Abstract
Previous studies have demonstrated that an acute implantation of lesioned lumbosacral ventral roots into the rat conus medullaris (CM) results in functional reinnervation of the lower urinary tract (LUT). Although the root implantation procedure results in a return of reflexive micturition, voiding efficiency (VE) remains incompletely recovered. Here, we performed a detailed urodynamic analysis of cystometry and external urethral sphincter (EUS) electromyography (EMG) recordings to determine underlying mechanisms for the incompletely recovered VE. For this purpose, adult female rats were studied at 12 weeks after a bilateral L5-S2 ventral root avulsion injury followed by an acute surgical implantation of the avulsed L6 and S1 ventral roots into the CM (n=6). Age-matched sham-operated rats (n=6) were included for control purposes. Compared to sham-operated controls, rats of the implanted series showed 1) reflex bladder contractions with a significantly shortened urine expulsion phase, 2) markedly decreased phasic EUS EMG activity during micturition, and 3) a pronounced bladder-sphincter dys-coordination, as demonstrated by a significantly delayed onset of the switch from low-amplitude tonic EUS EMG activity to either phasic EUS EMG activity or a large-amplitude tonic EUS EMG activity during the urine expulsion phase. Our findings provide a mechanistic explanation for the incomplete recovery of the VE following implantation of avulsed ventral roots into the spinal cord. Our future studies will aim to increase successful axonal regeneration in attempts to augment the recovery of the LUT after cauda equina injury and repair.
Keywords: bladder, external urethral sphincter, cystometrogram, electromyography, cauda equina
Introduction
Traumatic injuries to the spinal cord commonly cause an upper motoneuron syndrome, which includes bladder hyperreflexia and detrusor-sphincter dyssynergia (Schalow et al., 1995; Fowler, 1999). In contrast, traumatic injuries to the conus medullaris/cauda equina (CM/CE) may result in a lower motoneuron syndrome, which includes a flaccid weakness of the lower extremities, a sensory disturbance, neuropathic pain, as well as bladder, bowel and reproductive dysfunction (Taylor and Coolican, 1988; Sindou et al., 2001; Hoang and Havton, 2006). Following the direct injury to segmental efferent and afferent connections, an areflexic bladder, atrophy of the external urethral sphincter (EUS), and an inability to initiate voiding typically present (Pavlakis et al., 1983; Beric and Light, 1992). Unfortunately, no effective treatments are presently available for the neural repair of traumatic CM/CE injury.
However, we have recently developed a rat lumbosacral ventral root avulsion (VRA) injury model, which mimics key features of the clinical CM syndrome, including denervation of pelvic targets and the development of neuropathic pain (Hoang et al., 2003, 2006a; Bigbee et al., 2007). A bilateral L5-S2 VRA injury in the rat is followed by urinary retention and absence of both reflex bladder contractions and EUS activity detected by electromyography (EMG), as well as increased bladder weight and size (Hoang et al., 2006a). In contrast, an acute bilateral surgical implantation of the lesioned ventral roots into the CM for repair purposes resulted in innervation of implanted roots by regenerating axons, reduced urinary retention, return of reflexive voiding, as well as normalization of the bladder size and weight (Hoang et al., 2006a, b). However, the voiding efficiency (VE), calculated as the percentage of bladder volume voided per reflex bladder contraction, and maximum intravesical pressure (IVP) remained significantly below normal levels when compared with corresponding data obtained in the shamoperated series in spite of a normal duration of voiding contractions and the intercontraction interval in the implanted series (Hoang et al., 2006a). Similarly, the amplitude of EUS EMG activity during the bladder pre- and full contraction and the firing rate were not significantly different between the implanted and sham-operated series (Hoang et al., 2006a).
Therefore, the aim of the present study was to investigate plausible mechanisms for the above discrepancy between normalized key urodynamic parameters, including both cystometrogram (CMG) and EUS EMG recordings, on one hand, and a persistently reduced VE, on the other, in the implanted series. For this purpose, we performed an expanded analysis of the above CMG and EUS EMG recordings, taking into consideration also different aspects of the functional state of the lower urinary tract (LUT). For instance, special attention was paid to the bladder expulsion phase, indicated by a period of high frequency oscillations (HFO), which normally takes place during a bladder contraction and is detectable during CMG recordings (Maggi et al., 1986a). Bladder distension at volumes below the threshold for evoking micturition elicits high frequency tonic activity of the EUS, whereas bladder distension at volumes sufficient to initiate micturition elicits a prolonged phasic activity of the EUS at lower frequencies between 6–8 Hz in the rats. (Maggi et al., 1986a, b; Kruse et al., 1993; Cheng and de Groat, 2004).
Interestingly, we demonstrated that the implanted series exhibit significantly shorter HFO periods and thus a reduced expulsion time during voiding compared to the sham-operated series. The phasic EUS EMG activity was also significantly reduced, and the ratio of EUS EMG amplitudes during the voiding and bladder filling were significantly decreased as well. Furthermore, a pronounced bladder-sphincter dys-coordination was encountered in the implanted series, as demonstrated by a significantly delayed onset of the switch from low-amplitude tonic EUS EMG activity to either phasic EUS EMG activity or large-amplitude tonic EUS EMG activity during voiding. We conclude that the above differences in effective bladder expulsion time, in the phasic EUS EMG activity, and in the detrusor-EUS coordination between the implanted and sham-operated series are likely key contributors to the observed reduced VE in the implanted group.
