Abstract
Background
Seborrheic keratosis (SK) is one of the most common epidermal tumors of the skin. However, only a few large-scale clinicohistopathological investigations have been conducted on SK or on the possible correlation between histopathological SK subtype and location.
Objective
The aim of this study was to analyze the clinical and histopathological features of a relatively large number of cases of diagnosed SK.
Methods
Two hundred and seventy-one pathology slides of skin tissue from patients with clinically diagnosed SK and 206 cases of biopsy-proven SK were analyzed. The biopsy-proven cases of SK were assessed for histopathological subclassification. The demographic, clinical, and histopathological data of the patients were collected for analysis of associated factors.
Results
The most frequent histopathological subtype was the acanthotic type, followed by mixed, hyperkeratotic, melanoacanthoma, clonal, irritated, and adenoid types; an unexpectedly high percentage (9.2%) of the melanoacanthoma variant was observed. The adenoid type was more common in sun-exposed sites than in sun-protected sites (p=0.028). Premalignant and malignant entities together represented almost one-quarter (24.2%) of the clinicopathological mismatch cases (i.e., mismatch between the clinical and histopathological diagnoses). Regarding the location of SK development, the frequency of mismatch for the sun-exposed areas was significantly higher than that for sun-protected areas (p=0.043).
Conclusion
The adenoid type was more common in sun-exposed sites. Biopsy sampling should be performed for lesions situated in sun-exposed areas to exclude other premalignant or malignant diseases.
Keywords: Classification, Pathology, Seborrheic keratosis
INTRODUCTION
Seborrheic keratosis (SK) is one of the most common benign epidermal tumors that affects both sexes equally, and usually arises in individuals older than 50 years1,2,3,4. It presents as sharply demarcated, slightly raised brownish patches or plaques, usually on sun-exposed surfaces of the skin2,5. The clinical presentation can be quite variable and includes clinical variants, such as stucco keratosis and dermatosis papulosa nigra2. Despite the diverse clinical presentation of SK, diagnosis is most often clinically straightforward. However, the tumor may simulate other lesions, such as common warts, lentigines, melanocytic nevi, actinic keratosis, and Bowen disease, or occasionally more aggressive entities, such as basal cell and squamous cell carcinomas, or even cutaneous melanomas6,7,8.
It can be divided into six major histopathological variants: acanthotic, hyperkeratotic, adenoid, irritated, clonal, and melanoacanthoma. Histopathologically, all of the subtypes have three features in common: hyperkeratosis, acanthosis, and papillomatosis. On low- to moderate-power magnification, the base of the lesion lies roughly on an imaginary axis drawn between two dermoepidermal junctions at both ends of the surrounding normal tissue2.
Previous studies have investigated SKs according to subject age, sex, site of occurrence, and histological types; however, the clinical and pathological features of SKs relative to their location have yet to be established. The aim of this study was to determine the clinical and histopathological features of SKs, and the pattern of histopathological SK subclassification relative to its sites.
MATERIALS AND METHODS
Subjects and data collection
Slide-mounted sections of pathology samples harvested from outpatient cases between January 2011 and August 2013 at Konkuk University Medical Center (Seoul, Korea) were reviewed for inclusion in this study (KUH1120058). Tissue samples from 271 cases of clinically diagnosed SK (including 91 cases initially diagnosed clinically as SKs, but subsequently proven not to be on histological examination) and 206 cases of biopsy-proven SK (including 26 cases not initially clinically diagnosed as SKs, but subsequently proven to be so on histological examination) were ultimately included following electronic medical record review. The medical records were investigated retrospectively for any difference in age, sex, site of occurrence, and analysis of the clinicopathological mismatch cases (i.e., cases in which there was mismatch between the clinical and pathological diagnoses).
Clinical and histopathological assessment
Hematoxylin and eosin-stained pathology sections were subclassified according to their histological features2 as acanthotic, hyperkeratotic, adenoid, irritated, clonal, melanoacanthoma, or mixed. For the sake of convenience, the sites of the biopsy-proven cases were categorized as follows (these are the actual biopsy sites): facial area, neck, scalp, trunk, upper extremities, and lower extremities. The sites of the lesions were also categorized as either sun-exposed or unexposed according to whether they were locations that are readily exposed to the sun (i.e., facial area, neck, scalp, and upper extremities) and those less prone to frequent solar exposure (trunk and lower extremities). Cases of mismatch between clinical impression and histological diagnosis were examined; these involved 91 cases that initially resembled SK clinically but were proven otherwise on histopathological examination, and 26 cases that were initially not considered to resemble SKs clinically, but were subsequently proven to be so on histopathological examination. The existence of an association between frequency of clinicopathological mismatch and location of SK development was determined.
Statistical analysis
The patients' demographic data, lesion sites, distribution of histopathological SK subtypes, and frequency of SK misdiagnosis (i.e., clinical diagnosis of SK where there was not SK) were analyzed using the SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The data are presented as mean±standard deviation values when appropriate. Statistical significance was assessed using t-test, χ2 test, and Fisher's exact test. The cutoff for statistical significance was set at p<0.05.
RESULTS
Analysis of clinically diagnosed SKs
Of the 271 clinically diagnosed SK cases, 180 were subsequently proven to be SK following histological analysis of biopsy specimens (biopsy-proven SKs), and 91 were proven to be something other than SK.
