock BN, Jochim AL, Arora PS. Assessing Helical Protein Interfaces for Inhibitor Design. J Am Chem Soc. 2011;133:14220–14223. doi: 10.1021/ja206074j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burte F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol. 2015;11:11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38:794–796. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Everall I, Vaida F, Khanlou N, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Rockenstein E, et al. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J Neurovirol. 2013;19:89–101. doi: 10.1007/s13365-012-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BD, Liu QH, Del Corno M, Collman RG. HIV-1 gp120 chemokine receptor-mediated signaling in human macrophages. Immunol Res. 2003;27:261–276. doi: 10.1385/IR:27:2-3:261. [DOI] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. Faseb J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pagès M, et al. Huntingtin Controls Neurotrophic Support and Survival of Neurons by Enhancing BDNF Vesicular Transport along Microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gelman BB. Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Curr HIV/AIDS Rep. 2015;12:272–279. doi: 10.1007/s11904-015-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Huang CC, Tang M, Zhang MY, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013;23:64–71. doi: 10.1016/j.tcb.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahreiss L, Menzies FM, Rubinsztein DC. The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic. 2008;9:574–587. doi: 10.1111/j.1600-0854.2008.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Luchko T, Huzil JT, Stepanova M, Tuszynski J. Conformational analysis of the carboxy-terminal tails of human beta-tubulin isotypes. Biophys J. 2008;94:1971–1982. doi: 10.1529/biophysj.107.115113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117:43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, et al. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Steiner J. Synaptodendritic injury with HIV-Tat protein: What is the therapeutic target? Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Parodi A, Haddix SG, Taghipour N, et al. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8:9874–9883. doi: 10.1021/nn502807n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passeri E, Mocchetti I, Moussa C. Is Human Immunodeficiency Virus-mediated Dementia an Autophagic Defect that Leads to Neurodegeneration? CNS Neurol Disord Drug Targets. 2014;13:1571–1579. doi: 10.2174/1871527313666140806125841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier K, Saillour Y, Bahi-Buisson N, et al. Mutations in the neuronal ss-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum Mol Genet. 2010;19:4462–4473. doi: 10.1093/hmg/ddq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Dwyer T, Shao Q, Yang T, Huang H, Liu G. Direct binding of TUBB3 with DCC couples netrin-1 signaling to intracellular microtubule dynamics in axon outgrowth and guidance. J Cell Sci. 2013;126:3070–3081. doi: 10.1242/jcs.122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi SJ, Borelli G, Ryan K, Steiner JP, Reglodi D, Mocchetti I, Avdoshina V. PACAP27 is protective against tat-induced neurotoxicity. J Mol Neurosci. 2014;54:485–493. doi: 10.1007/s12031-014-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z-H, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- Taraballi F, Natalello A, Campione M, Villa O, Doglia SM, Paleari A, Gelain F. Glycine-spacers influence functional motifs exposure and self-assembling propensity of functionalized substrates tailored for neural stem cell cultures. Front Neuroeng. 2010;3:1. doi: 10.3389/neuro.16.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorof C, Divakar S, Soontornniyomkij B, Achim CL, Kaul M, Singh KK. Intracellular mannose binding lectin mediates subcellular trafficking of HIV-1 gp120 in neurons. Neurobiol Dis. 2014;69:54–64. doi: 10.1016/j.nbd.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield MA, Baris HN, Wu C, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle G, Henry S, Todoruk T, et al. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]