Materials and methods
Animal preparations
Twelve adult female Sprague-Dawley rats (200–220 g, Charles River Laboratories, Raleigh, NC) were included in the study. The animals were divided into two groups: 1) Laminectomy and dura opening (sham-operated rats, n=6), and 2) Bilateral L5-S2 VRA injury followed by a bilateral acute implantation of the L6 and S1 ventral roots into the spinal cord (implanted rats, n=6). All animal procedures were carried out according to the standards established by the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996). The experimental protocols were approved by the Chancellor’s Animal Research Committee at UCLA.
A bilateral lumbar laminectomy (L1-L4) and opening of the dura mater were performed in all rats under general gas anesthesia with 2–2.5% isoflurane (Abbott Laboratories, North Chicago, IL). Anatomical landmarks were used to identify the L5, L6, S1 and S2 ventral roots under a surgical microscope. The sham-operated group did not undergo any further surgery. In the implanted group, all four ventral roots were avulsed bilaterally. These ventral roots were chosen as efferent parasympathetic neurons are primarily located in the L6 and S1 spinal cord segments and the EUS innervating motoneurons are located primarily in the L6 segment (Schrøder, 1980; Nadelhaft and Booth, 1984). A bilateral L5-S2 VRA injury in rats has also been demonstrated to abolish reflex bladder contractions and EUS EMG activity during urodynamic recordings (Hoang et al., 2006a). By applying constant traction with a pair of fine jeweler’s forceps along the normal course of each individual root, all rootlets of each root were separated from the anterior surface of the spinal cord. The rootlets of the avulsed L6 and S1 ventral roots were trimmed. Only about 1–2 mm of the tip of the avulsed roots were trimmed to obtain an even cut surface for implantation purposes. Two small longitudinal scalpel incisions were made bilaterally into the lateral funiculus of the L6 and S1 spinal cord segments. The roots were implanted into the spinal cord in their respective incision sites. As the L6 and S1 ventral roots are of small caliber, no tissue glue was necessary to hold the roots in place. An 8-0 suture was loosely tied around each implanted root to allow for easier identification during later dissections. For all animals, a titanium mesh cage was placed over the laminectomy site to stabilize the vertebral column and protect the spinal cord from compression by the overlying muscles (Nieto et al., 2005). The overlying paraspinous muscles and skin were subsequently sutured in layers, and all animals were allowed to recover. Bladders were manually expressed three times a day for two days after surgery, then twice a day until termination of the experiment at 12 weeks post-operatively.
Urodynamic evaluation
In the preparation for urodynamic recordings, each animal was under general gas anesthesia using 2–2.5% isoflurane. A catheter (PE-50) was inserted through the urethra into the bladder to measure IVP of the bladder. The transurethral catheter was connected to a pressure transducer (Model 041500503A, Maxxim Medical, Athens, TX) to record the CMG. For EMG, two 50 µm epoxy-coated platinum-iridium wire electrodes (A-M Systems, Everett, WA) were hooked percutaneously into the EUS to record EMG activity. For this purpose, a 30 gauge needle with a hooked electrode at the tip was inserted into the urethra along the side of the catheter to the level of the EUS and subsequently withdrawn, leaving the electrodes embedded in both sides of the EUS. CMG and EUS EMG data were amplified and digitized (CMG at 5 samples/sec and EUS EMG at 6250 samples/sec) using a 12-bit data acquisition system (DataLab 2000, Model 70754, Lafayette Instruments, Lafayette, IN). Animals were placed in a prone position and allowed to acclimate to a decreased concentration of isoflurane (0.6–0.8%) for about one hour before infusion of saline (13.2 ml/hr) into the bladder through the catheter. The rats were kept only lightly anesthetized. Prior to urodynamic recordings, all rat bladders were expressed and found to retain no or a minimal amount of residual urine, a finding typical of normal rats. The recording sessions lasted for 2–3 hours for each animal. After the completion of the urodynamic recordings, 0.5 µl of 2% Fast Blue (Sigma, St. Louis, MO) was injected into the EUS. Animals were allowed to recover and survive for another 5 days to allow for retrograde transport and labeling of spinal cord neurons. All animals were perfused intravascularly using a paraformaldehyde solution.
