Abstract
Background
Lung transplantation is an intervention that improves survival for adult patients with cystic fibrosis (CF). Some patients with CF are never referred for lung transplant evaluation despite meeting physiologic criteria for referral.
Methods
We performed a retrospective analysis of adult patients (≥ 18 years of age) in the Cystic Fibrosis Foundation Patient Registry (CFFPR), eligible for their first evaluation for lung transplantation during the years 2001–2008 based on FEV1 <30% predicted in two consecutive years.
Results
Within the CFFPR, 1240 patients met eligibility criteria. Eight hundred and nine (65.2%) were referred for lung transplant evaluation, and 431 (34.8%) were not referred. In a multivariable model, Medicaid insurance (OR 1.79, 95% CI 1.29–2.47), older age (per 5 year increase; OR 1.25, 95% CI 1.13–1.39), lack of high school graduate education (OR 2.27, 95% CI 1.42–3.64), and Burkholderia cepacia complex sputum culture positivity (OR 2.48, 95% CI 1.50–4.12) were associated with non-referral, while number of pulmonary exacerbations (OR 0.93, 95% CI 0.87–0.99) and supplemental oxygen use (OR 0.59, 95% CI 0.43–0.81) were associated with increased referral.
Conclusions
Despite meeting lung function criteria for lung transplant evaluation, 35% of patients with CF had not yet been referred to a lung transplant center. Predictors of non-referral included markers of low socioeconomic status, older age and Burkholderia cepacia complex sputum culture. Further work is needed to understand the outcomes for non-referred patients in order to refine referral recommendations in this population.
Background
Progressive respiratory failure causes death in approximately 80% of patients with cystic fibrosis (CF).(1–3) Lung transplantation (LTx) is a treatment option for certain patients with CF and end-stage lung disease. CF is currently the third most common indication for LTx in the United States (US).(4) Appropriate candidates for LTx have advanced lung disease with impaired quality of life and are adherent to medical recommendations, while lacking contraindications to transplant. Patients deemed good candidates for LTx are referred to a lung transplant center. The evaluation for LTx involves: assessing a patient’s indication for transplant; identifying potential contraindications or barriers to transplant; and providing the patient with information about the LTx process.(5) If the candidate is determined to be appropriate for lung transplantation, he/she is placed on the United Network for Organ Sharing (UNOS) waitlist in rank-order by Lung Allocation Score (LAS). The LAS was adopted in May 2005 in the US in an attempt to maximize net benefit of transplant, considering a patient’s waitlist urgency and 1-year post-transplant survival.
The International Society for Heart and Lung Transplantation (ISHLT) recommends referral for lung transplant evaluation when a patient has a 2- to 3-year predicted survival of <50%.(6) Historically, forced expiratory volume in one second (FEV1) <30% of the predicted value was considered the strongest independent predictor of 2-year mortality in patients with CF, but this finding has not been uniform.(7–9) FEV1 <30% predicted has been an ISHLT-recommended indication for consideration of referral for lung transplant evaluation since 1998.(10) Several other clinical factors have also been recommended for consideration of referral, including a rapid decline in FEV1 despite optimal therapy, 6-minute walk distance <400 meters, development of pulmonary hypertension, or clinical decline (pulmonary exacerbations with intensive care unit admission, refractory or recurrent pneumothorax, life-threatening hemoptysis not controlled by embolization), particularly if present prior to a fall in FEV1 to below 30% predicted.(6)
Despite meeting current medical indications for referral for transplant evaluation, some CF patients are not referred to a lung transplant center for evaluation. Listing for LTx for patients with CF (once referred to a transplant center) has been shown to differ based on socioeconomic status (SES), including Medicaid insurance status and driving time to nearest lung transplant center.(11) We hypothesized that markers of low SES would also be associated with non-referral of CF patients for lung transplant evaluation. The purpose of this study was to identify predictors of non-referral for lung transplant evaluation in CF patients with advanced lung disease.
Methods
The Institutional Review Board at the University of Washington determined that this research has Exempt Status based on the proposed project’s use of a de-identified data set, the US Cystic Fibrosis Foundation Patient Registry (CFFPR). Our request to use data from the CFFPR was reviewed and accepted by the Cystic Fibrosis Foundation.
