Abstract
Objectives
Autoantibodies are used clinically to phenotype and subset patients with autoimmune rheumatic diseases. We detected a novel 60 kDa autoantibody specificity by immunoblotting using a dermatomyositis patient’s serum. Our objective was to identify the targeted autoantigen and to evaluate disease specificity and clinical significance of this new autoantibody.
Methods
A new 60 kDa specificity was detected by immunoblotting HeLa cell lysates. The targeted autoantigen was identified as poly(U)-binding-splicing factor (PUF60) using (i) a human protein array and (ii) 2D gel electrophoresis and LC/MS/MS peptide sequencing. Anti-PUF60 antibodies were assayed by ELISA using sera from patients with primary Sjögren’s syndrome (SS; n=84), systemic lupus erythematosus (SLE; n=71), dermatomyositis (DM; n=267), polymyositis (n=45), inclusion body myositis (n=45) and healthy controls (n=38).
Results
PUF60 was identified as a new autoantigen. Anti-PUF60 antibodies were present in 25/84 (30%) SS patients, 6/71 (8.5%) SLE patients, and 2/38 (5.0%) control subjects (SS vs. controls, p=0.002; SLE vs. controls, p=0.711). Anti-PUF60 antibodies were present in 48/267 (18.0%) DM patients versus 4/45 (8.9%) and 5/45 (11.1%) patients with inclusion body myositis and polymyositis, respectively. The antibody was significantly associated with anti-Ro52 antibodies, rheumatoid factor, and hyperglobulinemia in the primary SS patients. In DM patients, the antibody was associated with anti-transcription intermediary factor 1 gamma (TIF-1γ) seropositivity and Caucasian race.
Conclusions
PUF60 represents a novel autoantigen in SS and DM patients. PUF60 antibodies are associated with distinct clinical features and different immune responses in different diseases.
Keywords: poly-U binding splicing factor 60KDa, dermatomyositis, Sjögren’s Syndrome, autoantibodies
INTRODUCTION
Autoimmune rheumatic diseases have highly variable and overlapping clinical presentations. Autoantibodies are useful because they can identify distinct clinical subsets with unique patterns of organ involvement, natural history and potentially, response to therapy[1–3]. This is well-illustrated in dermatomyositis (DM), a chronic inflammatory disease affecting the skin, muscle and lungs, where recent investigations have identified several autoantibody specificities, each associated with distinct clinical features[3, 4]. Targets of these DM-specific autoantibodies include melanoma differentiation–associated protein-5 (MDA5)[5, 6], transcription intermediary factor 1 gamma (TIF-1γ) [7, 8], sumoyl-activating enzyme subunit 1/2 (SAE 1)[9] and nuclear matrix protein NXP-2[10].
Sjögren’s Syndrome (SS) is a systemic autoimmune disease characterized by lymphocytic infiltration of salivary and lacrimal glands, diverse extraglandular manifestations affecting the lungs, kidneys, liver and nervous system, and circulating autoantibodies. Antibodies against Ro52 and La/SSB are present in 60–90% and 30–60% of SS patients[11], respectively and are associated with more severe glandular and extraglandular manifestations[12]. Other antibodies associated with specific rheumatic diseases are found in SS, albeit rarely; in these cases, patients may have clinical features that overlap with the other disease-associated antibodies[1]. Interestingly, for those antigens targeted in multiple autoimmune diseases, clinical associations often vary by disease[13, 14], likely reflecting the distinct immune response microenvironments.
Although autoantibodies have been useful in defining clinical phenotypes in rheumatic diseases, considerable heterogeneity exists within a given antibody subgroup. For example, although anti-TIF-1γ antibodies are associated with internal malignancy in DM, only a proportion of patients with these antibodies are diagnosed with cancer[15, 16]. It is likely that other factors shape the clinical disease, including genetics, environmental exposures, and, possibly, the emergence of additional antigen-specific immune responses[17, 18].
In addition to their clinical utility, autoantibodies can provide important clues to disease pathogenesis. This is well illustrated by recent studies examining the relationship between systemic sclerosis and cancer. Shah et al [17] noted the close temporal relationship between cancer onset and systemic sclerosis in patients with antibodies against RNA polymerase III (POLR3A). Building on this knowledge, Joseph et al [18] subsequently showed that cancers are associated with systemic sclerosis because they trigger POLR3A-specific immune responses by harboring mutations in the POLR3A gene.
For these reasons, identification of novel, disease-specific antigens remains a high priority. Our objective was to characterize the target of a novel autoantibody present in both SS and DM and to evaluate its disease specificity and clinical significance.