Data and statistical analysis
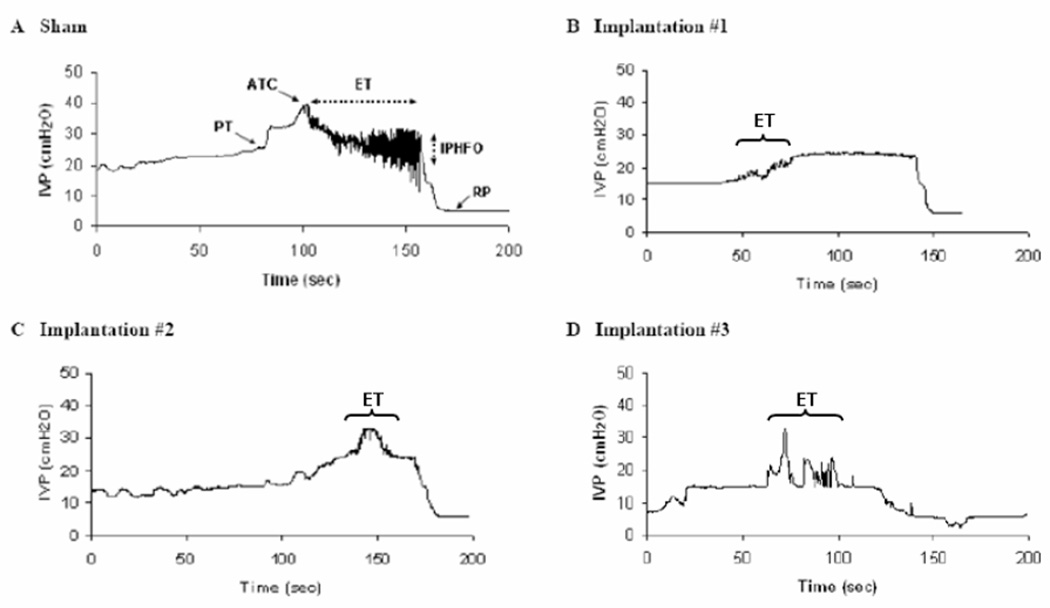
A minimum of 20 minutes of recordings and 6 voiding cycles were analyzed for each animal. In each experiment the pressure threshold (PT), resting pressure (RP), expulsion time (ET), amplitude of trigger contraction (ATC) as well as amplitude of intraluminal pressure and period of high frequency oscillations (IPHFO) were determined (Maggi et al., 1986b) (Figure 1A). Here, the EUS EMG recordings may be affected by the wire electrode placement, which can be the source of significant variation in baseline amplitude values (Callsen-Cencic and Mense, 1998). Therefore, the baseline fluctuations caused by a low-frequency component of the EMG (which may be attributed to the activity of the internal urethral sphincter) were filtered out by the use of a baseline zero correction at 100 ms intervals. EUS EMG activity was analyzed using a custom software written in Visual Basic (Microsoft Corporation, Redmond, WA) with the Measurement Studio ActiveX components (National Instruments, Austin, TX). The amplitude and frequency of EUS EMG activity was determined during bladder filling and voiding in all rats. As a marker for the coordinated function of the bladder and EUS, the time delay between the PT of the bladder contraction and the onset of tonic EUS EMG activity during the same voiding cycle was determined. Quantitative data were expressed as mean ± SE. The data obtained in sham-operated and implanted groups were statistically compared using the t-test. We considered p<0.05 as statistically significant.
Figure 1.
The urine expulsion phase is shortened after lumbosacral VRA injury and root re-implantation. Bladder contractions in a sham-operated (A) rat and three implanted (B–D) rats during bladder filling and voiding. High-frequency oscillations (HFOs) did not appear in each implanted rat, i.e., the animal (B).Two implanted rats (C and D) showed the shorter duration of HFOs than that in the sham-operated rat (A). ET: expulsion time. ATC: amplitude of trigger contraction. PT: pressure threshold. RP: resting pressure. IPHFO: amplitude of intraluminal pressure of HFOs.
Results
Urodynamic recordings were performed at 12 weeks post-operatively in adult female rats of both the implanted and sham-operated series. The implanted group had undergone a prior bilateral L5-S2 VRA injury and acute implantation of the avulsed L6 and S1 ventral roots into the CM. Only a modest leg weakness was typically detected in distal muscle groups affecting ankle movements. The rats were able to weight bear on hindlimbs and ambulate using all four extremities (Hoang et al., 2006a).
Analysis of bladder contractions by cystometry
In all animals of the implanted (n=6) and sham-operated series (n=6), CMG recordings demonstrated reflex voiding bladder contractions, a readily identified PT at the beginning of the bladder contraction and a RP following the completion of the contraction (Figure 1A).
Although the overall duration of bladder contractions was indistinguishable between the implanted and sham-operated series, several properties of the bladder expulsion phase were different between the two groups (Table 1). Specifically, the ET was significantly shorter in the implanted series (17 ± 10 seconds, n=6) compared to sham-operated rats (33 ± 17 seconds, n=6, p<0.05). Consequently, the ET/CD ratio was lower in the implanted series (0.25 ± 0.17, n=6) compared to sham-operated rats (0.42 ± 0.22, n=6, p<0.05). The ATC, indicating the onset of the expulsion phase of the bladder contraction, was also reduced in the implanted series (15 ± 6 cm H2O, n=6) compared to the sham-operated rats (24 ± 3 cm H2O, n=6, p<0.05). In addition, the amplitudes of both the PT and RP were significantly smaller in the implanted series (n=6) compared to the sham-operated group (n=6, p<0.05, Table 1).
Table 1.
Parameters of bladder activity in the sham-operated and implanted series.
| Sham-operated (n = 6) |
Implanted (n = 6) |
|
|---|---|---|
| ET (s) | 33 ± 17 | 17 ± 10* |
| CD (s) | 66 ± 19 | 91 ± 31 |
| Ratio (ET/CD) | 0.42 ± 0.22 | 0.25 ± 0.17* |
| ATC (cm H2O) | 24 ± 3 | 15 ± 6* |
| PT (cm H2O) | 12 ± 1 | 8 ± 3* |
| RP (cm H2O) | 4 ± 2 | 2 ± 1* |
ET: expulsion time. CD: contraction duration (Hoang et al., 2006a). ATC: amplitude of trigger contraction. PT: pressure threshold. RP: resting pressure.
: p<0.05 significantly changed compared with sham-operated series.