Study population and data source
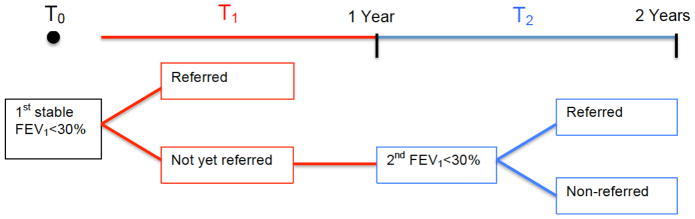
We performed a retrospective cohort study to evaluate predictors of non-referral for lung transplant evaluation in patients with CF using the CFFPR. The CFFPR captures demographic and encounter-based clinical data for approximately 85–90% of the US CF population.(1) Inclusion criteria for this study included: age 18 years and older with a valid residential zip code, eligible for first lung transplant evaluation during the years 2001–2008 based on FEV1 <30% predicted for two consecutive years when clinically stable (Figure 1). The physiologic inclusion criterion for this study was intended to capture an extreme phenotype for CF patients, those likely to be referred for lung transplant evaluation based on current ISHLT recommendations.(6) Subjects with less than two years of lung function data or with a prior lung transplant evaluation were excluded. Once meeting the eligibility requirement, the patient’s lung transplant referral status was ascertained from the CFFPR.
Figure 1. Patient inclusion occurs after two consecutive years of FEV1 <30% and referral status is identified from annual data entries.
T0 = time of first stable FEV1 measurement <30% predicted
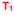 = first annual data entry after T0 (actual time between T0 and T1 will vary from patient to patient but will be less than one year)
= first annual data entry after T0 (actual time between T0 and T1 will vary from patient to patient but will be less than one year)
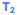 = second annual entry after T0 (actual time between T0 and T2 will vary from patient to patient, but will be more than 1 year for everyone and up to 2 years for some patients); this is the time of eligibility; FEV1 remains <30%
= second annual entry after T0 (actual time between T0 and T2 will vary from patient to patient, but will be more than 1 year for everyone and up to 2 years for some patients); this is the time of eligibility; FEV1 remains <30%
Outcome and exposures
The primary outcome of interest was non-referral (yes/no) for lung transplant evaluation, irrespective of the decision to list for transplant following a referral. The primary covariates of interest focused on SES. Receipt of any Medicaid insurance at the time of eligibility for lung transplant was our primary indicator of low SES and a common proxy for low SES in the CF literature.(12) We also examined other markers of low SES, including educational attainment (high school graduate vs. did not complete high school), median household income based on the patient’s residential ZIP code [relative to the 2000 federal poverty level (FPL)] and driving time to the nearest lung transplant center, defined as the driving time from the center of the patient’s residential zip code to the nearest adult lung transplant center.(11)
Additional covariates included demographics (race, age, gender), markers of disease severity (FEV1 % predicted, number of acute exacerbations [requiring intravenous antibiotics] per year, Pseudomonas aeruginosa and Burkholderia cepacia complex sputum culture status, use of supplemental oxygen or noninvasive positive pressure ventilation [NPPV], body mass index [BMI, kg/m2], insulin-requiring CF-related diabetes), comorbidities (CF-related liver cirrhosis with associated portal hypertension, renal failure requiring dialysis, osteoporosis, depression, tissue-proven cancer, smoking), adherence to medical follow-up (frequency of outpatient visits per year), and LAS implementation period (pre-2005, 2005 or later). Covariate values were ascertained in the same year as the patient met physiologic inclusion criteria. Current clinical recommendations include quarterly clinical visits for all CF patients; therefore, having fewer than four outpatient visits per year was used as a marker of non-adherence. There was significant missing data for smoking (67%) and NPPV (68%) and they were, therefore, excluded from analyses.
Statistical analyses
Descriptive statistics were produced for and compared between patients who were referred and those who were not referred for lung transplant evaluation. Additionally, patient characteristics were compared amongst those receiving and not receiving Medicaid. Continuous variables were compared using Student’s t test allowing for unequal variances and categorical variables with a Chi-squared or Fisher’s exact test when appropriate. To evaluate whether differences in referral for lung transplant evaluation occurred during the time period, a Chi-squared test of referral status and year was performed and a nonparametric test of trend was used to evaluate a linear change in referral over time. A priori power calculations can be found in the online supplement.
