Abstract
Resveratrol is a natural polyphenol with plethora of biological activities. Resveratrol has previously shown to decrease DNA methyltransferase (DNMT) enzymes expression and to reactivate silenced tumor suppressor genes. Currently, it seems that no resveratrol analogues have been developed as DNMT inhibitors. Recently, we reported the synthesis of resveratrol-salicylate derivatives and by examining the chemical structure of these analogues, we proposed that these compounds could exhibit DNMT inhibition especially that they resembled NSC 14778, a compound we previously identified as DNMT inhibitor by virtual screening. Indeed, using in vitro DNMT inhibition assay, some of the resveratrol-salicylate analogues we screened in this work showed selective inhibition against DNMT3 enzymes which was greater than resveratrol. A molecular docking study revealed key binding interactions with DNMT3A and DNMT3B enzymes. Additionally, the most active analogues, 10 showed considerable cytotoxicity against three human cancer cells; HT-29, HepG2 and SK-BR-3 which was greater than resveratrol. Further studies are needed to understand the anticancer mechanisms of these derivatives.
INTRODUCTION
Resveratrol (3,4′,5-trans-trihydroxystilbene; Figure 1) is a naturally occurring polyphenol with a wide variety of biological properties. Resveratrol has been regarded as a phytoalexin (plant antibiotic), and it is produced by several plant species. The biological effects of resveratrol have been extensively studied in vitro and in vivo (1–5); some of the reported effects of resveratrol include its anti-inflammatory (6), anticancer (7), antioxidant (8), cardio-protective (9), modulation of the estrogen receptor (10), and chemopreventive activity (11). In this regard, resveratrol possesses an attractive chemopreventive profile, because it inhibits the proliferation of cancer cells in vitro without exerting significant cytotoxicity to normal cells (12) ; it induces cancer cell apoptosis in several cell lines from different tissue types (13–15), and it significantly decreases tumor size in vivo using different cancer cells in xenograft models of rodents (16, 17). The mechanisms of action associated with the chemopreventive profile of resveratrol are varied and rather complex. In accordance with the current paradigm involving the design of “multi-target” drugs, and the relatively new concept known as polypharmacology (18), there is evidence supporting the multi-target profile of resveratrol. In this regard, resveratrol downregulates the expression or inhibits the activity of key enzymes and transcription factors involved in carcinogenesis, including (but not limited to) cyclooxygenase (COX) enzymes, inducible nitric oxide synthase (iNOS), lipoxygenase (LOX), PI3-kinases, NF-κB, PPARγ, Sirt1, DNA-methyltransferases (DNMTs) and others (19).
Figure 1.
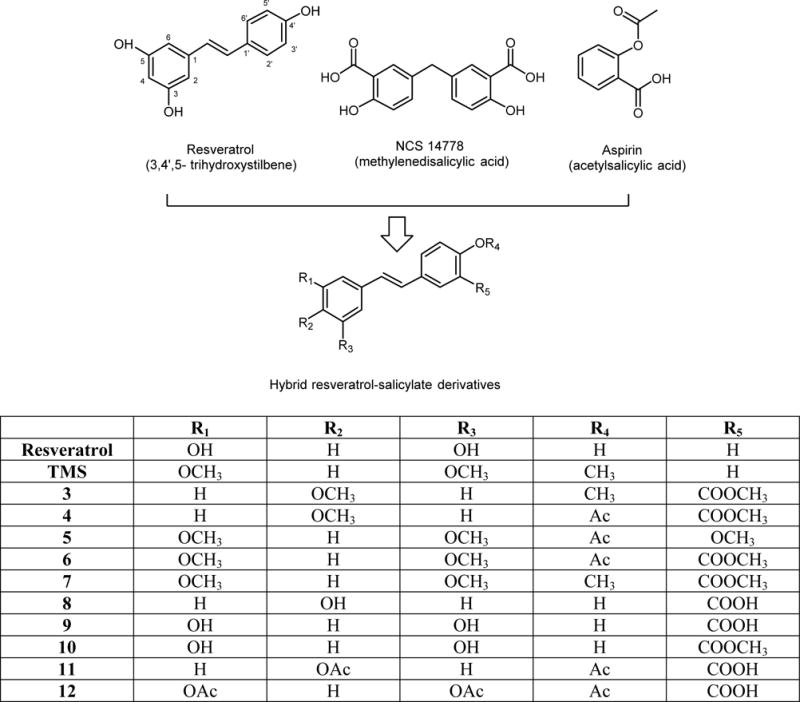
Chemical structures of resveratrol, NSC 14778, and aspirin. The hybrid resveratrol-salicylate derivatives possess the combined chemical features of these three different types of agents; the methylene bridge in NCS 14778 is replaced by an ethylene linkage between a phenol on one side, and the salicylate on the other.
DNMTs are a group of enzymes expressed by mammals in three active isoforms, namely the DNMT1, DNMT3A, and DNMT3B (and one more regulatory enzyme identified as DNMT3L) (20). Under normal physiological conditions, DNMTs are crucial for DNA methylation at cytosine residues (20); specifically, DNMT3 functions as initial (de novo) methylator, while DNMT1 is responsible for “maintenance” of the methylation during cell division (20). However, aberrant methylation patterns (referred as “epigenetic”) affecting certain genes and/or overexpression of DNMTs have been associated with many cancer types including lung, colorectal, prostate, breast, endometrial, gastric, hepatocellular, cervical, and pancreatic cancers (21, 22).
Experimentally, the selective inhibition of different DNMT enzymes has provided important clues to determine their role in physiology and pathophysiology. For example, it has been observed that DNMT inhibition reactivates “silenced” or hypermethylated genes, particularly tumor suppressor genes (genes associated with the expression of proteins that prevent tumor formation) (23, 24). Another important observation is that the concomitant incubation of DNMT inhibitors with chemotherapeutic agents (25, 26), as well as radiotherapy (27), has shown significant synergistic effects of both of these therapeutic strategies. Finally, the inhibition of DNMT1 and DNMT3B has been shown to abrogate hepatitis C infection in hepatocellular carcinoma cells (28). Consequently, it has been proposed that targeting the aberrant enzymatic activity of DNMTs could restore otherwise hypermethylated tumor suppressor genes, which is considered a promising strategy to prevent cancer initiation and cancer development (29, 30).