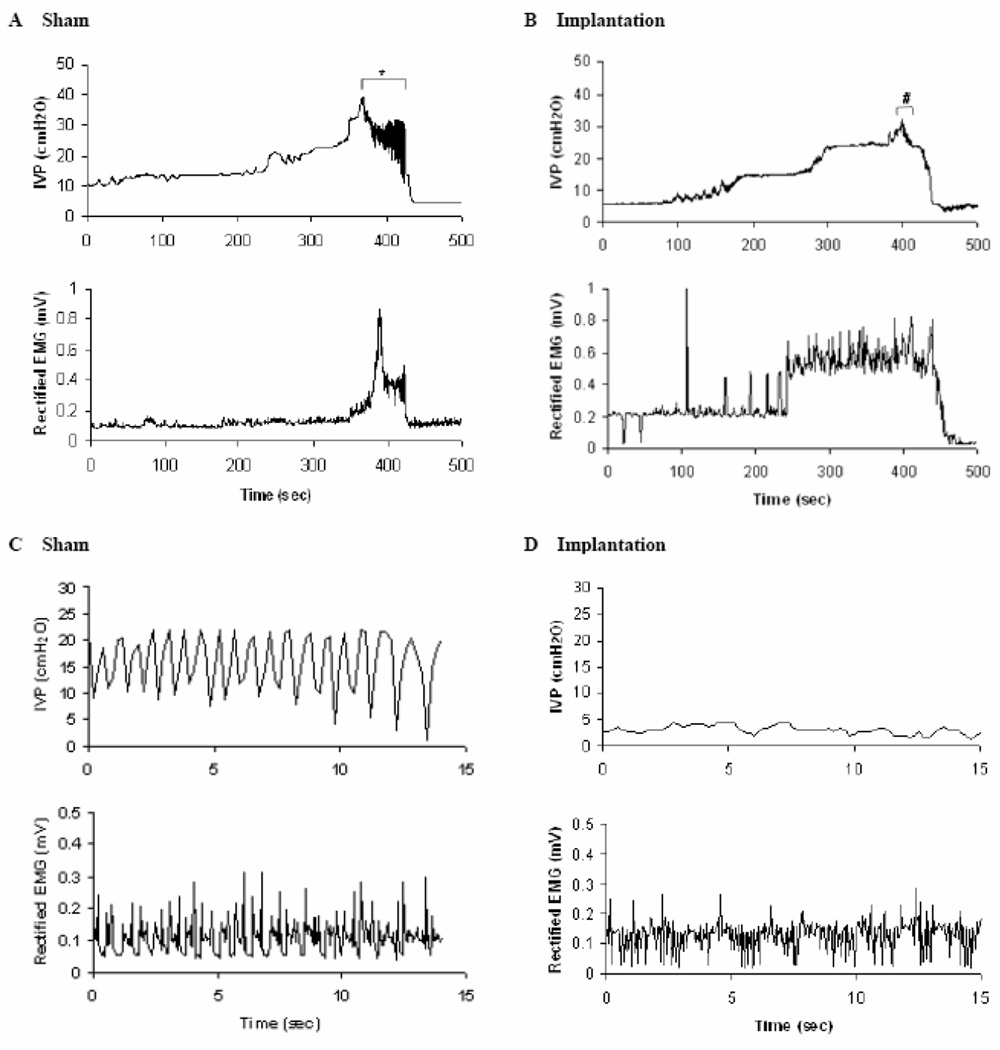
A period of HFOs was associated with the expulsion phase of the bladder contraction in all rats of the sham-operated group but was detected in only 2 of 6 rats in the implanted series (Figures 1A–D). Furthermore, the amplitude of the IPHFO in the implanted series was 2.2 cm H2O and 2.1 cm H2O for the two rats and appeared markedly smaller than the corresponding measurements in the sham-operated series (7 ± 4 cm H2O, n=6, Table 1; Figure 3).
Figure 3.
Phasic EUS EMG activity occurs during bladder contractions in sham-operated rats and after lumbosacral VRA injury and root re-implantation. Coordination between bladder contractions and EUS EMG activity may be assessed in rats of the sham series (A and C) and after implantation (B and D). Top traces show the cystometrogram. Bottom traces show the rectified EUS EMG activity elicited by continuously infusing saline to the bladder. In panels A and B, tonic EUS EMG activity is detected during the bladder filling phase performed at the onset of the micturition reflex. The phasic EUS EMG activity replaced tonic EUS EMG activity during voiding. In C and D cystometrogram recordings and EUS EMG activity are shown at an expanded time base for the sequence of recordings indicated by symbols in A (*) and B (#), respectively. The amplitude of HFOs appears larger in the sham-operated group (C) compared to corresponding data from the implanted group (D).
Studies of EUS activity patterns by EMG
Quantitative analysis of EMG recordings obtained from the EUS was performed in rats of both the sham-operated and implanted series (Table 2). EUS EMG recordings demonstrated tonic activity during bladder filling in all rats of both the implanted and sham-operated series (Figures 2A–D). The amplitude of the tonic EUS EMG activity obtained during bladder filling was 0.14 ± 0.07 mV (n=6) in the implanted animals and was not statistically significant (p=0.12) from the corresponding amplitude of 0.08 ± 0.01 mV (n=6) in the sham-operated series.
Table 2.
Analysis of EUS EMG activity during bladder filling and voiding in the sham-operated and implanted series.
| Sham-operated (n = 6) |
Implanted (n = 6) |
|
|---|---|---|
| Tonic activity during filling (mV) | 0.08 ± 0.01 | 0.14 ± 0.07 |
| Tonic and phasic activity during voiding (mV) | 0.22 ± 0.11 | 0.24 ± 0.21 |
| Ratio (voiding/filling) | 2.72 ± 1.28 | 2.09 ± 0.73* |
: p<0.05 significantly decreased compared with sham-operated group. The phasic EUS EMG activity during voiding was shown in sham-operated group and two of six animals in implanted group. Four animals in implanted group showed large-amplitude tonic EUS EMG activity during voiding.