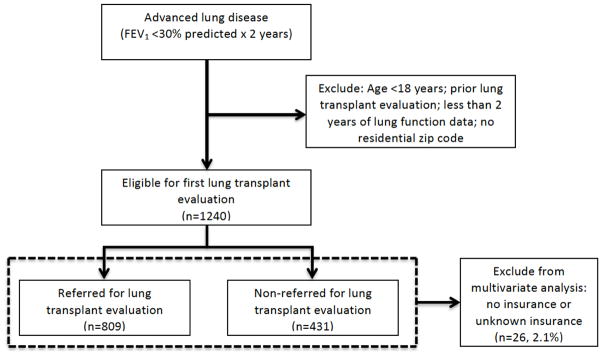
Unadjusted logistic regression was used to evaluate the association between each predictor of interest and non-referral for lung transplant evaluation. Multivariable logistic regression models with robust standard errors were used to evaluate the independent predictors of non-referral. Initially, the model included only Medicaid status and referral outcome. Due to the low numbers, patients with no insurance or unknown insurance status were excluded from analyses (Figure 2). Potential confounding covariates were sequentially forced into the model as sets of related variables: demographics; disease severity characteristics; potential contraindications to referral; driving time to nearest lung transplant center; and lung transplant period. The final model included all sets of covariates. Each model was adjusted for cluster effects at the level of the CF care center. Results of logistic regression models are presented as odds ratios (OR) with corresponding 95% confidence intervals (CI). Two sided P values <0.05 were considered statistically significant. All analyses were performed using STATA version 13.0 (StataCorp LP).
Figure 2.
Study cohort selection of adult patients with cystic fibrosis eligible for first lung transplant evaluation based on lung function criteria, 2001–2008
Results
A total of 1240 patients met our eligibility criteria for lung transplant evaluation during the years 2001–2008, of which 809 (65%) eligible patients were referred for LTx evaluation and 431 (35%) were not referred (Figure 2). Overall, the mean FEV1 % predicted was 24.6% (standard deviation [SD]=3.7%) and mean age of subjects was 31 years (SD=9 years) (Table 1). The majority of subjects were male (59%) and Caucasian (94%). Timing of eligibility (pre/post-LAS implementation) was not significantly associated with referral status. Year of eligibility for lung transplant referral was associated with referral status (p = 0.013, see online supplement Table E1); however, there was no significant trend in referral pattern over time (p = 0.236).
Table 1.
Characteristics of CF patients eligible for referral for lung transplant evaluation based on FEV1 <30% predicted for two consecutive years
| All Eligible Patients | Non-Referred | Referred | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Observed | N | Observed | N | Observed | N | P Value | |
|
| |||||||
| Age – mean (SD), years | 30.6 (9.4) | 1240 | 31.9 (11.0) | 431 | 29.9 (8.4) | 809 | 0.002 |
| Male gender | 736 (59.4%) | 1240 | 275 (63.8%) | 431 | 461 (57.0%) | 809 | 0.020 |
| Race, white vs. non-white | 1164 (93.9%) | 1239 | 404 (94.0%) | 430 | 760 (94.0%) | 809 | 0.994 |
| FEV1 - % predicted (SD) | 24.6 (3.7) | 1240 | 24.7 (3.8) | 431 | 24.5 (3.7) | 809 | 0.513 |
| Acute exacerbations per year – N (SD) | 2.4 (2.4) | 1240 | 2.0 (2.1) | 431 | 2.6 (2.6) | 809 | <0.001 |
| Sputum culture positive | 1176 | 399 | 777 | ||||
| Pseudomonas aeruginosa | 1000 (85.0%) | 330 (82.7%) | 670 (86.2%) | 0.109 | |||
| Burkholderia cepacia complex | 96 (8.2%) | 46 (11.5%) | 50 (6.4%) | 0.003 | |||
| Nontuberculous mycobacterium | 23 (2.0%) | 4 (1.0%) | 19 (2.5%) | 0.091 | |||
| Supplemental oxygen | 693 (56.2%) | 1233 | 205 (47.9%) | 428 | 488 (60.6%) | 805 | <0.001 |
| BMI – kg/m2 (SD) | 19.4 (3.1) | 1236 | 19.3 (3.2) | 429 | 19.5 (3.0) | 807 | 0.383 |
| CF-related diabetes on insulin | 330 (26.6%) | 1240 | 107 (24.8%) | 431 | 223 (27.6%) | 809 | 0.299 |
| CF-related liver cirrhosis | 48 (3.9%) | 1240 | 15 (3.5%) | 431 | 33 (4.1%) | 809 | 0.603 |
| Renal failure requiring dialysis | 10 (0.8%) | 1240 | 1 (0.2%) | 431 | 9 (1.1%) | 809 | 0.179a |
| Osteoporosis | 142 (11.5%) | 1240 | 39 (9.1%) | 431 | 103 (12.7%) | 809 | 0.052 |
| Depression | 291 (23.5%) | 1240 | 112 (26.0%) | 431 | 179 (22.1%) | 809 | 0.127 |