The chemical structure and structural features required for a compound to display DNMT inhibition are described in the literature. According to the chemical structure, DNMT inhibitors can be classified in two main groups, namely the nucleos(t)ide and the non-nucleos(t)ide DNMT inhibitors (31–33). Azacitidine (Vidaza®, Celgene) and Decitabine (Dacogen®, Astex) are two US FDA clinically-approved nucleoside DNMT inhibitors (33), whereas the compound MG98 represents an oligonucleotide. Representative examples of the non-nucleos(t)ide class of DNMT inhibitors are tryptophan derivative (RG108), quinoline derivatives (SGI-1027), alkyne derivatives, cyclopenta- and cyclohexathiophene derivatives, procainamide derivatives, genistein (natural flavonoid), curcumin, Psammaplin A (a marine natural compound) and hydralazine (see Figure 2 for chemical structures) (33).
Figure 2.
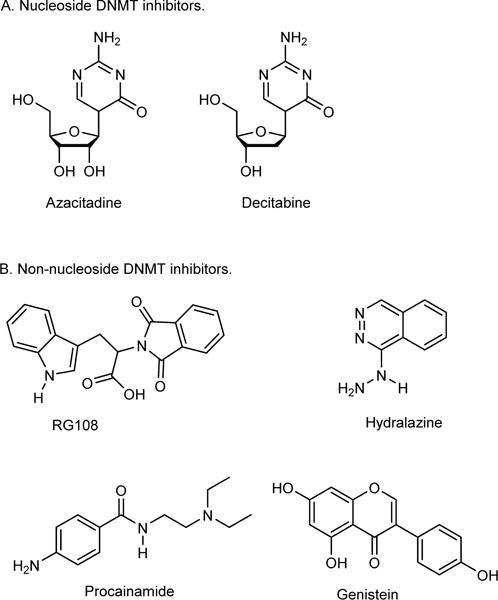
Chemical structures of representative examples of nucleoside and non-nucleoside DNMT inhibitors.
Based on the observation that DNA methylation can be reversed by specific DNA repair mechanisms, the inhibition of hypermethylation of tumor suppressor genes is a promising strategy to prevent cancer initiation and development. This inhibition may take place over a long-period of time after administering either synthetic (34) or naturally occuring chemopreventive drugs (35). Computational approaches have demonstrated the ability to identify DNMT inhibitors or compounds with demethylating properties that have novel scaffolds (32, 36). In this regard, a recent work published by Kuck et al. (37) reported the docking-based, virtual screening, and in vitro evaluation of more than 26,000 compounds from the National Cancer Institute (NCI) database on DNMT enzymes. In that paper, authors reported a series of small molecules with relatively high biochemical selectivity towards individual human DNMT enzymes. Using a multistep docking approach of lead-like compounds with a homology model of the catalytic site of DNMT1, followed by experimental testing, authors identified seven new molecules with detectable DNMT1 inhibitory activity. The molecules identified in this study had diverse scaffolds, some of them not previously reported as DNMT inhibitors, such as a series of methylenedisalicylic acids, among which, the compound NSC 14778 (Figure 1) was one of the most potent compounds tested on DNMT1 and DNMT3B enzymes (37).
By analysing the chemical structure of the scaffold present in methylenedisalicylic acids, and compare it to that of our recently reported resveratrol-salicylate analogues, in which we added a carboxylic acid group to one of the aromatic rings present in the polyphenol (38), we hypothesized that, in addition to the CYP1A1 inhibitory activity reported previously, these hybrid drugs could also inhibit the enzymatic activity of DNMT (Figure 1).
To the best of our knowledge, there are no reports in the literature describing the direct inhibitory effect of resveratrol on DNMT enzymes, and the only report we could find on this regard, was that published by Qin et al., who reported the effects of resveratrol on the expression of DNMT enzymes (39). As part of an ongoing research work aimed at developing new cancer chemopreventive agents, we now report in vitro biological evaluation and the molecular modeling (docking) studies of a new series of resveratrol-salicylate derivatives with DNMT inhibitory activity. Our hypothesis was based on the idea that the addition of a carboxylic acid or its methyl ester, attached ortho to one of the phenol groups present in hydroxystilbenes, might confer resveratrol with a novel DNMT inhibitory profile, similar to that exerted by methylenedisalicylic acids described above. In this report, we identified compound 10 as the most active analogue which showed greater than four-fold potency compared to resveratrol in inhibiting the DNMT3A enzyme. Additionally, compound 10 exerted cell proliferation inhibition on three different human cancer cell lines (HT-29, HepG2, and SK-BR-3), suggesting that this chemical compound was more effective than the parent resveratrol under the same experimental conditions.
MATERIALS AND METHODS
Chemistry
We carried out the synthesis of hybrid resveratrol-salicylate derivatives 3–12 as described in our previous paper (38).
Inhibition of DNMT enzymes
The catalytic domains of DNMT3A/3B and full length DNMT 3L were purified as described previously by Hemeon, I. et al (40). Full length DNMT1 was purified as previously described (41). The dose response experiments were performed against DNMT1 and DNMT3A/3B using the radiometric assay described by Hemeon et al. (40). Briefly, the assay was conducted in the buffer containing 50 mM HEPES, 50 mM KCl, 5% glycerol and 1 mM DTT, pH = 8.0. The inhibitors were preincubated in the buffer containing 1 μM of the corresponding enzyme or enzyme complex for 30 min, and the reaction was initiated by the addition of the substrate mix (1 μg dIdC substrate and 1.83 μM 3H-S-adenosyl-L-methionine). The methylation reactions were allowed to proceed at ambient room temperature of 22 °C for 4 h (DNMT3B/3L) and overnight (DNMT3A/3L). Subsequently, 6 μL of the reaction was spotted on a 1.2 cm × 1.2 cm DE81 Anion Exchanger exchange filter paper squares. Each reaction was spotted three times. The filter paper was allowed to dry for 15 minutes, and washed twice with 0.2 M ammonium bicarbonate, followed by deionized double distilled water and ethanol. The filter paper was then put in scintillation vials, followed by the addition of 0.5 mL of deionized double distilled water and then 5 mL of scintillation fluid. The signal was monitored using a Liquid Scintillation Analyzer (Perkin Elmer Tri-Carb 2910 TR) and percent inhibition was calculated as previously described (42).