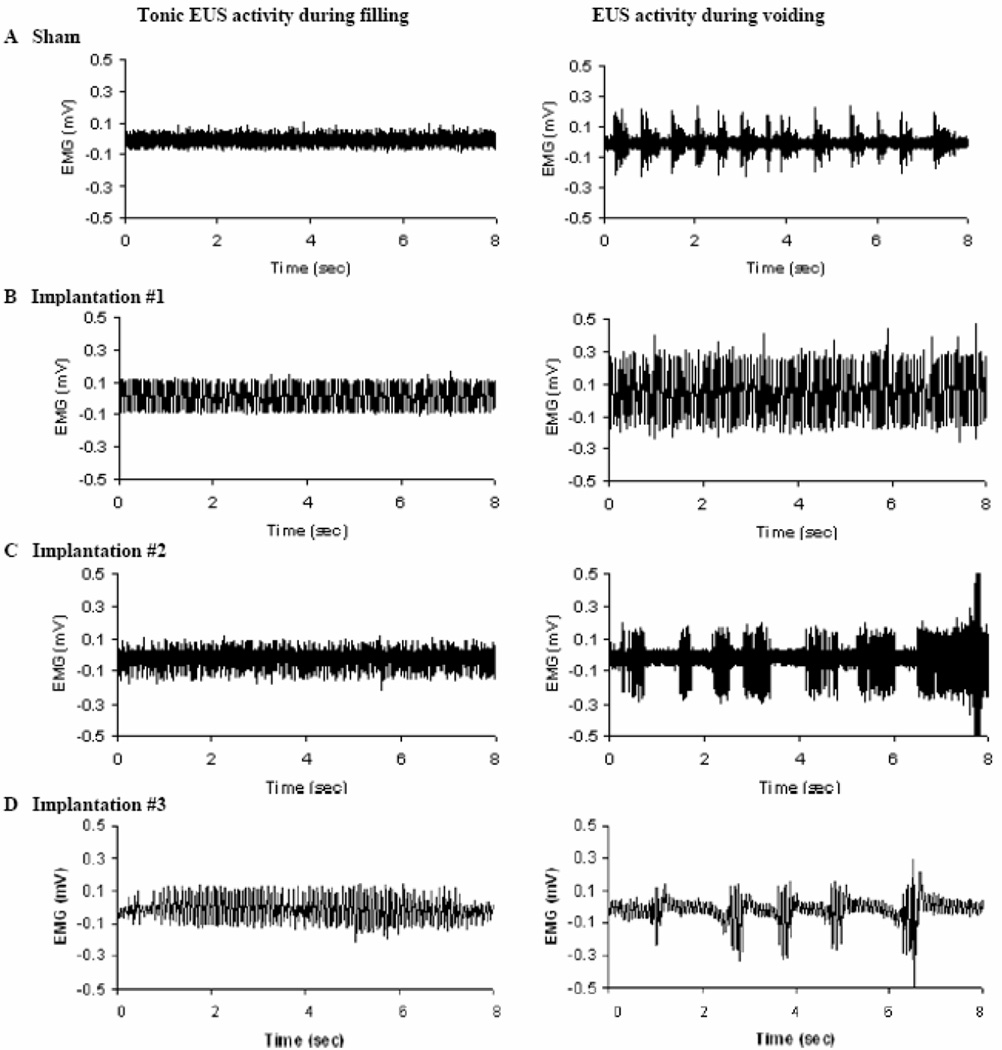
Figure 2.
The phasic EUS EMG activity is decreased after lumbosacral VRA injury and root re-implantation. Representative examples show EUS activity during bladder filling (left) and voiding (right) in sham (A) and implanted (B–D) series. Tonic EUS EMG activity takes place during filling in sham operated (A) and implanted rats (B–D). Sham-operated rats show EUS EMG burst activity during voiding, whereas implanted rats show either large-amplitude tonic EUS EMG activity (B) or EUS EMG bursting activity (C, D) during voiding.
All rats of the sham-operated series demonstrated phasic EUS EMG activity during bladder emptying. The amplitude of the phasic EUS EMG activity in these sham-operated rats measured 0.22 ± 0.11 mV (n=6, Figures 3A and C). In the implanted series, however, only two of the six rats demonstrated detectable phasic EUS EMG activity during voiding (Figures 2C–D). One of these two rats demonstrated the best voiding efficiency of the implanted series (Hoang et al., 2006a). The remaining four of the six rats of the implanted series instead exhibited a switch to large-amplitude tonic EUS EMG activity, but without the brief periodic interruptions in bursting characterized by the phasic EUS EMG activity, during the bladder expulsion phase (Figure 2B). The amplitude of the phasic or tonic EUS EMG activity during the bladder expulsion phase was 0.24 ± 0.21 mV (n=6) in the implanted series (Figures 3B and D). However, the ratio of EUS EMG amplitude during voiding and filling was 2.09 ± 0.73 (n=6) in the implanted group and significantly lower than the corresponding ratio of 2.72 ± 1.28 in the sham-operated rats (n=6, p<0.05, Table 2).
Furthermore, the mean firing frequency of the phasic EUS EMG activity in the sham-operated group was 2.24 ± 0.10 Hz (n=6). In two of six rats with demonstrable phasic EUS EMG activity in the implanted series, the firing frequency was 1.33 Hz and 1.58 Hz, and in both cases appeared slower than the corresponding firing rate of all rats in the sham-operated group.