| Cancer, tissue proven | 5 (0.4%) | 1240 | 1 (0.2%) | 431 | 4 (0.5%) | 809 | 0.664a |
| <4 outpatient visits per year | 281 (22.7%) | 1240 | 132 (30.6%) | 431 | 149 (18.4%) | 809 | <0.001 |
| No marital partner | 752 (63.1%) | 1192 | 268 (65.2%) | 411 | 484 (62.0%) | 781 | 0.271 |
| High school graduate | 972 (90.5%) | 1074 | 311 (85.7%) | 363 | 661 (93.0%) | 711 | <0.001 |
| Insurance status | 1214 | 420 | 794 | 0.002 | |||
| Medicaid | 587 (48.4%) | 229 (54.5%) | 358 (45.1%) | ||||
| No Medicaid | 627 (51.7%) | 191 (45.5%) | 436 (54.9%) | ||||
| Median income relative to FPL | 1181 | 412 | 769 | 0.046 | |||
| >300% FPL | 309 (26.2%) | 95 (23.1%) | 214 (27.8%) | ||||
| 250–300% FPL | 237 (20.1%) | 78 (18.9%) | 159 (20.7%) | ||||
| 200–250% FPL | 318 (26.9%) | 109 (26.5%) | 209 (27.2%) | ||||
| <200% FPL | 317 (26.8%) | 130 (31.6%) | 187 (24.3%) | ||||
| Driving time: lung transplant center – min (SD) | 112 (106) | 1203 | 120.6 (107.3) | 423 | 108.0 (105.3) | 780 | 0.050 |
| <30 min (1st quartile) | 281 (23.4%) | 90 (21.3%) | 191 (24.5%) | ||||
| 30–75 min (2nd quartile) | 290 (24.1%) | 97 (22.9%) | 193 (24.7%) | ||||
| 75–150 min (3rd quartile) | 313 (26.0%) | 107 (25.3%) | 206 (26.4%) | ||||
| >150 min (4th quartile) | 319 (26.5%) | 129 (30.5%) | 190 (24.4%) | ||||
| Pre-LAS (prior to 2005) | 672 (54.2%) | 1240 | 222 (51.5%) | 431 | 450 (55.6%) | 809 | 0.166 |
| Post-LAS (2005 or later) | 568 (45.8%) | 1240 | 209 (48.5%) | 431 | 359 (44.4%) | 809 | 0.166 |
Data are presented as No. (%) unless indicated otherwise.
CF = cystic fibrosis, FEV1 = forced expiratory volume in 1 second, SD = standard deviation, BMI = body mass index, FPL = federal poverty level, min = minutes, LAS = lung allocation score
Fisher’s exact test
Despite being younger, subjects with Medicaid insurance had more advanced disease, increased rates of depression, less social support, and more markers of low SES (Table 2). Longer driving time to the nearest lung transplant center was significantly associated with Medicaid insurance status (p <0.001). Additionally, driving time to the nearest lung transplant center was significantly associated with median income relative to the FPL (p<0.001), with an increased frequency of median income <200% FPL in the highest quartile of driving time (See online supplement, Table E2). Despite these findings among patients with Medicaid insurance, there is no evidence for a difference in the proportion of patients with <4 outpatient visits per year based on Medicaid insurance status (Table 2).
Table 2.
Characteristics of CF patients eligible for referral for lung transplant evaluation, by Medicaid insurance status (N = 1214)
| Any Medicaid (N = 587) | No Medicaid (N = 627) | ||||
|---|---|---|---|---|---|
|
| |||||
| Observed | N | Observed | N | P Value | |
| Age – mean (SD), years | 27.7 (7.9) | 587 | 33.4 (10.0) | 627 | <0.001 |
| Male gender | 349 (59.5%) | 587 | 375 (59.8%) | 627 | 0.900 |
| Race, white vs. non-white | 536 (91.5%) | 586 | 605 (96.5%) | 627 | <0.001 |
| FEV1 - % predicted (SD) | 24.2 (3.9) | 587 | 24.9 (3.5) | 627 | 0.001 |
| Acute exacerbations per year – N (SD) | 2.8 (2.6) | 587 | 2.1 (2.3) | 627 | <0.001 |
| Positive sputum culture: | |||||
| Pseudomonas aeruginosa | 477 (84.9%) | 562 | 509 (85.7%) | 594 | 0.696 |
| Burkholderia cepacia complex | 45 (8.0%) | 562 | 50 (8.4%) | 594 | 0.800 |
| Nontuberculous mycobacterium | 10 (1.8%) | 562 | 13 (2.2%) | 594 | 0.619 |
| Supplemental oxygen | 366 (62.6%) | 585 | 318 (51.0%) | 623 | <0.001 |
| BMI – kg/m2 (SD) | 18.9 (2.8) | 586 | 19.9 (3.3) | 624 | <0.001 |
| CF-related diabetes on insulin | 178 (30.3%) | 587 | 150 (23.9%) | 627 | 0.012 |
| CF-related liver cirrhosis | 24 (4.1%) | 587 | 23 (3.7%) | 627 | 0.704 |
| Renal failure requiring dialysis | 4 (0.7%) | 587 | 6 (1.0%) | 627 | 0.754a |