Molecular Modeling
Proteins
The crystal structures of human DNMT1 (PDB ID: 3SWR), and DNMT3A (PDB ID: 2QRV) were retrieved form the Protein Data Bank (PDB), whereas for the DNMT3B structure we used the homology model we previously published for this isozyme (37). The structures were prepared and submitted to a geometry optimization protocol (OPLS force field) by using the Protein Preparation Wizard protocol of Schrödinger software using the default settings (43).
Ligands
Compounds 3 to 12, 3,4′,5-trans-trimethoxystilbene (TMS) as well as resveratrol were built and submitted to a geometry optimization protocol employing the AMBER99SB force field in UCSF Chimera 1.9 (44).
Docking
Molecular docking studies were performed using AutoDock 4.2 software (45). In these studies we evaluated the compounds in the DNMT catalytic site in the presence and absence of the co-factor. We used a grid box of 80 × 80 × 80 points with a grid spacing of 0.375 Å that covers the catalytic pocket and the co-factor binding site. The Lamarckian genetic algorithm was used as a search method. A total of 20 runs were carried out with a maximal number of 5,000,000 energy evaluations and initial population of 150 conformers. The best binding modes for each molecule were selected for the analysis. We have previously used AutoDock to model DNMT inhibitors (37).
Cytotoxicity
Human colorectal adenocarcinoma HT-29 (ATCC HTB-38, Manassas, VA), human hepatoma HepG2 cells (ATCC HB-8065, Manassas, VA), and human mammary gland/breast SK-BR-3 cells (ATCC HTB-30, Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium, supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Cells were grown in 75-cm2 tissue culture flasks at 37°C in a 5% CO2 humidified incubator. To evaluate the antiproliferative effect of the most active compound 10, resveratrol, and its natural analogue TMS, we carried out a series of MTT assays using a published procedure (46) with minor modifications. All test compounds were dissolved in DMSO and tested at a final concentration range of 0.03 to 125 μM, over a 24-hour incubation period. The final concentration of DMSO in culture media was fixed at 0.5% (v/v). The corresponding IC50 values were calculated from the cell growth inhibition curve using GraphPad Prism software (IC50 values represent an average of three different experiments, in triplicate).
RESULTS AND DISCUSSION
During the last decade, the evolution of epigenetics and the validated association of this biological process with many disorders such as cancer (47), Alzheimer (48), cardiovascular diseases (49), and diabetes (50) have been the subject of scientific research. These epigenetic mechanisms are regulated by multiple proteins including DNA methyltransferase enzymes (DNMTs) (51). DNMTs catalyse the transfer of a methyl group from the substrate S-adenosylmethionine (SAM), to DNA cytosine residues (called “CG sites”) (20). DNMT1 specifically methylates hemi-methylated DNA, while DNMT3A and DNMT3B bind to unmethylated DNA to carry out de novo methylation (20). It has been proposed that small molecule inhibitors of DNMT enzymes can bind either at the catalytic binding pocket binding site of (DNA) or at the binding site of the cofactor S-adenosylhomocysteine (SAH) (52), or both, depending on the structure of the inhibitor. The latter is particularly applicable if the structure of the inhibitor has a ‘long’ scaffold such as SGI-1027 (53).
Compound NSC14778 (Figure 1), a methylenedisalicylic acid was reported by Kuck et al. after implementing a virtual screening protocol on more than 26,000 compounds from a NCI database, provided a useful lead scaffold to design new molecules with potential DNMT inhibitory activity (37). In this regard, we have recently reported the chemical synthesis and CYP1A1 inhibitory profile of a new series of hybrid resveratrol-salicylate analogues with promising chemopreventive activity (38); after we re-examined the chemical structures of these derivatives, we recognized a potentially useful pattern: by replacing the central methylene group in NSC14778 for the ethylene (CH=CH) moiety present in stilbenes. It is noteworthy that we identified some structural similarities between NSC14778, and our recently reported salicylate-resveratrol analogues, so that it was reasonable to predict certain degree of DNMT inhibition by our molecules (however, please note that we did not start the design of our salicylate-resveratrol derivatives based on the docking pose of NSC14778). We hypothesized that these compounds could exert significant inhibition of DNMT enzymes, for two reasons.
First, the new salicylate moiety on resveratrol (Figure 1) would resemble the salicylate group in NSC14778, which has been reported as “essential” for DNMT inhibition (37); second, literature reports have shown that resveratrol is capable of reducing the expression of DNMT enzymes, reactivating previously hypermethylated tumor-suppressor genes (23, 24, 34).
In vitro DNMT inhibition
To study the in vitro DNMT inhibition exerted by the salicylate-resveratrol analogues reported previously (38), we used a filter paper based Scintillation Proximity Assay (SPA) (42). We used the well-known DNMT inhibitor (altough structurally unrelated) S-adenosyl-L-homocysteine (SAH)(54, 55) as the standard which showed IC50 = 2 μM on DNMT1. For comparison purposes, we also used the parent stilbene resveratrol, which showed inhibition on DNMT3B (IC50 = 65 μM) and DNMT3A (IC50 = 105 μM), but no activity on DNMT1 (IC50 higher than 300 μM, Table 1). These results suggest that the parent polyphenol shows selective inhibition of the DNMT3B isozyme. This observation is somewhat related to a recent study reported by Qin et al. (39) in which his group reported a significant reduction in the expression of DNMT3B after a 21-week treatment period to rats with resveratrol. Interestingly, this treatment did not significantly reduce the expression of the DNMT1 enzyme (39). Nevertheless, it is important to distinguish that our study measured enzyme activity, whereas that of Qin et al. determined protein expression.
Table 1.
Concentration (μM) of test compounds required to inhibit by 50% the enzymatic activity of DNMT1, DNMT3A, and DNMT3B. Results are expressed as IC50 values using a cell-free biochemical assay. To generate the enzyme inhibition curves, duplicate reactions were performed for each concentration; IC50 values were calculated using the GraphPad Prism v6 software.
| Compounds | IC50 (μM) | ||
|---|---|---|---|
| DNMT3A/3L | DNMT3B/3L | DNMT1 | |
| 3 | 282 | >300 | NI1 |
| 4 | >300 | >300 | NI1 |
| 5 | >300 | >300 | NI1 |
| 6 | >300 | >300 | NI1 |
| 7 | >300 | >300 | NI1 |
| 8 | 281 | 156 | NI1 |
| 9 | 40 | 52 | NI1 |
| 10 | 25 | 62 | NI1 |
| 11 | 186.6 | 190 | NI1 |
| 12 | 100 | 215 | NI1 |
| TMS | >300 | >300 | NI1 |
| Resveratrol | 105 | 65 | >300 |
NI = no inhibition at the maximum test compound concentration (300 μM).