Coordination between bladder contractions and EUS activation
Although the above analyses demonstrated quantitative and qualitative differences in the morphology of bladder contractions and EUS EMG activity patterns between the implanted and sham-operated groups, additional factors may also affect the VE. For instance, the bladder voiding contractions and the onset of phasic EUS EMG activity are normally well coordinated in time in control preparations, whereas a lumbosacral VRA injury followed by an acute implantation of lesioned roots into the CM may affect the coordination between the bladder contraction and EUS activation.
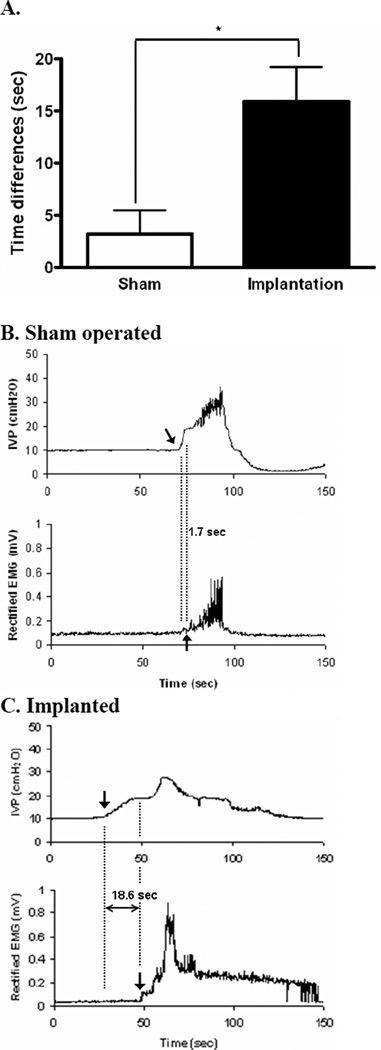
To determine quantitatively the degree of coordination between bladder contractions and EUS activation during reflexive voiding, we measured the time interval separating the start of a bladder contraction, identified as the point of PT using cystometry, and the onset of phasic or large amplitude tonic EUS EMG activity during the same voiding cycle. In sham-operated rats, there was a slight delay between the onset of a voiding bladder contraction and the start of the phasic EUS EMG activity (3.2 ± 2.3 seconds, n=6, Figures 4A and B). However, rats of the implanted series demonstrated a markedly prolonged delay between the onset of bladder contractions and the start of phasic EUS EMG activity or large-amplitude tonic EUS EMG activity (15.9 ± 3.3 seconds, n=6, p<0.05, Figures 4A and C).
Figure 4.
The onset of the phasic EUS EMG activity is delayed during voiding after lumbosacral VRA injury and root re-implantation. Time delay between the onset of bladder contractions and phasic EUS EMG activity was assessed by cystometry and EUS EMG, respectively, in the sham-operated and implanted series. Note the statistically significant different delay in the implanted series as indicated in A. Representative urodynamic recordings in rats of sham-operated and implanted series are shown in B and C, respectively.
Discussion
In the present urodynamic study, we used a bilateral CE injury and repair model to examine detailed features of functional reinnervation of the LUT. The study expands on our prior demonstration that an acute implantation of avulsed lumbosacral ventral roots into the CM promotes functional and anatomical reinnervation of both the bladder detrusor and EUS in the adult rat (Hoang et al., 2006a). All rats were studied at 12 weeks post-operatively to allow for axonal regeneration to occur and bridge the approximately 40 mm distance between the lumbosacral spinal cord and the LUT. Although the implantation procedure results in bladder contractions with normal duration and with normal intercontraction intervals, the VE is only incompletely restored (Hoang et al., 2006a). Here, we demonstrate in the implanted series three key urodynamic mechanisms, which individually and together may contribute to the limited recovery of the VE in these animals. Specifically, CMG recordings exhibited a shortened expulsion phase, the phasic EUS EMG activity was reduced, and there was marked dys-coordination between the bladder contractions and phasic EUS EMG activation in rats of the implanted series.
Effects of ventral root injury and repair on bladder contractions
The intravesical pressure of the rat bladder normally remains relatively stable during the bladder filling phase due to stretching and dilatation of the bladder wall but increases acutely in response to an active reflex bladder contraction (Maggi et al., 1986b; de Groat, 2006). During a bladder contraction, urine expulsion begins when the trigger contraction has produced a threshold IVP, the ATC, and coincides with the onset of a period of voiding-associated HFO (Maggi et al., 1986a, b). Here, we demonstrate that the absolute duration of the bladder expulsion time and its proportion of the duration of the bladder contraction are reduced in the implanted series during bladder contractions. Therefore, the shortened urine expulsion phase in the implanted series may contribute to the incompletely restored VE in these rats.
Effects of ventral root injury and repair on EUS EMG activity
The EUS makes an important to contribution to normal voiding in the rat. For instance, coordinated micturition is characterized by EUS bursting during voiding (Cheng and de Groat, 2004). The presence of the phasic EUS EMG activity is an important index for a functional micturition reflex also after neurological injuries. For instance, the phasic EUS EMG activity may disappear acutely following a traumatic spinal cord injury (SCI) but may show signs of recovery at later time points in rodent models (Maggi et al., 1986b; Kruse et al., 1993; Kruse and de Groat, 1993; Cheng and de Groat, 2004; Chang et al., 2006, 2007). However, when the phasic EUS EMG activity was abolished in SCI rats, VE was also reduced (Maggi et al., 1986b; Cheng et al., 1997; Cheng and de Groat, 2004; Kruse et al., 1993). Although early studies on the effects of SCI on micturition reflexes in the rat suggested phasic EUS EMG activity to be dependent on the presence supraspinal pathways (Kruse et al., 1993), recent investigations have shown that phasic EUS EMG activity may be detected also following a complete transection of the thoracic spinal cord in adult rats (Cheng and de Groat, 2004).