METHODS
Patient cohorts
Both the Johns Hopkins University and Stanford University Institutional Review Boards approved the collection of clinical data, serum and other biospecimens from patients for these studies. All patients were >18 years old and gave informed consent. Patients with primary Sjögren’s syndrome (n=84) were seen in the Johns Hopkins Jerome L. Greene Sjögren’s Syndrome Center. SS was diagnosed with the American-European consensus group criteria[19]. The DM cohort consisted of 165 patients from Stanford and 102 patients from the Johns Hopkins Myositis Center. All patients had a diagnosis of probable or definite DM based on the criteria of Bohan and Peter[20], or, for clinically amyopathic patients, based on characteristic skin findings[21]. The inclusion body myositis (IBM) (n=45) and polymyositis (PM) (n=45) cohorts consisted of patients evaluated at the Johns Hopkins Myositis Center. Subjects were diagnosed with IBM according to Data Derived Criteria[22] and/or “probable” or “definite” European Neuro Musuclar Center Criteria[23]. The PM subjects were diagnosed with “probable” or “definite” PM based on the criteria of Bohan and Peter[20, 24]. Serum samples were obtained from 71 patients with SLE from the Johns Hopkins Lupus Cohort. Clinical diagnosis of SLE was made by a member of the Rheumatology Division; 94% of the patients satisfied at least 4 of the 1982 American College of Rheumatology revised criteria for the classification of SLE[25, 26]. Sera from 38 healthy laboratory personnel were used as controls. These were confirmed negative for classic myositis autoantibodies in previous studies[15, 27–29].
Patient Tissue
Salivary gland, skin and muscle biopsies from SS and DM patients and healthy controls were collected for immunohistochemistry and lysate generation (detailed in online supplementary text).
PUF60 ELISA assay
Recombinant full-length human PUF60 (Origene, Rockville, MD) was first validated by immunoblotting with a polyclonal anti-PUF60 (Novus, Fig 1B). 96-well ELISA plates were coated overnight at 4°C with 50 ng/well of PUF60. Plates were washed and developed as described in online supplementary text. An arbitrary positive serum (serum #7012, 1:400 dilution with an OD in the linear range) was included as a reference in every ELISA; all absorbances were calibrated relative to this.
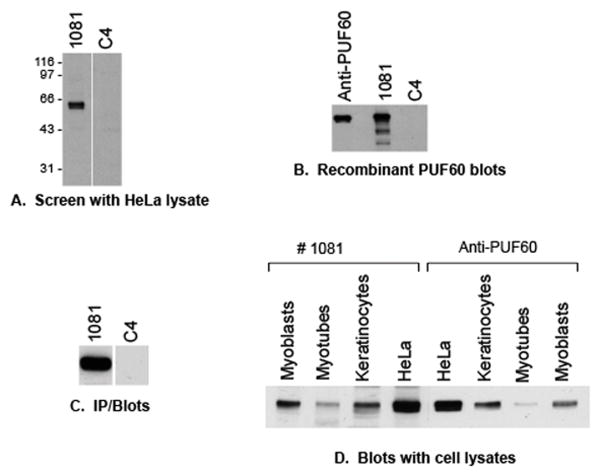
Figure 1. Identification of a new 60 kDa autoantigen, and confirmation that it is PUF60.
(A) HeLa lysates were immunoblotted with serum from a DM patient (#1081), and a control serum (C4). An unidentified 60 kDa band was blotted with serum #1081. (B) Recombinant PUF60 (30 ng/lane) was immunoblotted with the indicated antibodies and patient sera. The commercial anti-PUF60 antibody, as well as serum 1081 blotted PUF60, but not the control serum. (C) HeLa lysates were immunoprecipitated with serum 1081 or a control serum, then immunoblotted with an anti-PUF60 rabbit antibody. Endogenous PUF60 was immunoprecipitated only by serum #1081. (D) Equal protein amounts of various cell lysates were immunoblotted with the prototype serum (#1081) and the rabbit anti-PUF60 antibody. The blotted bands co-migrated, and the expression levels in the various lysates were the same.
Protein array
The prototype serum (#1081) was profiled using a human protein array (~18,000 human GST-HisX6 tagged proteins). Arrays were blocked, then probed with patient serum and rabbit anti-GST. After incubating with appropriate secondary antibodies, arrays were imaged and signal intensity was calculated (detailed in online supplementary text).
Proteomic identification
HeLa lysates were run on two-dimensional gels. The protein spot of interest was plucked and subjected to liquid chromatography tandem mass spectrometry (LC/MS/MS) peptide sequencing (detailed in online supplementary text).
Statistical Analysis
Wilcoxon rank-sum test was used to compare continuous variables and two-sided Fisher exact test was used to compare categorical variables. P values less than 0.05 were considered statistically significant. Analyses were conducted using SAS (Version 9.3, SAS Institute Inc, Cary, NC).
Please see online supplementary text for additional cohort details, and other methods
RESULTS
Identification of PUF60 as a new autoantigen
In routine screens performed by immunoblotting HeLa lysates with DM sera (1:5,000 dilution), we noted that serum #1081 blotted an ~60 kDa specificity (Fig 1A). Since this sample did not have Ro52/60 antibodies, and since this was a specificity we had noticed in several other DM samples routinely screened by both immunoblotting and immunoprecipitation, we sought to identify this new specificity. Two approaches were performed simultaneously, which, along with the known molecular weight, allowed us to identify the targeted protein. We first interrogated a human protein array and found that the PUF60 protein (poly(U)-binding splicing factor 60 kDa) was one of the top ranked proteins on the list of potential autoantigens. A healthy control serum was run in parallel and PUF60 was not represented as an autoantigen. In a second approach, we performed a proteomic analysis by selecting the spot corresponding to the unidentified 60 kDa protein from a 2-dimensional gel run of HeLa lysates. Amongst the peptides identified by mass spectrometric sequencing, only PUF60 was also present in the protein array dataset. We therefore prioritized PUF60 as the candidate autoantigen.