Another compound that could be a potential DNMT inhibitor is the methylated version of resveratrol, or 3,4′,5-trans-trimethoxystilbene (TMS), which has previously displayed an enhanced anticancer profile compared to resveratrol (46). In this regard, we observed no significant inhibition of DNMT enzymes at the highest test compound concentration (300 μM). This suggests that the free hydroxyl groups present in resveratrol are essential to exert inhibition of the DNMT enzymatic activity. As far as we are concerned, our study is the first one reporting the apparent lack of activity of TMS on DNMT1, DNMT3A, and DNMT3B enzymes (Table 1). A similar effect (i.e., loss of enzymatic inhibitory activity with DNMT1 upon methylation of a hydroxyl group), was noted for a sulfonamide DNMT inhibitor recently identified by high-throughput screening (56, 57).
Once we analyzed the inhibitory profile of compounds described above, we started the screening evaluation of the new hybrid salicylate-resveratrol derivatives. According to our results, derivatives possessing methoxy groups (3–7) at any position on the stilbene structure, were practically inactive at the highest test compound concentration (300 μM), which is in accordance with the results obtained for TMS. However, we also observed that derivatives possessing free hydroxyl groups (8–10) were significantly more potent than their methoxylated counterparts, but their inhibitory profile was significant only on DNMT3A and DNMT3B enzymes, not on DNMT1. In this regard, the most active compounds in the series were compounds 9 and 10, which showed significant inhibition on both DNMT3A (IC50 = 40 μM and 25 μM respectively), and DNMT3B (IC50 values = 52 μM and 62 μM respectively).
Compounds possessing an acetyl group (mimicking an acetylsalicylic acid moiety), either on a 4′- or a 3,5- pattern, are not as potent as those having the free hydroxyl groups. Interestingly, the negative effect of adding acetyl groups on DNMT3 inhibition is milder than that of adding methoxy groups, given that compounds 11 and 12 still showed some degree of inhibition on both DNMT3A and DNMT3B enzymes (IC50’s in the 100–215 μM range; Table 1). The observation that methoxy groups reduce DNMT inhibition seems to be in agreement with a recent report by Rilova et al., in which they reported that dimethoxytriazine groups decreased the DNMT3A inhibitory activity of quinolone-based DNMT inhibitors (58).
A detailed comparison between molecules 8 and 9, both possessing free hydroxyl groups, suggests that two phenol groups at positions 3- and 5- (compound 9 IC50 values = 40 μM –on DNMT3A–, and 52 μM –on DNMT3B–), exert a better enzyme inhibitory profile than having only one at the 4- position (compound 8 IC50 values = 281 μM –on DNMT3A–, and 156 μM –on DNMT3B–).
To complement the structural analysis of compounds 3–12, we studied the effects of the carboxylic acid group on the stilbene scaffold, which according to previous reports, seems to be an essential requirement for DNMT1 inhibition. This requirement has been previously described in different molecules. Analogue series of drugs having a carboxyl group are in general more potent DNMT1 inhibitors than those not having it (34, 59). This observation has been studied using molecular modeling (docking) studies, and it has been predicted that carboxylate anions are able to form hydrogen bonds with key amino acid residues in the active site of DNMT1 (34, 59). Nevertheless, according to our results and the experimental conditions we used, for DNMT3A and DNMT3B, the presence of the carboxylic acid group on the stilbene scaffold seems to be significant only when the phenol groups are free. As it can be observed with our small library of hybrid salicylate-resveratrol derivatives, compounds possessing a free carboxylic acid (8, 9, 11 and 12), a carboxylate methyl ester (3, 4, 6, 7 and 10), or no carboxylic acid at all (compound 5) did not show any inhibition on DNMT1, even at concentrations as high as 1 mM (results not shown). In this regard, a recent study by Asgatay et al. showed that a N-phthaloyl-L-tryptophan derivative, in which a carboxylate group was replaced by an amide function, can still display some activity towards DNMT1. Therefore, authors proposed that the essential role of the carboxyl group is still “inconclusive” (60).
As far as DNMT3A/3B inhibition is concerned, it is still not clear if the presence of a carboxyl group is required for a drug to exert binding interactions in the active site of DNMT3 enzymes. Nevertheless, recent developments with small molecule inhibitors have showed that in vitro DNMT3A inhibition is possible without the presence of carboxylate groups (58). Thus, results of this work showed that compounds bearing either a free carboxylic acid (9), or a carboxylate methyl ester (10), exerted a better inhibitory profile than resveratrol against both DNMT3 enzymes (see Table 1).
Molecular Modeling
To test in silico the ability of the test compounds to interact with the catalytic site of DNMT enzymes, we carried out molecular modeling (docking) simulations, in which we assessed the ability of hybrid salicylate-resveratrol derivatives to exert binding interactions with key amino acid residues in the enzyme’s active site. We did these experiments in the presence and in the absence of the co-factor SAH according to a previously reported protocol (52).
Figure 3 shows the binding mode for the parent compound (resveratrol), and the active compounds 9 and 10 within the human DNMT enzyme binding sites, in the presence and in the absence of the co-factor. The table below Figure 3 summarizes the calculated binding free energies for each binding mode. The binding free energies as calculated by Autodock, and the binding modes of the remaining compounds are reported in Table 2 and Figure 4, respectively. According to our molecular modeling results, the docking scores calculated for resveratrol, compound 9 and compound 10 in the active sites of both DNMT3A and DNMT3B (in the presence and absence of the co-factor) are, overall, more favorable (more negative), than those values obtained with DNMT1. Despite the well-known number of approximations considered in calculating docking scores (61), this is in good qualitative agreement with the trend observed experimentally. In the docking study performed in the absence of the co-factor, the presence of a π-π interaction with Trp889 and Trp834 was observed in the DNMT3A and DNMT3B structures, respectively. It is noteworthy that the tryptophan is absent in the structure of the DNMT1, which may explain the differences in binding energies and the lack of activity on the DNMT1 isoform.
Figure 3.