In the present injury model, the lumbosacral VRA injury resulted in a lower motoneuron syndrome, characterized by the absence of inducible micturition reflexes (Hoang et al., 2006a). Here, the root implantation procedure resulted in reinnervation of the LUT but limited return of the phasic EUS EMG activity. Although phasic EUS EMG activity was detected in two of six rats in the implanted group at twelve weeks postoperatively, the majority of rats exhibited absence of phasic EUS EMG activity associated with the bladder contraction phase. Another indicator for a functional difference in the micturition reflex between the rats of the implanted and sham-operated groups was the significantly reduced ratio in the implanted series for the EUS EMG amplitude measured during bladder filling and voiding. We speculate that the limited return of phasic EUS EMG activity in the implanted group may contribute to the incompletely restored VE in the implanted series.
Functional recovery may differ following repair of proximal root and peripheral nerve injuries. For instance, a lumbosacral VRA injury results in marked and progressive death of motor and autonomic neurons (Hoang et al., 2003), whereas lesions to peripheral nerves are associated with no or limited motoneuron loss (Piehl et al., 1995; Vanden Noven et al., 1993; Herdegen et al., 1997; Ma et al., 2001). Any injury-induced loss of motoneurons normally innervating the lower urinary tract is likely to have a detrimental effect on the recovery of functional micturition, as motoneuron survival in the dorsolateral (DL) nucleus of the L6 segment is positively correlated with improved VE following lumbosacral VRA injury and repair in the adult rat (Hoang et al., 2006a). The degree of functional recovery may also be affected by the number of motoneurons that successfully reinnervate its target muscle. Specifically, in our experimental model results in successful reinnervation of the EUS by approximately 25% of the lesioned DL motoneurons (Hoang et al., 2006a). For comparison, approximately 54% of lesioned motoneurons were assessed as having successfully reinnervated the EUS following a crush injury to the proximal pudendal nerve (Kerns et al., 2000).
Coordination in time between bladder contraction and EUS phasic activity
Normally, there is a close time relationship between the onset of the bladder voiding contraction and the EUS EMG pattern change from tonic to phasic activity (Maggi et al., 1986b; Cheng and de Groat, 2004; Chang et al., 2006, 2007). Increased IVP during a bladder contraction and the near simultaneous peristaltic activity of the EUS contribute to efficient voiding in the rat (de Groat, 2006). Interestingly, the present study demonstrates that a lumbosacral ventral root injury and repair may result in a disturbed synchronization in time between the detrusor and EUS. Here, this dys-coordination is characterized by a significant time delay between the reflex bladder contraction and the switch from tonic to phasic EUS EMG activity in the implanted series compared to the sham-operated rats. In our implanted series the switch to phasic activity was characterized by the onset of phasic EUS EMG activity or to larger-amplitude tonic EUS EMG activity. We speculate that the delayed onset of the phasic EUS EMG activity in the implanted series may contribute to the decreased VE.
Methodological considerations
Previous EMG studies from the neurologically intact rat EUS have demonstrated the phasic activity component during micturition to exhibit a frequency of approximately 6 Hz (Kruse et al., 1993; Cheng and de Groat, 2004; Chang et al., 2006, 2007). In those earlier studies, the EUS EMG recordings were part of a terminal procedure and the animal preparations for the electrophysiological recordings included a surgical exposure of the EUS and placement of the EMG electrodes under visual guidance. However, the urodynamic recordings in the present study were part of a survival procedure. This difference in electrode placement techniques used between studies may explain, at least in part, the apparent lower frequency of 2.2 Hz for the phasic EUS EMG activity in our sham-operated series. It is therefore possible that the data collected from the EMG recordings in the present study may represent a conservative underestimate of the phasic EUS EMG activity in both our sham-operated and implanted series.
Concluding remarks
Earlier studies have demonstrated that functional reinnervation of the LUT is possible following CE injury and repair (Hoang et al., 2006a). However, the re-establishment of the micturition reflex in this model was associated with an incomplete recovery of the VE. In the present study, we investigated possible contributors to the limited recovery of VE following a bilateral VRA injury and surgical implantation of lesioned ventral roots into the CM in the rat. The implanted series showed three distinct electrophysiological differences, which may affect functional micturition: First, reflex bladder contractions showed a significantly shortened urine expulsion phase; second, phasic EUS EMG activity during micturition was markedly decreased; third, there was a pronounced bladder-sphincter dys-coordination, as demonstrated by a significantly delayed onset of the switch from low-amplitude tonic EUS EMG activity to either phasic EUS EMG activity or a large-amplitude tonic EUS EMG activity during voiding. Our findings identify plausible mechanisms and provide a functional explanation for the incomplete recovery of the VE following implantation of avulsed ventral roots into the spinal cord. Future studies will evaluate the effects of the addition of a variety of therapeutic agents in attempts to augment the functional recovery of micturition after CE injury and repair.