We confirmed that the 60 kDa protein is indeed PUF60 in several ways. Immunoblots were performed using recombinant human PUF60 as the source material. This was detected robustly by both a rabbit anti-PUF60 antibody and by serum #1081, but not by a control human serum (Fig 1B). We also immunoblotted a panel of cultured cell lysates with serum #1081 and the commercial anti-PUF60 antibody. The 60 kD bands blotted by both antibodies comigrated, and the expression patterns were identical (Fig 1D). In a third approach, unlabeled HeLa lysates were immunoprecipitated with the prototype serum or a control serum, followed by detection by immunoblotting with the polyclonal anti-PUF60 antibody. PUF60 was detected only in the immunoprecipitation performed with serum #1081 (Fig 1C). Together, these assays confirm that the 60 kDa autoantigen detected by serum #1081 is PUF60.
Anti-PUF60 antibodies are commonly found in DM and SS patients
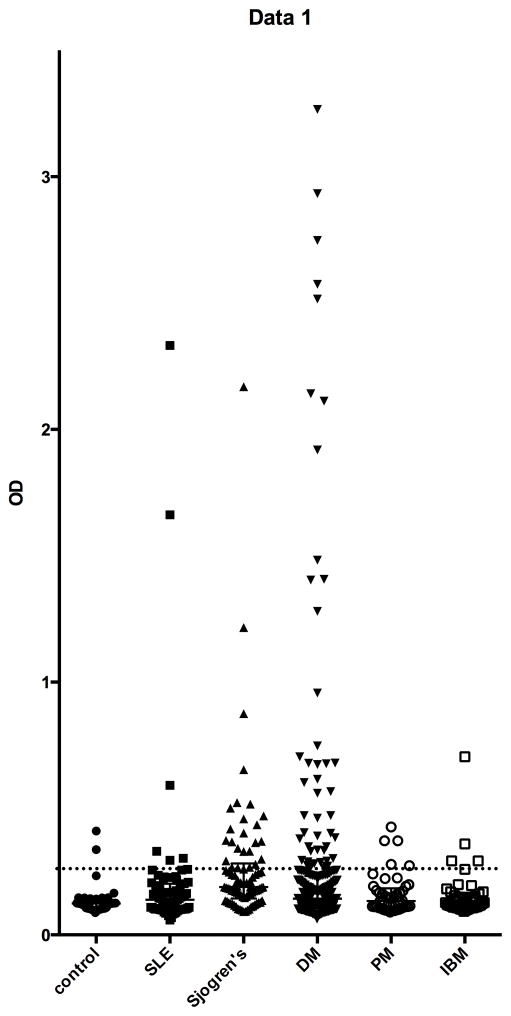
We next determined the prevalence of anti-PUF60 antibodies in patients with DM as well as other rheumatic diseases and healthy controls. For this, we developed and optimized an ELISA assay. Sera were assigned a positive antibody status if the relative absorbance value was >2 SD higher than the mean value in 38 healthy controls. The levels of anti-PUF60 antibodies in the patient cohorts and control group are shown in Figure 2. The median OD values for both DM and SS patients (and no other disease groups) were significantly higher than healthy controls (p=0.0169 and <0.0001, respectively). Anti-PUF60 antibodies were present in 25/84 (29.8%) SS patients, 48/267 (18%) DM patients, 6/71 (8.5%) SLE patients, 4/45 (8.9%) of IBM patients, 5/45 (11.1%) of PM patients and in 2/38 (5.3%) of controls. The prevalence of anti-PUF60 antibodies in SS patients was significantly greater than healthy controls (p=0.002). When compared to healthy controls, DM patients, but not other myositis patients, tended to have a higher prevalence of anti-PUF60 antibodies (p=0.059, 0.68 and 0.45 for DM, IBM, and PM, respectively). Furthermore, there was a large number of higher titer (OD>0.5) anti-PUF60 antibodies in DM (23/267) compared to IBM (1/45) or PM (0/45) (Figure 2). Compared to DM, most of the anti-PUF-60 positive SS patients had relatively low titers; only 5/25 (20%) of SS patients had OD values >4 SD above the mean value of the controls, compared to 23/48 (48%) for DM patients (p=0.024).
Figure 2. Antibodies against PUF60 are detected in patients with SS, but not in healthy controls.