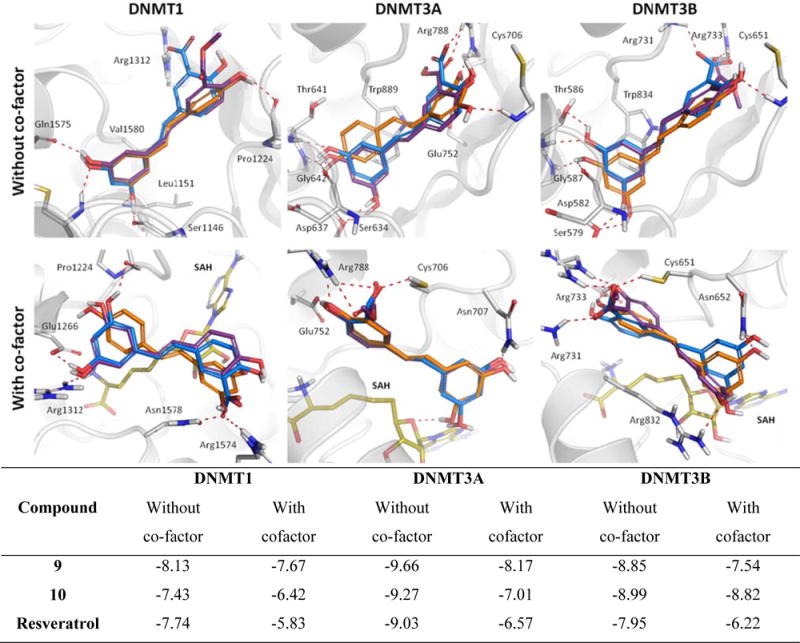
Comparison of the binding modes calculated for compounds 9 (blue), 10 (purple), and resveratrol (orange) as predicted by AutoDock 4.2, in the presence and in the absence of the co-factor SAH (yellow) in the active site of DNMT1, DNMT3A and DNMT3B enzymes.
Table 2.
Calculated binding free energies of resveratrol-salicylate analogues in human DNMTs.
| Compound | DNMT1
|
DNMT3A
|
DNMT3B
|
|||
|---|---|---|---|---|---|---|
| Without cofactor | With cofactor | Without cofactor | With cofactor | Without cofactor | With cofactor | |
| 3 | −7.52 | −6.58 | −8.11 | −7.08 | −7.32 | −7.16 |
| 4 | −8.47 | −5.83 | −8.99 | −5.84 | −8.02 | −6.78 |
| 5 | −8.26 | −6.26 | −8.52 | −6.27 | −8.76 | −6.17 |
| 6 | −7.05 | −6.39 | −5.06 | −6.29 | −8.28 | −7.02 |
| 7 | −7.44 | −6.14 | −8.40 | −5.76 | −7.82 | −7.85 |
| 8 | −8.37 | −6.91 | −9.41 | −7.84 | −9.05 | −8.54 |
| 9 | −8.13 | −7.67 | −9.66 | −8.17 | −8.85 | −7.54 |
| 10 | −7.43 | −6.42 | −9.27 | −7.01 | −8.99 | −8.82 |
| 11 | −8.94 | −7.21 | −7.65 | −7.72 | −9.33 | −8.23 |
| 12 | −7.95 | −8.96 | −8.42 | −7.50 | −7.88 | −8.44 |
| TMS | −7.36 | −6.02 | −7.95 | −5.67 | −8.23 | −7.53 |
| Resveratrol | −7.74 | −5.83 | −9.03 | −6.57 | −7.95 | −6.22 |
Figure 4.
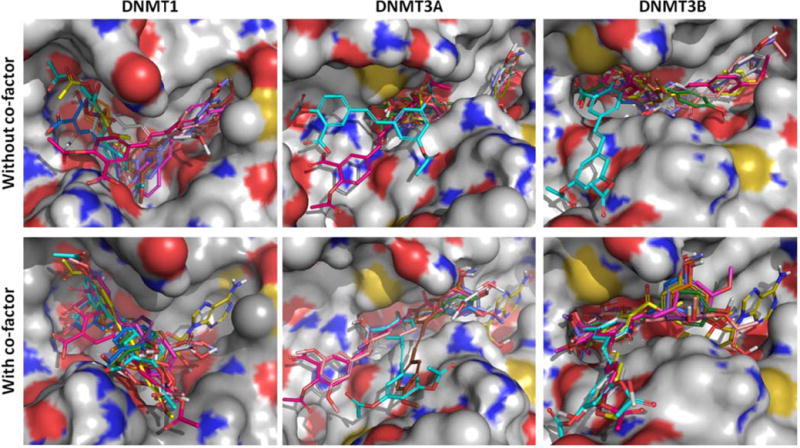
Comparison of the binding modes of compounds 3 (magenta), 4 (yellow), 5 (pink), 6 (gray), 7 (violet), 8 (green), 9 (blue), 10 (purple), 11 (pink), 12 (cyan), TMS (brown) and resveratrol (orange) predicted by AutoDock 4.2 in the presence and absence of co-factor SAH of DNMT1, DNMT3A and DNMT3B.
In the study carried out in the presence of the co-factor, we observed interactions of the ligands with the catalytic cysteine, glutamic acid and arginine in both DNMT3A (Cys706, Glu752 and Arg788) and DNMT3B (Cys651, Glu697 and Arg733) active sites, which in previous studies have proven to be a primary interaction for enzyme inhibition. In this regard, Cys651 has previously showed to be a key site for binding interactions between the antibiotic Nanaomycin and the DNMT3B enzyme (62). Consequently, these docking results allowed us to hypothesize that regardless of the operating inhibition mechanism (with or without the co-factor), these binding interactions may offer a plausible explanation for the observed selectivity toward DNMT3 enzymes by the test compounds, including the parent resveratrol. It is noteworthy that the presence of the 3,5-dihydroxyphenyl group (also called resorcinol) is important for the interaction of the ligands in both studies, suggesting that the test compounds should have this group for the inhibition of DNMT3 isoforms.