Acknowledgments
HYC was supported by a post-doctoral fellowship from the NIH-funded Neural Repair Training Program at UCLA (T32 NS07449); LAH was supported by NIH/NINDS (NS042719), The Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, The Paralysis Project of America, and The Roman Reed Funds for Spinal Cord Injury Research of California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beric A, Light JK. Function of the conus medullaris and cauda equina in the early period following spinal cord injury and the relationship to recovery of detrusor function. J. Urol. 1992;148:1845–1848. doi: 10.1016/s0022-5347(17)37047-7. [DOI] [PubMed] [Google Scholar]
- 2.Bigbee A, Hoang TX, Havton LA. At-level neuropathic pain is induced by lumbosacral ventral root avulsion injury and ameliorated by root reimplantation into the spinal cord. Exp. Neurol. 2007;204:273–282. doi: 10.1016/j.expneurol.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callsen-Cencic P, Mense S. Abolition of cystitis-induced bladder instability by local spinal cord cooling. J. Urol. 1998;160:236–241. [PubMed] [Google Scholar]
- 4.Chang HY, Cheng CL, Chen JJ, de Groat WC. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R224–R234. doi: 10.1152/ajpregu.00780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am. J. Physiol. Renal Physiol. 2007;292:F1044–F1053. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng CL, Chai CY, de Groat WC. Detrusor-sphincter dyssynergia induced by cold stimulation of the urinary bladder of rats. Am J Physiol. 1997;272:R1271–R1282. doi: 10.1152/ajpregu.1997.272.4.R1271. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp. Neurol. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 8.de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br. J. Pharmacol. 2006;147:S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler CJ. Neurological disorders of micturition and their treatment. Brain. 1999;122:1213–1231. doi: 10.1093/brain/122.7.1213. [DOI] [PubMed] [Google Scholar]
- 10.Herdegen T, Skene P, Bähr M. The c-Jun transcription factor – bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 11.Hoang TX, Nieto JH, Tillakaratne NJ, Havton LA. Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model of cauda equina injury. J. Comp. Neurol. 2003;467:477–486. doi: 10.1002/cne.10928. [DOI] [PubMed] [Google Scholar]
- 12.Hoang TX, Pikov V, Havton LA. Functional reinnervation of the rat lower urinary tract after cauda equina injury and repair. J. Neurosci. 2006a;26:8672–8679. doi: 10.1523/JNEUROSCI.1259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoang TX, Nieto JH, Dobkin BH, Tillakaratne NJ, Havton LA. Acute implantation of an avulsed lumbosacral ventral root into the rat conus medullaris promotes neuroprotection and graft reinnervation by autonomic and motor neurons. Neuroscience. 2006b;138:1149–1160. doi: 10.1016/j.neuroscience.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 14.Hoang TX, Havton LA. Novel repair strategies to restore bladder function following cauda equina/conus medullaris injuries. Prog. Brain Res. 2006;152:195–204. doi: 10.1016/S0079-6123(05)52012-0. [DOI] [PubMed] [Google Scholar]
- 15.Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, Brubaker L. Effects of pudendal nerve injury in the female rat. Neurourol. Urodyn. 2000;19:53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993;264:R1157–R1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- 17.Kruse MN, de Groat WC. Spinal pathways mediate coordinated bladder urethral sphincter activity during reflex micturition in decerebrate and spinalized neonatal rats. Neurosci. Lett. 1993;152:141–144. doi: 10.1016/0304-3940(93)90503-d. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Novikov LN, Wiberg M, Kellerth JO. Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp. Brain Res. 2001;139:216–223. doi: 10.1007/s002210100769. [DOI] [PubMed] [Google Scholar]
- 19.Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1986a;251:R250–R257. doi: 10.1152/ajpregu.1986.251.2.R250. [DOI] [PubMed] [Google Scholar]
- 20.Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J. Pharmacol. Methods. 1986b;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- 21.Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J. Comp. Neurol. 1984;226:238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- 22.Nieto JH, Hoang TX, Warner EA, Franchini BT, Westerlund U, Havton LA. Titanium mesh implantation--a method to stabilize the spine and protect the spinal cord following a multilevel laminectomy in the adult rat. J. Neurosci. Methods. 2005;147:1–7. doi: 10.1016/j.jneumeth.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Pavlakis AJ, Siroky MB, Goldstein I, Krane RJ. Neurourologic findings in conus medullaris and cauda equina injury. Arch. Neurol. 1983;40:570–573. doi: 10.1001/archneur.1983.04050080070014. [DOI] [PubMed] [Google Scholar]
- 24.Piehl F, Tabar G, Cullheim S. Expression of NMDA receptor mRNAs in rat motoneurons is down-regulated after axotomy. Eur. J. Neurosci. 1995;7:2101–2110. doi: 10.1111/j.1460-9568.1995.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 25.Schalow G, Bersch U, Michel D, Koch HG. Detrusor-sphincteric dyssynergia in humans with spinal cord lesions may be caused by a loss of stable phase relations between and within oscillatory firing neuronal networks of the sacral micturition center. J Auton Nerv Syst. 1995;52:181–202. doi: 10.1016/0165-1838(94)00155-d. [DOI] [PubMed] [Google Scholar]
- 26.Schrøder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J. Comp. Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- 27.Sindou M, Mertens P, Wael M. Microsurgical DREZotomy for pain due to spinal cord and/or cauda equina injuries: long-term results in a series of 44 patients. Pain. 2001;92:159–171. doi: 10.1016/s0304-3959(00)00487-5. [DOI] [PubMed] [Google Scholar]
- 28.Taylor TK, Coolican MJ. Injuries of the conus medullaris. Paraplegia. 1988;26:393–400. doi: 10.1038/sc.1988.60. [DOI] [PubMed] [Google Scholar]
- 29.Vanden Noven S, Wallace N, Muccio D, Turtz A, Pinter MJ. Adult spinal motoneurons remain viable despite prolonged absence of functional synaptic contact with muscle. Exp. Neurol. 1993;123:147–156. doi: 10.1006/exnr.1993.1147. [DOI] [PubMed] [Google Scholar]