| Osteoporosis | 79 (13.5%) | 587 | 61 (9.7%) | 627 | 0.042 |
| Depression | 166 (28.3%) | 587 | 116 (18.5%) | 627 | <0.001 |
| Cancer, tissue proven | 2 (0.3%) | 587 | 3 (0.5%) | 627 | 1.00 a |
| <4 outpatient visits per year | 130 (22.2%) | 587 | 136 (21.7%) | 627 | 0.848 |
| No marital partner | 428 (74.7%) | 573 | 318 (52.3%) | 608 | <0.001 |
| High school graduate | 441 (85.0%) | 519 | 523 (96.0%) | 545 | <0.001 |
| Median income relative to FPL | 565 | 590 | <0.001 | ||
| >300% FPL | 92 (16.3%) | 214 (36.3%) | |||
| 250–300% FPL | 109 (19.3%) | 125 (21.2%) | |||
| 200–250% FPL | 169 (29.9%) | 141 (23.9%) | |||
| <200% FPL | 195 (34.5%) | 110 (18.6%) | |||
| Driving time: lung transplant center – min (SD) | 129.9 (111.7) | 573 | 97.1 (98.8) | 604 | <0.001 |
| <30 min (1st quartile) | 101 (17.6%) | 169 (28.0%) | |||
| 30–75 min (2nd quartile) | 117 (20.4%) | 168 (27.8%) | |||
| 75–150 min (3rd quartile) | 166 (29.0%) | 142 (23.5%) | |||
| >150 min (4th quartile) | 189 (33.0%) | 125 (20.7%) | |||
| Pre-LAS (prior to 2005) | 310 (52.8%) | 587 | 352 (56.1%) | 627 | 0.244 |
| Post-LAS (2005 or later) | 277 (47.2%) | 587 | 275 (43.9%) | 627 | 0.244 |
Data are presented as No. (%) unless indicated otherwise.
CF = cystic fibrosis, FEV1 = forced expiratory volume in 1 second, SD = standard deviation, BMI = body mass index, FPL = federal poverty level, min = minutes, LAS = lung allocation score
Fisher’s exact
In unadjusted analyses, predictors for non-referral included: Medicaid insurance, older age, male gender, positive Burkholderia cepacia complex sputum culture, <4 outpatient visits per year, no high school education, lower median household income, and farthest quartile (>150 minutes) of driving time to a lung transplant center (Table 3). Patients with increased frequency of exacerbations or use of supplemental oxygen were less likely to have non-referral.
Table 3.
Univariate analysis of predictors for non-referral for transplant evaluation
| Predictors | OR (95% CI) | P Value |
|---|---|---|
|
| ||
| Age (per 5 year increase) | 1.11 (1.04, 1.18) | 0.001 |
| Male gender | 1.33 (1.05, 1.69) | 0.020 |
| Acute exacerbations per year | 0.90 (0.85, 0.95) | <0.001 |
| Positive sputum culture: | ||
| Burkholderia cepacia complex | 1.89 (1.24, 2.88) | 0.003 |
| Supplemental oxygen | 0.60 (0.47, 0.76) | <0.001 |
| <4 outpatient visits per year | 1.96 (1.49, 2.57) | <0.001 |
| Not a high school graduate | 2.21 (1.47, 3.33) | <0.001 |
| Insurance status – Medicaid vs. no Medicaid | 1.46 (1.15, 1.85) | 0.002 |
| Median income relative to FPL | ||
| >300% FPL | REF | |
| 250–300% FPL | 1.11 (0.77, 1.59) | 0.590 |
| 200–250% FPL | 1.17 (0.84, 1.64) | 0.346 |
| <200% FPL | 1.57 (1.13, 2.18) | 0.008 |
| Driving time to lung transplant center | ||
| <30 min (1st quartile) | REF | |
| 30–75 min (2nd quartile) | 1.07 (0.75, 1.51) | 0.718 |
| 75–150 min (3rd quartile) | 1.10 (0.78, 1.55) | 0.577 |
| >150 min (4th quartile) | 1.44 (1.03, 2.02) | 0.033 |
OR = odds ratio; CI = confidence interval; FPL = federal poverty level; min = minutes
The following covariates had non-significant associations with referral status: Race (OR 1.00, p=0.994), FEV1 (OR 1.01, p=0.515), BMI (OR 0.98, p=0.389), CF-related diabetes on insulin (OR 0.87, p=0.299), NTM culture positive (OR 0.40, p=0.102), CF-related liver cirrhosis (OR 0.85, p=0.603), Renal failure requiring dialysis (OR 0.21, p=0.136), Osteoporosis (OR = 0.68, p=0.054), Cancer, tissue proven (OR 0.47, p=0.498), Depression (OR 1.23, p=0.127), No marital partner (OR 1.15, p=0.272), pre-LAS (OR 0.85, p=0.166)