Antibodies against PUF60 were assayed by ELISA in sera from patients with dermatomyositis (DM, n = 267), primary Sjögren’s syndrome (SS, n = 86), SLE (n = 71), polymyositis (PM, n = 45), inclusion body myositis (IBM, n = 45) and healthy individuals (n = 38), as described in the Methods and online supplementary text. Each symbol represents the PUF60 antibody level in a single patient serum and the dotted line marks the cutoff for assignment of a positive score. Antibodies were detected in 25/82 (30%) SS patients, 48/267 (18.0%) DM patients, 6/71 (8.5%) SLE patients, 4/45 (8.9%) IBM patients, 5/45 (11.1%) PM patients, and 2/38 (5.0%) control subjects 38/133 (29%) of SS patients, and 1/47 (2.1%) of controls.
Characterization of Sjögren’s syndrome patients with anti-PUF60 antibodies
We compared characteristics of SS patients with and without anti-PUF60 antibodies (Table 1). Anti-PUF60 positive patients were significantly more likely to be of either Asian or African descent. In addition, patients with anti-PUF60 antibodies had a higher prevalence of ANA positivity (≥1:320), rheumatoid factor, hypergammaglobulinemia, and anti-thyroid antibodies. Anti-PUF60 antibodies were highly associated with anti-Ro52, anti-Ro60, and anti-SSB/La antibodies—every anti-PUF60 positive patient also had antibodies to Ro52 (Table 1).
Table 1.
Comparison of phenotypic features of primary SS patients with and without anti-PUF60 antibodies
| Phenotypic feature | PUF60 positive (n=25) | PUF60 negative (n=59) | P value* |
|---|---|---|---|
| Age, median (range) | 55 (30–78) | 57 (20–82) | 0.55 |
| Female | 24/25 (96) | 51/59 (86) | 0.1953 |
| Race | 0.01 | ||
| Caucasian | 17/25 (68) | 55/59 (93) | |
| Asian | 3/25 (12) | 1/59 (2) | |
| African-American | 4/25 (16) | 2/59 (3) | |
| Positive Schirmer (<5 mm/5 min) | 12/19 (63) | 37/44 (84) | 0.0742 |
| Positive lip biopsy | 8/8 (100) | 25/36 (69) | 0.1702 |
| WBC<4000/μl | 6/25 (24) | 8/58 (14) | 0.2546 |
| C3<90 mg/dl | 4/25 (16) | 3/56 (5) | 0.1941 |
| C4<16 mg/dl | 3/24 (13) | 10/56 (18) | 0.7445 |
| ANA ≥1:320 | 22/25 (88) | 28/57 (49) | 0.0011 |
| Monoclonal protein | 2/25 (8) | 8/54 (15) | 0.3818 |
| Rheumatoid factor positive | 23/25 (92) | 10/56 (18) | <.0001 |
| IgG>1445 mg/dl | 23/25 (92) | 18/53 (34) | <.0001 |
| Anti-SSB/La | 16/25 (64) | 18/59 (31) | 0.0042 |
| Anti-Ro52 | 25/25 (100) | 40/59 (68) | 0.0005 |
| Anti-Ro60 | 22/25 (88) | 40/59 (68) | 0.0623 |
| Anti-Ro52 and/or Ro60 | 25/25 (100) | 48/59 (81) | 0.0289 |
| Anti-thyroid antibodies | 2/20 (10) | 13/29 (45) | 0.0121 |
Pearson chi-square unless cell frequency ≤5, in which case Fisher’s exact test used
Continuous variable (age) tested with Wilcoxon rank sum.
Characterization of DM patients with anti-PUF60 antibodies
In DM patients, PUF60 antibody status was not associated with age of disease onset, gender, or cancer risk (Table 2), Unlike SS patients, anti-PUF60 antibodies were absent in Asian patients and more common in Caucasians (p=0.0055) and were not associated with anti-Ro52 or anti-Ro60 antibodies (Table 2; data not shown). Seventy-one percent of patients with anti-PUF60 antibodies also had anti-TIF-1γ antibodies (the latter is a DM-specific antibody associated with cancer, low ILD risk and certain cutaneous features[8, 30]). This frequency was significantly higher than the anti-TIF-1γ prevalence in the anti-PUF60 negative group (p<0.0001, Table 2). Interestingly, PUF60 antibodies were rarely found in DM patients with other DM-associated serotypes (see online supplementary Table S1). Using more detailed data available for the Stanford cohort, we found that PUF60 antibodies were not associated with dysphagia, Raynaud’s, arthralgia/arthritis, peak CK values, or any characteristic cutaneous DM manifestation (see online supplementary Table S1; not shown).
Table 2.