Cytotoxicity in culture cells
The promising inhibitory profile observed for compound 10, along with the corresponding molecular modeling (docking) studies, and the observation that epigenetic modifications in cancer cells are essential for cell proliferation, we evaluated the effects of compound 10 on in vitro cell proliferation. To carry out this, we used three different human cancer cell lines, namely HT-29 cells (colorectal), HepG2 cells (liver), and SK-BR-3 cells (breast). In these cell lines, DNMT-mediated epigenetic regulations have been recently confirmed (25, 63, 64); the results are summarized in Table 3. Interestingly, compound 10 exerted a stronger cell proliferation inhibition than that exerted by the parent resveratrol in all three cancer cells, and it was more active than TMS on HepG2 and SK-BR-3 cells. In this regard, it has been reported that TMS, being a more lipophilic stilbene than resveratrol (and consequently, more likely to cross cell membranes), demonstrated a higher cell proliferation inhibition than resveratrol in cancer cells (46). The ability of TMS (as well as compound 10) to inhibit DNMT3 activity, does not exclude other mechanisms by which this hybrid molecule could decrease cell proliferation. In fact, there is considerable evidence backing up the multi-target profile exerted by resveratrol, which may be applicable to its salicylate hybrid 10; nevertheless, further studies are needed to investigate other mechanisms of anti-proliferative activity exerted by this compound.
Table 3.
Concentration (μM) of the test compounds required to inhibit cell proliferation by 50 % (IC50) using the MTT assay. Each IC50 value represents the mean of three different experiments in triplicate. To generate the cell proliferation inhibition curves, six concentrations (in the 0.03 to 125 μM range) were used for each compound. IC50 values were generated using the GraphPad v6 Prism software.
| Compounds | IC50 (μM) | ||
|---|---|---|---|
| HT-29 | HepG2 | SK-BR-3 | |
| 10 | 44.3 | 18.9 | 11.3 |
| Resveratrol | 130.0 | 54.9 | 110.3 |
| TMS | 14.0 | >100 | 111.8 |
In a previous study (38), we reported the CYP1A1 inhibitory profile of compounds 3–12, in which we elaborated on the chemical features required for hybrid molecules to exert inhibitory activity on CYP1A1. Compound 10 was not as effective as other molecules inhibiting the CYP isoform; however, the binding interactions make this molecule an effective DNMT3 inhibitor, despite its lack of activity on CYP enzymes. These observations suggest that the overall design of hybrid salicylate-resveratrol analogues is flexible enough to offer preferential inhibition against at least these two proteins (CYP1A1 and DNMT3).
CONCLUSION
We showed that the hybrid salicylate-resveratrol scaffold is a promising alternative to the parent stilbene resveratrol and its methylated analogue TMS as a DNMT inhibitor. Derivatives 9 and 10 showed a significant and selective inhibitory profile on DNMT3A and DNMT3B enzymes, which is 2–4 times more potent than that exerted by resveratrol under the same experimental conditions. Structure-activity relationships showed that free hydroxyl groups are required to exert DNMT3 inhibition, and this pattern is better in analogues having phenols in positions 3- and 5- of a stilbene. The presence of the salicylate group in resveratrol’s structure produced an enhanced inhibitory profile only when there are free phenol groups on the stilbene. Compound 10 showed an improved in vitro cell proliferation inhibition compared to resveratrol and TMS on at least two human cancer cells, suggesting that compound 10, and possibly compound 9, are promising candidates worth evaluating in vivo, to further understand their potential anticancer/chemopreventive properties.
Acknowledgments
The authors acknowledge the financial support provided by the Saudi Cultural Bureau in Canada, for a graduate scholarship to (F.S.A)., the National Institute of General Medical Sciences (1R01GM096056) (M. L.), the National Institute of Health (NIH) Director’s New Innovator Award Program (1DP2-OD007335) (M. L.), the Starr Cancer Consortium (M. L.), and Mr. William H. Goodwin and Mrs. Alice Goodwin, the Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center (M. L.) and the Department of Pharmacy, School of Chemistry at UNAM (Mexico) for their support. The authors would like also to acknowledge Prof. Vern Schramm (Albert Einstein College of Medicine) for providing plasmids for heterologous expression of DNMT3A, 3B and DNMT3L, Prof. Dinshaw Patel (Memorial Sloan Kettering Cancer Center) for hDNMT1 plasmid and Glorymar Ibañez for purifying DNMT3A/3B and 3L.
Abbreviations
- COX
cyclooxygenase
- DNMT
DNA methyltransferase
- iNOS
inducible nitric oxide synthase
- LOX
lipoxygenase
- NF-κB
nuclear factor-kappa B
- PI3K
Phosphoinositide 3-kinase
- PPAR
γ – peroxisome proliferator activated receptor gamma
- SAH
S-adenosyl-L-homocysteine
- SAM
S-adenosyl-L-methionine
- Sirt1
Sirtuin type 1 (silent information regulator type 1)
- TMS
3,4′,5-trans-trimethoxystilbene
Footnotes
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Fulda S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discovery Today. 2010;15:757–65. doi: 10.1016/j.drudis.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, et al. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–83. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009;284:1–6. doi: 10.1016/j.canlet.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 5.Kundu JK, Surh Y-J. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008;269:243–61. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 6.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 7.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–66. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Sengottuvelan M, Senthilkumar R, Nalini N. Modulatory influence of dietary resveratrol during different phases of 1,2-dimethylhydrazine induced mucosal lipid-peroxidation, antioxidant status and aberrant crypt foci development in rat colon carcinogenesis. BBA-Gen Subjects. 2006;1760:1175–83. doi: 10.1016/j.bbagen.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Das S, Das DK. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2007;2:133–8. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 10.Gehm BD, Levenson AS, Liu H, Lee E-J, Amundsen BM, Cushman M, et al. Estrogenic effects of resveratrol in breast cancer cells expressing mutant and wild-type estrogen receptors: role of AF-1 and AF-2. J Steroid Biochem Mol Biol. 2004;88:223–34. doi: 10.1016/j.jsbmb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Juan ME, Alfaras I, Planas JM. Colorectal cancer chemoprevention by trans-resveratrol. Pharmacol Res. 2012;65:584–91. doi: 10.1016/j.phrs.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Gwak H, Haegeman G, Tsang BK, Song YS. Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of Akt/GLUT1 axis in ovarian cancer cells. Mol Carcinog. 2014 doi: 10.1002/mc.22133. [DOI] [PubMed] [Google Scholar]