A statistically significant association was present between patient insurance status (any Medicaid vs. no Medicaid) and non-referral for transplant evaluation after adjustment for demographics, disease severity, potential contraindications to referral, and driving time to the nearest lung transplant center (OR 1.79, 95% CI 1.29–2.47) (Table 4; see online supplement Table E3 for complete results of the model fitting procedures). Medicaid insurance, lack of high school graduation, older age (per 5 year increase) and Burkholderia cepacia complex sputum culture positivity were associated with non-referral for lung transplant evaluation. Increased number of pulmonary exacerbations and supplemental oxygen use were associated with decreased odds of non-referral (these patients were more likely to be referred). A sensitivity analysis that included smoking status and NPPV did not change the significant predictors of non-referral (See online supplement, Table E4).
Table 4.
Multivariate analysis - Insurance status as a predictor of non-referral for lung transplant evaluation, adjusted for all covariates listed in the table
| Models
| ||
|---|---|---|
| Primary SES Indicator | OR (95% CI) | P Value |
|
| ||
| Medicaid | 1.79 (1.29, 2.47) | <0.001 |
| No Medicaid | REF | |
| Demographics | ||
| Age (per 5 year increase) | 1.25 (1.13, 1.39) | <0.001 |
| Male gender | 1.19 (0.86, 1.65) | 0.291 |
| Race, white vs. non-white | 0.85 (0.44, 1.66) | 0.640 |
| Not a high school graduate | 2.27 (1.42, 3.64) | 0.001 |
| Disease Severity | ||
| FEV1 % predicted | 1.01 (0.97, 1.06) | 0.652 |
| # Acute Exacerbations | 0.93 (0.87, 0.99) | 0.031 |
| Supplemental oxygen | 0.59 (0.43, 0.81) | 0.001 |
| BMI | 0.95 (0.90, 1.00) | 0.067 |
| CF-related diabetes on insulin | 0.87 (0.62, 1.20) | 0.390 |
| Potential Contraindications | ||
| Positive sputum culture: | ||
| Burkholderia cepacia complex | 2.48 (1.50, 4.12) | <0.001 |
| Nontuberculous mycobacterium | 0.69 (0.18, 2.64) | 0.590 |
| CF-related liver cirrhosis | 0.80 (0.39, 1.64) | 0.538 |
| Renal failure requiring dialysis | 0.24 (0.04, 1.38) | 0.111 |
| Osteoporosis | 0.78 (0.51, 1.20) | 0.257 |
| Cancer, tissue proven | 1.02 (0.08, 13.82) | 0.987 |
| Depression | 1.41 (0.98, 2.03) | 0.065 |
| No marital partner | 1.36 (1.00, 1.87) | 0.053 |
| <4 outpatient visits per year | 1.33 (0.90, 1.95) | 0.152 |
| Driving time to Lung Transplant Center | ||
| <30 min (1st quartile) | REF | |
| 30–75 min (2nd quartile) | 0.96 (0.60, 1.54) | 0.878 |
| 75–150 min (3rd quartile) | 0.99 (0.65, 1.50) | 0.947 |
| >150 min (4th quartile) | 1.23 (0.81, 1.86) | 0.343 |
| Period of Lung Transplant | ||
| Pre-LAS (<2005) | 0.87 (0.65, 1.16) | 0.332 |
All models adjusted for cluster effects at the level of the CF care center
OR = odds ratio, SES = socioeconomic status, FEV1 = forced expiratory volume in 1 second, BMI = body mass index, CF = cystic fibrosis, min = minutes, LAS = lung allocation score
Discussion
The timing of referral of CF patients for lung transplant evaluation is part of the “art” of caring for patients with CF. The ISHLT guidelines are intended to help physicians decide when to refer patients for transplant evaluation. Our study of the predictors of non-referral for lung transplant evaluation for CF patients with advanced lung disease revealed that despite meeting lung function criteria for lung transplant evaluation, 35% of CF patients had not yet been referred to a transplant center. It is not surprising that the sicker patients (increased number of acute exacerbations, increased supplemental oxygen use) are being referred for lung transplant evaluation. Our findings imply one of three things: physicians are not following ISHLT guidelines that identify appropriate patients for referral, patients are not interested in referral despite their advanced disease, or the ISHLT guidelines are not identifying appropriate patients for referral (and the physicians are correct to wait to refer these patients).