Phenotypic comparison of DM patients with and without anti-PUF-60 antibodies*
| Phenotypic feature | PUF60 positive (n=48) | PUF60 negative (n=219) | P valueχ |
|---|---|---|---|
| Female | 39 (81) | 158 (72) | 0.21 |
| Mean age at diagnosis μ | 49 (14) | 47 (16) | 0.41 |
| Race | 0.04 | ||
| Caucasian | 45 (94) | 168 (77) | |
| Asian | 0 | 22 (10) | |
| African-American | 3 (6) | 16 (7) | |
| Pacific Islander | 0 | 10 (5) | |
| Internal malignancy | 8 (17) | 30 (14) | 0.65 |
| ILD | 2 (4) | 36 (16) | 0.036 |
| Anti-TIFγ antibodies | 34 (71) | 78 (36) | <0.0001 |
| Anti-Ro52 antibodies | 14 (29) | 51 (23) | 0.39 |
Values are the number (%) of patients, denominators vary due to missing data
Value reported as mean. Student’s t-test used to calculate p-value
Pearson chi-square unless cell frequency ≤5, in which case Fisher’s exact test used
PUF60 expression in disease target tissues
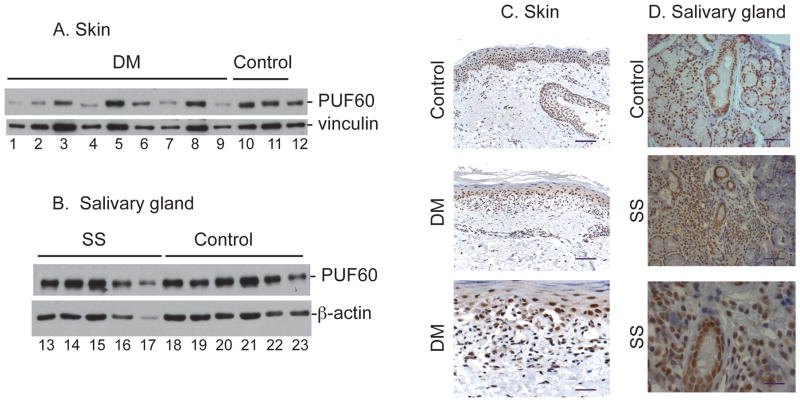
We quantitated PUF60 protein expression in lysates made from frozen minor salivary glands, skin and muscle (target tissues affected by SS and DM) by immunoblotting, and visualized PUF60 in these tissues by immunohistochemical staining of paraffin sections. Robust PUF60 levels were detected in tissue from SS patients and controls by immunoblotting (Fig 3B); quantitation confirmed that levels were similar (p=0.25). Immunohistochemical staining was consistent with this, and showed extensive nuclear staining throughout both SS and control salivary glands in acinar cells, ductal cells and infiltrating leukocytes (Fig 3D). Similarly, PUF60 levels in affected skin of DM patients did not differ significantly from that of healthy controls by immunoblotting, although there was a trend for lower expression in DM patients (p=0.07, Fig 3A). Immunohistochemical staining of skin from DM patients revealed a nuclear PUF60 pattern in most keratinocytes and endothelial cells, and in a significant proportion of infiltrating lymphocytes and mononuclear cells. This pattern and intensity did not differ from that seen in skin from healthy controls (Fig. 3C).
Figure 3. PUF60 expression in skin and salivary gland.
A & B: Immunoblots performed on lysates made from frozen tissues. Equal protein amounts of lysates made from skin biopsies (dermatomyositis and control subjects, panel A lanes 1–12) and salivary glands (SS and control subjects, panel B lanes 13–23) were immunoblotted with a polyclonal anti-PUF60 antibody. Vinculin (skin) and -actin (salivary gland) were included as loading controls. C & D: Immunohistochemical PUF60 staining. Skin (Panel C, control and dermatomyositis) and salivary gland (Panel D, control and SS) paraffin sections were stained with a rabbit anti-PUF60 antibody. Representative images are shown. In Panels C & D, the scale bar represents 50 μM in the 2 upper images, and 20 μM in the lowest images. No staining was detected when controls were performed by incubating serial sections with rabbit IgG instead of the rabbit anti-PUF60 polyclonal antibody (data not shown).
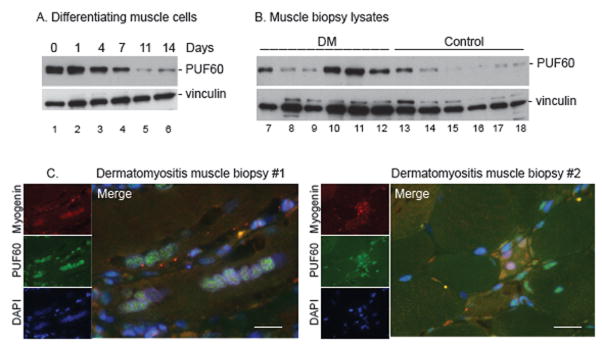
Our initial data suggested that PUF60 was expressed at higher levels in myoblasts compared to myotubes (Fig.1D). This is consistent with our earlier observation that other myositis autoantigens are highly expressed in undifferentiated muscle cells relative to their differentiated counterparts[31]. We tested this by quantitating PUF60 expression in a tissue culture model of differentiation in which myoblasts differentiate into myotubes. PUF60 expression was high in myoblasts, but decreased significantly during differentiation (Fig. 4A). When we immunoblotted muscle biopsy lysates, we found that PUF60 expression was higher in DM compared to control muscle (p=0.04, Fig 4B). We therefore hypothesized that the higher PUF60 levels in DM muscle could be due to regenerating myofibers (“myoblasts”) which are present in diseased muscle and express high PUF60 levels. To test this, we evaluated expression of PUF60 in DM muscle by immunohistochemistry, and co-stained with myogenin, a marker of regenerating muscle. Interestingly, PUF60 was expressed at low levels in myogenin negative muscle cells but at high levels in most myogenin-positive cells (Fig. 4C).