- 13.Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 14.Tinhofer I, Bernhard D, Senfter M, Anether G, Loeffler M, Kroemer G, et al. Resveratrol, a tumor-suppressive compound from grapes, induces apoptosis via a novel mitochondrial pathway controlled by Bcl-2. FASEB J. 2001;15:1613–5. doi: 10.1096/fj.00-0675fje. [DOI] [PubMed] [Google Scholar]
- 15.Joe AK, Liu H, Suzui M, Vural ME, Xiao DH, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- 16.Bai Y, Mao Q-Q, Qin J, Zheng X-Y, Wang Y-B, Yang K, et al. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010;101:488–93. doi: 10.1111/j.1349-7006.2009.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin HT, Tian QZ, Guan L, Zhou Y, Huang XE, Zhang H. In vitro and in vivo Evaluation of the Antitumor Efficiency of Resveratrol Against Lung Cancer. Asian Pac J Cancer Prev. 2013;14:1703–6. doi: 10.7314/apjcp.2013.14.3.1703. [DOI] [PubMed] [Google Scholar]
- 18.Medina-Franco JL, Giulianotti MA, Welmaker GS, Houghten RA. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discovery Today. 2013;18:495–501. doi: 10.1016/j.drudis.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallauf K, Giller K, Huebbe P, Rimbach G. Nutrition and healthy ageing: calorie restriction or polyphenol-rich “MediterrAsian” diet? Oxid Med Cell Longev. 2013;2013:707421. doi: 10.1155/2013/707421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 2011;12:647–56. doi: 10.1038/embor.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnyszka A, Jastrzebski Z, Flis S. DNA Methyltransferase Inhibitors and Their Emerging Role in Epigenetic Therapy of Cancer. Anticancer Research. 2013;33:2989–96. [PubMed] [Google Scholar]
- 22.Gao J, Wang L, Xu J, Zheng J, Man X, Wu H, et al. Aberrant DNA methyltransferase expression in pancreatic ductal adenocarcinoma development and progression. J Exp Clin Cancer Res. 2013;32:86. doi: 10.1186/1756-9966-32-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu W, Qin W, Zhang K, Rottinghaus GE, Chen Y-C, Kliethermes B, et al. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012;64:393–400. doi: 10.1080/01635581.2012.654926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capobianco E, Mora A, La Sala D, Roberti A, Zaki N, Badidi E, et al. Separate and combined effects of DNMT and HDAC inhibitors in treating human multi-drug resistant osteosarcoma HosDXR150 cell line. PloS one. 2014;9:e95596. doi: 10.1371/journal.pone.0095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flis S, Gnyszka A, Flis K. DNA methyltransferase inhibitors improve the effect of chemotherapeutic agents in SW48 and HT-29 colorectal cancer cells. PloS one. 2014;9:e92305. doi: 10.1371/journal.pone.0092305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandhu R, Rivenbark AG, Coleman WB. Enhancement of chemotherapeutic efficacy in hypermethylator breast cancer cells through targeted and pharmacologic inhibition of DNMT3b. Breast Cancer Res Treat. 2012;131:385–99. doi: 10.1007/s10549-011-1409-2. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Kim JH, Chie EK, Young PD, Kim IA, Kim IH. DNMT (DNA methyltransferase) inhibitors radiosensitize human cancer cells by suppressing DNA repair activity. Radiat Oncol. 2012;7:39. doi: 10.1186/1748-717X-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Pan D, Deng AM, Huang F, Sun B-L, Yang R-G. DNA methyltransferases 1 and 3B are required for hepatitis C virus infection in cell culture. Virology. 2013;441:57–65. doi: 10.1016/j.virol.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Sin-Chan P, Huang A. DNMTs as potential therapeutic targets in high-risk pediatric embryonal brain tumors. Expert Opin Ther Targets. 2014;18:1103–7. doi: 10.1517/14728222.2014.938052. [DOI] [PubMed] [Google Scholar]
- 30.Subramaniam D, Thombre R, Dhar A, Anant S. DNA methyltransferases: a novel target for prevention and therapy. Front Oncol. 2014;4:80. doi: 10.3389/fonc.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erdmann A, Halby L, Fahy J, Arimondo PB. Targeting DNA methylation with small molecules: what’s next? J Med Chem. 2014 doi: 10.1021/jm500843d. [DOI] [PubMed] [Google Scholar]
- 32.Medina-Franco JL, Méndez-Lucio O, Yoo J, Dueñas A. Discovery and development of DNA methyltransferase inhibitors using in silico approaches. Drug Discovery Today. 2014 doi: 10.1016/j.drudis.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Fahy J, Jeltsch A, Arimondo PB. DNA methyltransferase inhibitors in cancer: a chemical and therapeutic patent overview and selected clinical studies. Expert Opin Ther Pat. 2012;22:1427–42. doi: 10.1517/13543776.2012.729579. [DOI] [PubMed] [Google Scholar]
- 34.Brueckner B, Boy RG, Siedlecki P, Musch T, Kliem HC, Zielenkiewicz P, et al. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 2005;65:6305–11. doi: 10.1158/0008-5472.CAN-04-2957. [DOI] [PubMed] [Google Scholar]
- 35.Fang MZ, Wang YM, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- 36.Mendez-Lucio O, Tran J, Medina-Franco JL, Meurice N, Muller M. Toward drug repurposing in epigenetics: olsalazine as a hypomethylating compound active in a cellular context. Chem Med Chem. 2014;9:560–5. doi: 10.1002/cmdc.201300555. [DOI] [PubMed] [Google Scholar]