Clinically significant predictors of non-referral in the multivariate model included: Medicaid insurance, older age, lack of high school graduate education, and Burkholderia cepacia complex sputum culture positivity. Given that older age continues to be a significant predictor of non-referral among patients with low lung function, it is possible that there is a sub-population with the phenotype of stable severe lung disease, and these patients could benefit from deferring referral for transplant evaluation. Burkholderia cenocepacia (genomovar III) sputum culture positivity is a known marker of worse prognosis regardless of transplant status and is a contraindication to LTx at most centers.(13–15) Other genomovars of Burkholderia cepacia complex have not been found to be associated with increased mortality and should not preclude referral for transplant evaluation.(16) The finding of a strong association between a positive Burkholderia cepacia complex culture and non-referral in our study highlights the possibility that some patients may be inappropriately denied referral, but this analysis does not provide further insight into this problem because genomovar subtypes were not available in the CFFPR until 2010.
An evaluation of the eligible cohort stratified by insurance status confirms findings from other studies of patients with Medicaid insurance suggesting Medicaid insurance status is associated with more severe disease (Table 2).(11, 12) The patients with markers of the lowest SES (median income <200% FPL, lack of high school education, Medicaid insurance, farthest driving time from transplant center) are less likely to be referred for lung transplant evaluation despite meeting current referral guidelines. Medicaid patients had more severe disease, but were also more likely to have depression and lack a marital partner, which may be markers of factors considered to be contraindications to LTx by the referring provider. Interestingly, Medicaid patients were not more likely to have <4 outpatient visits per year, highlighting that a lack of access to a doctor is not the primary reason for increased severity of disease and decreased rates of referral.
In summary, multivariate adjustment confirms the significant relationship between markers of low SES and non-referral for transplant evaluation. The odds of non-referral for the main covariate of interest (Medicaid insurance status) remained clinically and statistically significant after adjustment in the multivariate analysis. These findings highlight potential disparities in access to care at the time of referral for lung transplant evaluation, in addition to previous findings of disparities at the time of listing for transplant, among CF patients of lower SES.(11)
There are several limitations with the present investigation. First, our primary exposures of interest were proxy variables for SES, which may not fully capture the true individual SES. In addition, several other proxy variables were used in the analysis to evaluate social support (marriage status) and adherence (frequency of annual outpatient visits). Lack of social support as noted by having no marital partner may be a poor surrogate for social support in this population. The average age of patients with no marital partner in our eligible cohort was 27.6 years, while patients with a marital partner had an average age of 35.4 years (p <0.001). Having more severe illness may limit the ability to find a marital partner and lacking social support, if appropriately measured, may more strongly be associated with non-referral. Second, there was significant missing data for smoking status and NPPV; given the substantial missing data, multivariable imputation is inappropriate. In lung transplant medicine, smoking and NPPV use are important variables and it is a limitation that they have been excluded from the analyses. Third, the time period examined spans a time when a major change to the allocation of lungs occurred (LAS implemented in 2005). Our analysis did not reveal a difference in patterns of non-referral pre/post-2005 or a linear trend over time (2001–2008). Finally, despite our efforts to adjust for meaningful confounders, residual confounding could exist.
Conclusions
In conclusion, a significant number of CF patients with advanced lung disease were not referred for lung transplant evaluation despite meeting current referral guidelines. Predictors of non-referral included Medicaid insurance status, lack of high school graduate education, older age, and Burkholderia cepacia complex sputum culture positivity. Patients with supplemental oxygen requirement or more frequent pulmonary exacerbations were more likely to be referred for transplant evaluation. Further work is needed to describe the outcomes for non-referred patients in order to refine referral guidelines in this patient population.
Supplementary Material
Highlights.