Figure 4. PUF60 expression is higher in cultured myoblasts, and in dermatomyositis muscle compared to control muscle.
(A) Equal protein amounts of lysates made from myoblasts (lane 1) harvested at various stages of in vitro differentiation to myotubes (lanes 2–6) were immunoblotted with an anti-PUF60 antibody. Vinculin was included as a loading control. (B) Equal protein amounts of muscle biopsy lysates made from dermatomyositis muscle (lanes 7–12) or control muscle (lanes 13–18) were immunoblotted as above. (C) Muscle paraffin sections from subjects with dermatomyositis were stained with a rabbit polyclonal antibody against PUF60 (green) and a mouse monoclonal antibody against myogenin (red). DNA was visualized by staining with DAPI (blue). Merged images are shown in the large panels; many of the regenerating muscle cells (with myogenin positive nuclei) express high PUF60 levels in the nuclei. The scale bar represents 20 μM. No specific staining was detected when serial muscle sections were stained with rabbit and mouse IgG (instead of the PUF60/myogenin antibodies above) and photographed using the same camera settings (data not shown). Minimal/no myogenin and PUF60 staining was detected when control muscle sections were similarly stained and photographed with the same camera settings (not shown).
DISCUSSION
In these studies, we report on the discovery of a novel autoantibody specificity in DM and primary SS patients, and have identified the target as PUF60. We characterized the target using a combination of human protein array profiling and mass spectrometry, and its identity was confirmed using recombinant PUF60 and a polyclonal PUF60 antibody. This specificity is relatively common in primary SS and DM patients (29% and 18%, respectively). However, several SLE, PM, and IBM patients also had anti-PUF60 antibodies, whose significance at present is unclear—anti-PUF60 is not simply a marker of myositis, however, as none of the SLE or SS patients had evidence of myositis (not shown). In addition, we wondered if high titer (e.g. OD>1) PUF60 antibodies had distinct significance (regardless of diagnosis), but we were unable to detect any clinical or laboratory associations in these patients (not shown).
PUF60 was originally characterized as a polyU-binding factor required for optimal RNA splicing in either polyU-adsorbed extracts or under other suboptimal conditions for in vitro splicing[32, 33]. PUF60 has subsequently been shown to be involved in various processes, including RNA splicing, transcription, and possibly modulating the location or function of non-coding RNA such as hYRNA[37]. This is interesting because many of the other well-defined, prominent targets of the autoimmune response bind nucleic acid and function in similar pathways[38]. Additionally, PUF60 may have relevance in cancer, as multiple cancers harbor amplification of the PUF60 locus and express elevated levels of PUF60 protein[39, 40].
PUF60 interacts with several proteins, many of them known autoantigens; for example, it interacts with Ro60 in biologic and biochemical assays[33, 35]. In its role at the splicing branch site, PUF60 is part of the large U2 snRNP complex, consisting of many splicing factors and U2RNAs, including U170K and La/SSB. Protein-protein interactions may explain the frequent targeting of these antigens due to epitope spreading. Interestingly, aside from 2 weak positives, we did not detect U170K antibodies when we tested the 32 sera with the highest levels of PUF60 antibodies (not shown).
In SS, there is a striking association between anti-PUF60 antibodies and antibodies against Ro52, Ro60 and La. By contrast, in DM PUF60 antibodies are largely found in patients with anti-TIF-1γ antibodies but not with other well-characterized DM-associated antibodies, including Ro52. Thus, anti-PUF60 immune responses are associated with specific and distinct autoantibodies in DM and SS. There is some precedence for this phenomenon for anti-Ro52 antibodies, as they are commonly associated with anti-Ro60 antibodies in SLE and SS but not in myositis or systemic sclerosis[42]. One explanation for our data is that PUF60 is found in different immunogenic complexes depending on the disease and/or tissue. Co-targeting of PUF60 and Ro60 (and La) could be explained by their biochemical association, but there is currently no evidence that TIF-1γ is a binding partner for PUF60. Investigating these interactions in appropriate diseased tissue may yield new and important insights[43]. It is noteworthy that when we immunodepleted PUF60 antibodies from sera that also contained antibodies against TIF-1γ, Ro52 or Ro60, the other reactivities remained unchanged, indicating that these associations are not due to antibody cross-reactivity (see online supplementary text and supplementary Figure S1).