- 37.Kuck D, Singh N, Lyko F, Medina-Franco JL. Novel and selective DNA methyltransferase inhibitors: Docking-based virtual screening and experimental evaluation. Bioorg Med Chem. 2010;18:822–9. doi: 10.1016/j.bmc.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 38.Aldawsari FS, Elshenawy OH, El Gendy MA, Aguayo-Ortiz R, Baksh S, El-Kadi AO, et al. Design and synthesis of resveratrol-salicylate hybrid derivatives as CYP1A1 inhibitors. J Enzyme Inhib Med Chem. 2014:1–12. doi: 10.3109/14756366.2014.979347. [DOI] [PubMed] [Google Scholar]
- 39.Qin W, Zhang K, Clarke K, Weiland T, Sauter ER. Methylation and miRNA effects of resveratrol on mammary tumors vs. normal tissue. Nutr Cancer. 2014;66:270–7. doi: 10.1080/01635581.2014.868910. [DOI] [PubMed] [Google Scholar]
- 40.Hemeon I, Gutierrez JA, Ho M-C, Schramm VL. Characterizing DNA Methyltransferases With An Ultrasensitive Luciferase-Linked Continuous Assay. Anal Chem. 2011;83:4996–5004. doi: 10.1021/ac200816m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J, Rechkoblit O, Bestor TH, Patel DJ. Structure of DNMT1-DNA complex reveals a role for autoinhibition in maintenance DNA methylation. Science. 2011;331:1036–40. doi: 10.1126/science.1195380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng W, Ibáñez G, Wu H, Blum G, Zeng H, Dong A, et al. Sinefungin derivatives as inhibitors and structure probes of protein lysine methyltransferase SETD2. J Am Chem Soc. 2012;134:18004–14. doi: 10.1021/ja307060p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macromdel, version 9.8. Schrödinger, LLC; New York, NY: 2010. [Google Scholar]
- 44.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 45.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Hu F, Gao Y, Jia S, Ji N, Hua E. Design, synthesis, and evaluation of methoxylated resveratrol derivatives as potential antitumor agents. Res Chem Intermed. 2013:1–14. [Google Scholar]
- 47.Mikeska T, Craig JM. DNA methylation biomarkers: cancer and beyond. Genes (Basel) 2014;5:821–64. doi: 10.3390/genes5030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cacabelos R, Torrellas C. Epigenetic drug discovery for Alzheimer’s disease. Expert Opin Drug Discov. 2014;9:1059–86. doi: 10.1517/17460441.2014.930124. [DOI] [PubMed] [Google Scholar]
- 49.Chaturvedi P, Tyagi SC. Epigenetic mechanisms underlying cardiac degeneration and regeneration. Int J Cardiol. 2014;173:1–11. doi: 10.1016/j.ijcard.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stankov K, Benc D, Draskovic D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics. 2013;132:1112–22. doi: 10.1542/peds.2013-1652. [DOI] [PubMed] [Google Scholar]
- 51.Ross SA. Diet and DNA methylation interactions in cancer prevention. Ann N Y Acad Sci. 2003;983:197–207. doi: 10.1111/j.1749-6632.2003.tb05974.x. [DOI] [PubMed] [Google Scholar]
- 52.Medina-Franco JL, Yoo J. Docking of a novel DNA methyltransferase inhibitor identified from high-throughput screening: insights to unveil inhibitors in chemical databases. Mol Divers. 2013;17:337–44. doi: 10.1007/s11030-013-9428-z. [DOI] [PubMed] [Google Scholar]
- 53.Yoo J, Choi S, Medina-Franco JL. Molecular modeling studies of the novel inhibitors of DNA methyltransferases SGI-1027 and CBC12: implications for the mechanism of inhibition of DNMTs. PloS one. 2013;8:e62152. doi: 10.1371/journal.pone.0062152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isakovic L, Saavedra OM, Llewellyn DB, Claridge S, Zhan L, Bernstein N, et al. Constrained (l-)-S-adenosyl-l-homocysteine (SAH) analogues as DNA methyltransferase inhibitors. Bioorg Med Chem Lett. 2009;19:2742–6. doi: 10.1016/j.bmcl.2009.03.132. [DOI] [PubMed] [Google Scholar]
- 55.Saavedra OM, Isakovic L, Llewellyn DB, Zhan L, Bernstein N, Claridge S, et al. SAR around (l)-S-adenosyl-l-homocysteine, an inhibitor of human DNA methyltransferase (DNMT) enzymes. Bioorg Med Chem Lett. 2009;19:2747–51. doi: 10.1016/j.bmcl.2009.03.113. [DOI] [PubMed] [Google Scholar]
- 56.Medina-Franco JL, Mendez-Lucio O, Yoo J. Rationalization of activity cliffs of a sulfonamide inhibitor of DNA methyltransferases with induced-fit docking. Int J Mol Sci. 2014;15:3253–61. doi: 10.3390/ijms15023253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kilgore JA, Du XL, Melito L, Wei SG, Wang CG, Chin HG, et al. Identification of DNMT1 selective antagonists using a novel scintillation proximity assay. J Biol Chem. 2013;288:19673–84. doi: 10.1074/jbc.M112.443895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rilova E, Erdmann A, Gros C, Masson V, Aussagues Y, Poughon-Cassabois V, et al. Design, synthesis and biological evaluation of 4-amino-N-(4-aminophenyl)benzamide analogues of quinoline-based SGI-1027 as inhibitors of DNA methylation. Chem Med Chem. 2014;9:590–601. doi: 10.1002/cmdc.201300420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki T, Tanaka R, Hamada S, Nakagawa H, Miyata N. Design, synthesis, inhibitory activity, and binding mode study of novel DNA methyltransferase 1 inhibitors. Bioorg Med Chem Lett. 2010;20:1124–7. doi: 10.1016/j.bmcl.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 60.Asgatay S, Champion C, Marloie G, Drujon T, Senamaud-Beaufort C, Ceccaldi A, et al. Synthesis and evaluation of analogues of N-phthaloyl-l-tryptophan (RG108) as inhibitors of DNA methyltransferase 1. J Med Chem. 2013;57:421–34. doi: 10.1021/jm401419p. [DOI] [PubMed] [Google Scholar]
- 61.Bello M, Martinez-Archundia M, Correa-Basurto J. Automated docking for novel drug discovery. Expert Opin Drug Discov. 2013;8:821–34. doi: 10.1517/17460441.2013.794780. [DOI] [PubMed] [Google Scholar]
- 62.Kuck D, Caulfield T, Lyko F, Medina-Franco JL. Nanaomycin A selectively inhibits DNMT3B and reactivates silenced tumor suppressor genes in human cancer cells. Mol Cancer Ther. 2010;9:3015–23. doi: 10.1158/1535-7163.MCT-10-0609. [DOI] [PubMed] [Google Scholar]
- 63.Sun Q, Xie Y, Wang G, Li J. Identification of genes in HepG2 cells that respond to DNA methylation and histone deacetylation inhibitor treatment. Exp Ther Med. 2014;8:813–7. doi: 10.3892/etm.2014.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costa FF, Verbisck NV, Salim AC, Ierardi DF, Pires LC, Sasahara RM, et al. Epigenetic silencing of the adhesion molecule ADAM23 is highly frequent in breast tumors. Oncogene. 2004;23:1481–8. doi: 10.1038/sj.onc.1207263. [DOI] [PubMed] [Google Scholar]