35% of patients with advanced lung disease were not referred for lung transplant
Low socioeconomic status, older age, B. cepacia complex associated with non-referral
Disparities in access to referral for transplant may exist for CF patients in the US
Acknowledgments
The authors thank Dr. Bruce Marshall, the Cystic Fibrosis Foundation and the Cystic Fibrosis Foundation Patient Registry Committee for providing the data for this analysis. Each author’s contribution: KJR, BSQ and CHG conceived the study question and design. KJR performed the final data analyses, wrote the initial draft of the manuscript and coordinated revisions. BSQ participated in data acquisition from the CFFPR, performed initial data analyses, and edited the manuscript. KJP participated in the design of the study, performed driving time calculations, informed statistical decisions and edited the manuscript. EDL provided clinical lung transplant expertise, refined the study question, and edited the manuscript. NMH was involved in refinement of statistical methods, data interpretation, and edited the manuscript. MLA provided clinical cystic fibrosis expertise, refined the interpretation of the results, and edited the manuscript. CHG is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. All authors have approved of the final version of the submitted manuscript. Funding CHG: Cystic Fibrosis Foundation, the NIH (R01HL103965, R01 AI101307, P30 DK089507) and the FDA (R01 FD003704); KJR: T32 HL007287, University of Washington Pulmonary and Critical Care Medicine Research Training Grant
Abbreviations
- BMI
body mass index
- CF
Cystic fibrosis
- CFFPR
Cystic Fibrosis Foundation Patient Registry
- CI
confidence interval
- FEV1
forced expiratory volume in one second
- FPL
federal poverty level
- ISHLT
International Society for Heart and Lung Transplantation
- LAS
Lung Allocation Score
- LTx
Lung transplantation
- min
minutes
- NPPV
noninvasive positive pressure ventilation
- OR
odds ratio
- SD
standard deviation
- SE
standard error
- SES
socioeconomic status
- UNOS
United Network for Organ Sharing
- US
United States
Footnotes
Conflict of interest: The authors have no financial conflicts of interest
Abstract presentation: American Thoracic Society, May 20, 2015, Denver, CO
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cystic Fibrosis Foundation Patient Registry, 2012 Annual Data Report. Bethesda, Maryland: 2013. [Google Scholar]
- 2.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 3.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. The New England journal of medicine. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Lund LH, Meiser B, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: Thirty-first Adult Lung and Heart-Lung Transplant Report-2014; Focus Theme: Retransplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2014;33:1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, Martinez FJ, Nathan S, Palmer S, Patterson A, Singer L, Snell G, Studer S, Vachiery JL, Glanville AR. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2006;25:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG, Snell GI, Verleden GM, Zamora MR, Glanville AR. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. American journal of respiratory and critical care medicine. 2002;166:1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 8.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. The New England journal of medicine. 1992;326:1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 9.Liou TG, Adler FR, Cahill BC, FitzSimmons SC, Huang D, Hibbs JR, Marshall BC. Survival effect of lung transplantation among patients with cystic fibrosis. Jama. 2001;286:2683–2689. doi: 10.1001/jama.286.21.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer JR, Frost AE, Estenne M, Higenbottam T, Glanville AR. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 1998;17:703–709. [PubMed] [Google Scholar]
- 11.Quon BS, Psoter K, Mayer-Hamblett N, Aitken ML, Li CI, Goss CH. Disparities in access to lung transplantation for patients with cystic fibrosis by socioeconomic status. American journal of respiratory and critical care medicine. 2012;186:1008–1013. doi: 10.1164/rccm.201205-0949OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. American journal of respiratory and critical care medicine. 2001;163:1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 13.Chaparro C, Maurer J, Gutierrez C, Krajden M, Chan C, Winton T, Keshavjee S, Scavuzzo M, Tullis E, Hutcheon M, Kesten S. Infection with Burkholderia cepacia in cystic fibrosis: outcome following lung transplantation. American journal of respiratory and critical care medicine. 2001;163:43–48. doi: 10.1164/ajrccm.163.1.9811076. [DOI] [PubMed] [Google Scholar]
- 14.Fauroux B, Hart N, Belfar S, Boule M, Tillous-Borde I, Bonnet D, Bingen E, Clement A. Burkholderia cepacia is associated with pulmonary hypertension and increased mortality among cystic fibrosis patients. Journal of clinical microbiology. 2004;42:5537–5541. doi: 10.1128/JCM.42.12.5537-5541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephenson AL, Sykes J, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, Stanojevic S. Clinical and Demographic Factors Associated with Post-lung Transplantation Survival in Individuals with Cystic Fibrosis. The Journal of Heart and Lung Transplantation. doi: 10.1016/j.healun.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Aris RM, Routh JC, LiPuma JJ, Heath DG, Gilligan PH. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. American journal of respiratory and critical care medicine. 2001;164:2102–2106. doi: 10.1164/ajrccm.164.11.2107022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.