Interestingly, antibodies to PUF60 are associated with different clinical features, depending on the disease. In SS, anti-PUF60 antibodies are associated with Asian and African-American race, hypergammaglobulinemia and rheumatoid factor—some (but not all) of these associations may be a consequence of association with anti-Ro52, although a paucity of anti-PUF60 SS patients not harboring antibodies to Ro-52 precluded further analysis. In DM, however, anti-PUF60 antibodies are largely absent in Asians and Pacific Islanders. This racial association could possibly be explained by HLA polymorphisms or other genetic factors. This trend, as well as the apparent inverse association of anti-PUF60 antibodies with ILD, may simply result from the high prevalence of anti-TIF-1γ antibodies in the anti-PUF60 population, as these features are associated with the latter serotype. Due to insufficient power, we were unable to discern a particular phenotype other than that conferred by anti-TIF-1γ antibodies. We note that these results are preliminary and require confirmation in other DM cohorts. It is likely that differences in the cellular and biochemical context associated with the anti-PUF60 immune response explain its unique clinical and autoantibody associations in a given autoimmune disease. We have identified PUF60 as a novel, commonly targeted autoantigen in SS and DM. Understanding the basis for its differential serologic and phenotypic associations in these two diseases will allow greater understanding of mechanisms that trigger and propagate autoimmunity.
Supplementary Material
Acknowledgments
These studies were supported by NIH grants R56A062615 (LCR, DF), R01-AR-44684 (LCR), R01 DE12354-15A1 (AR) and RO1-AR 43727 (MP). Drs. Baer and Casciola-Rosen’s work was additionally supported by the Jerome L. Greene Foundation and the Dorothy and Donald Stabler Foundation. Dr. Mammen’s work was supported [in part] by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Dr. Christopher-Stine’s work and The Johns Hopkins Myositis Cohort is supported by the The Huayi and Siuling Zhang Discovery Fund. The Johns Hopkins Rheumatic Disease Research Core Center, where the assays were performed, is supported by the NIH (grant P30-AR-053503). The authors thank the Johns Hopkins Mass Spectrometry and Proteomics Core Facility for performing the proteomic identification.
Footnotes
The authors have no relevant conflicts of interest to declare.
The data for this study were part of several research investigations that were approved by the IRB of both Stanford (protocols 12047 and 9357) and Johns Hopkins (protocols NA_00007454 and NA_00013201). All participants gave Informed Consent before taking part in the study.
AUTHOR CONTRIBUTORSHIP
Study planning: LCR, DFF, ANB
Data collection and/or presentation: LCR, DFF, MP, ANB, LCS, MP, KR
Manuscript drafting: DFF, LCR, ANB
Critical manuscript review: DFF, LCR, ANB, LCS, MP, KR, AR, AM
COMPETING INTERESTS
The authors do not have any competing interests to disclose.
References
- 1.Kyriakidis NC, Kapsogeorgou EK, Tzioufas AG. A comprehensive review of autoantibodies in primary Sjogren’s syndrome: clinical phenotypes and regulatory mechanisms. J Autoimmun. 2014;51:67–74. doi: 10.1016/j.jaut.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Mehra S, Walker J, Patterson K, et al. Autoantibodies in systemic sclerosis. Autoimmun Rev. 2013;12(3):340–54. doi: 10.1016/j.autrev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Casciola-Rosen L, Mammen AL. Myositis autoantibodies. Curr Opin Rheumatol. 2012;24(6):602–8. doi: 10.1097/BOR.0b013e328358bd85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rider LG, Shah M, Mamyrova G, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 2013;92(4):223–43. doi: 10.1097/MD.0b013e31829d08f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakashima R, Imura Y, Kobayashi S, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology. 2010;49(3):433–40. doi: 10.1093/rheumatology/kep375. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Kuwana M, Fujita T, et al. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Modern rheumatology/the Japan Rheumatism Association. 2012 doi: 10.1007/s10165-012-0663-4. [DOI] [PubMed] [Google Scholar]
- 7.Targoff I, Trieu E, Levy-Neto M, et al. Autoantibodies to transcriptional intermediary factor 1-gamma (TIF1-g) in dermatomyositis [abstract] Arthritis Rheum. 2006;54:S518. [Google Scholar]
- 8.Fujimoto M, Hamaguchi Y, Kaji K, et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 2012;64(2):513–22. doi: 10.1002/art.33403. [DOI] [PubMed] [Google Scholar]
- 9.Betteridge Z, Gunawardena H, North J, et al. Identification of a novel autoantibody directed against small ubiquitin-like modifier activating enzyme in dermatomyositis. Arthritis Rheum. 2007;56(9):3132–7. doi: 10.1002/art.22862. [DOI] [PubMed] [Google Scholar]
- 10.Gunawardena H, Wedderburn LR, Chinoy H, et al. Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum. 2009;60(6):1807–14. doi: 10.1002/art.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzioufas AG, Tatouli IP, Moutsopoulos HM. Autoantibodies in Sjogren’s syndrome: clinical presentation and regulatory mechanisms. Presse Med. 2012;41(9 Pt 2):e451–60. doi: 10.1016/j.lpm.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Molina G, Leal-Alegre G, Michel-Peregrina M. The meaning of anti-Ro and anti-La antibodies in primary Sjogren’s syndrome. Autoimmun Rev. 2011;10(3):123–5. doi: 10.1016/j.autrev.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Reed JH, Dudek NL, Osborne SE, et al. Reactivity with dichotomous determinants of Ro 60 stratifies autoantibody responses in lupus and primary Sjogren’s syndrome. Arthritis Rheum. 2010;62(5):1448–56. doi: 10.1002/art.27370. [DOI] [PubMed] [Google Scholar]
- 14.Defendenti C, Atzeni F, Spina MF, et al. Clinical and laboratory aspects of Ro/SSA-52 autoantibodies. Autoimmun Rev. 2011;10(3):150–4. doi: 10.1016/j.autrev.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Fiorentino DF, Chung LS, Christopher-Stine L, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 2013;65(11):2954–62. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trallero-Araguas E, Rodrigo-Pendas JA, Selva-O’Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64(2):523–32. doi: 10.1002/art.33379. [DOI] [PubMed] [Google Scholar]
- 17.Shah AA, Rosen A, Hummers L, et al. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum. 2010;62(9):2787–95. doi: 10.1002/art.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph CG, Darrah E, Shah AA, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 2014;343(6167):152–7. doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 21.Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. 2002;20(3):387–408. doi: 10.1016/s0733-8635(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd TE, Mammen AL, Amato AA, et al. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. 2014;83(5):426–33. doi: 10.1212/WNL.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady S, Squier W, Hilton-Jones D. Clinical assessment determines the diagnosis of inclusion body myositis independently of pathological features. J Neurol Neurosurg Psychiatry. 2013;84(11):1240–6. doi: 10.1136/jnnp-2013-305690. [DOI] [PubMed] [Google Scholar]
- 24.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292(8):403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 25.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 27.Hall JC, Casciola-Rosen L, Samedy LA, et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res (Hoboken) 2013;65(8):1307–15. doi: 10.1002/acr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd TE, Christopher-Stine L, Pinal-Fernandez I, et al. Cytosolic 5′-nucleotidase 1A is a common target of circulating autoantibodies in several autoimmune diseases. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiorentino DF, Kuo K, Chung L, et al. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1gamma antibodies in adults with dermatomyositis. J Am Acad Dermatol. 2015 doi: 10.1016/j.jaad.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casciola-Rosen L, Nagaraju K, Plotz P, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005;201(4):591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page-McCaw PS, Amonlirdviman K, Sharp PA. PUF60: a novel U2AF65-related splicing activity. RNA. 1999;5(12):1548–60. doi: 10.1017/s1355838299991938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hastings ML, Allemand E, Duelli DM, et al. Control of pre-mRNA splicing by the general splicing factors PUF60 and U2AF(65) PLoS ONE. 2007;2(6):e538. doi: 10.1371/journal.pone.0000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, He L, Collins I, et al. The FBP interacting repressor targets TFIIH to inhibit activated transcription. Mol Cell. 2000;5(2):331–41. doi: 10.1016/s1097-2765(00)80428-1. [DOI] [PubMed] [Google Scholar]
- 35.Bouffard P, Briere F, Wellinger RJ, et al. Identification of ribonucleoprotein (RNP)-specific protein interactions using a yeast RNP interaction trap assay (RITA) Biotechniques. 1999;27(4):790–6. doi: 10.2144/99274rr02. [DOI] [PubMed] [Google Scholar]
- 36.Bouffard P, Barbar E, Briere F, et al. Interaction cloning and characterization of RoBPI, a novel protein binding to human Ro ribonucleoproteins. RNA. 2000;6(1):66–78. doi: 10.1017/s1355838200990277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogg JR, Collins K. Human Y5 RNA specializes a Ro ribonucleoprotein for 5S ribosomal RNA quality control. Genes Dev. 2007;21(23):3067–72. doi: 10.1101/gad.1603907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann MH, Trembleau S, Muller S, et al. Nucleic acid-associated autoantigens: pathogenic involvement and therapeutic potential. J Autoimmun. 2010;34(3):J178–206. doi: 10.1016/j.jaut.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Cheng L, Wang P, Yang S, et al. Identification of genes with a correlation between copy number and expression in gastric cancer. BMC Med Genomics. 2012;5:14. doi: 10.1186/1755-8794-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramakrishna M, Williams LH, Boyle SE, et al. Identification of candidate growth promoting genes in ovarian cancer through integrated copy number and expression analysis. PLoS ONE. 2010;5(4):e9983. doi: 10.1371/journal.pone.0009983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsushita K, Tomonaga T, Shimada H, et al. An essential role of alternative splicing of c-myc suppressor FUSE-binding protein-interacting repressor in carcinogenesis. Cancer Res. 2006;66(3):1409–17. doi: 10.1158/0008-5472.CAN-04-4459. [DOI] [PubMed] [Google Scholar]
- 42.Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8(7):632–7. doi: 10.1016/j.autrev.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Rosen A, Casciola-Rosen L. Autoantigens in systemic autoimmunity: critical partner in pathogenesis. J Intern Med. 2009;265(6):625–31. doi: 10.1111/j.1365-2796.2009.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.