1) Demographic characteristics of patients with clinically diagnosed SKs
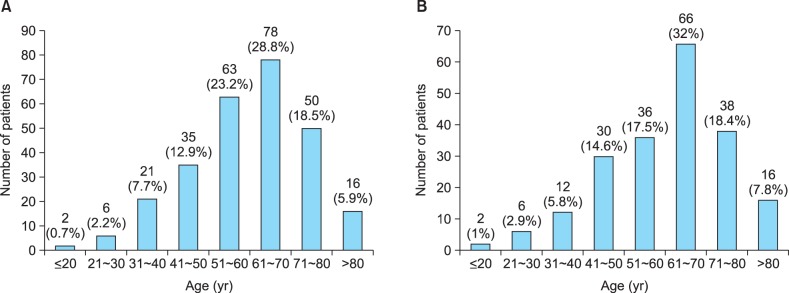
The age distribution was spread widely across all age groups; the age of the enrolled cases was 60.1±14.3 years (range, 16 to 86 years). There were 144 males and 127 females (male/female ratio, 1.13:1; Table 1). The mean age did not differ significantly with sex (p=0.094). The age distribution of the subjects is shown in Fig. 1A.
Table 1. Demographic data of patients.
| Variable | Patient (n) | Age (yr) |
|---|---|---|
| Clinically diagnosed SK | ||
| Men | 144 | 60.7±13.1 (31~85) |
| Women | 127 | 59.3±15.5 (16~86) |
| Overall | 271 | 60.1±14.3 (16~86) |
| Biopsy-proven SK | ||
| Men | 95 | 61.9±10.0 (19~88) |
| Women | 111 | 60.2±14.8 (23~98) |
| Overall | 206 | 60.8±14.6 (19~98) |
Values are presented as number of patients and mean of the age±standard deviation (range). SK: seborrheic keratosis.
Fig. 1. Age distribution of (A) 271 clinically diagnosed and (B) 206 biopsy-proven seborrheic keratosis patients.
2) Biopsy site for clinically diagnosed SKs
The most common biopsy site for the biopsy-proven SKs was the face and neck (101 cases, 37.3%), followed by the trunk (78 cases, 28.8%), scalp (51 cases, 18.8%), lower extremities (22 cases, 8.1%), and upper extremities (19 cases, 7.0%; Table 2). Of these lesion sites, 63.1% were categorized as sun-exposed (face, neck, scalp, and upper extremities) and 36.9% as nonexposed (i.e., areas not particularly prone to frequent solar exposure; trunk and lower extremities).
Table 2. Site of biopsy.
| Location | Clinically diagnosed SK (n=271) | Biopsy-proven SK (n=206) |
|---|---|---|
| Face and neck | 101 (37.3) | 69 (33.5) |
| Scalp | 51 (18.8) | 43 (20.9) |
| Upper extremities | 19 (7.0) | 4 (1.9) |
| Lower extremities | 22 (8.1) | 23 (11.2) |
| Trunk | 78 (28.8) | 67 (32.5) |
Values are presented as frequency (%). SK: seborrheic keratosis.
3) Clinicopathological mismatch among clinically diagnosed SKs
There were 91 clinicopathological mismatch cases. The lesions that were most commonly clinically misdiagnosed as SK were warts (verruca vulgaris; 25 cases, 27.5%), followed by actinic keratosis (13 cases, 14.3%), compound nevi (6 cases, 6.6%), intradermal nevi (5 cases, 5.5%), lichenoid dermatitis (5 cases, 5.5%), basal cell carcinoma (4 cases, 4.4%), squamous cell carcinoma (2 cases, 2.2%), Bowen disease (3 cases, 3.3%), and miscellaneous (urticaria, eccrine hidradenoma, fungal infestation, prurigo nodularis, or psoriasiform dermatitis; 28 cases, 30.8%). Thus, premalignant and malignant entities represented 17.6% and 6.6% of the mismatch cases, respectively (Table 3).
Table 3. Skin biopsy cases that were clinically mistaken for seborrheic keratosis.
| Diagnosis | Frequency (n=91) |
|---|---|
| Verruca vulgaris/warts | 25 (27.5) |
| Actinic keratosis | 13 (14.3) |
| Compound nevi | 6 (6.6) |
| Intradermal nevi | 5 (5.5) |
| Lichenoid dermatitis | 5 (5.5) |
| Basal cell carcinoma | 4 (4.4) |
| Bowen disease | 3 (3.3) |
| Squamous cell carcinoma | 2 (2.2) |
| Miscellaneous* | 28 (30.8) |
Values are presented as frequency (%). The sum of the percentages does not equal 100% because of rounding. *Miscellaneous includes other cases (urticaria, eccrine hidradenoma, fungal infestation, prurigo nodularis, psoriasiform dermatitis).
The correlation between frequency of clinicopathological mismatch among clinically diagnosed SK patients and the location of the SK lesions was analyzed. The proportion of mismatch cases was significantly lower in nonexposed areas (26/100, 26.0%) than in the sun-exposed body sites (65/171, 38.0%; χ2, p=0.043).
Analysis of biopsy-proven SKs
Of the 206 biopsy-proven SK cases, 180 and 26 were initially clinically diagnosed as SK and something other than SK, respectively.
1) Demographic characteristics of patients with biopsyproven SKs
The age distribution was widely spread across all age groups; the age of the enrolled cases was 60.8±14.6 years (range, 19 to 98 years). There were 95 men and 111 women (men/women ratio, 1:1.17; Table 1). The mean age did not differ significantly with sex (p=0.127). The age distribution of the subjects is shown in Fig. 1B.
2) Biopsy site for biopsy-proven SKs
The most common biopsy sites for the biopsy-proven SKs were the face and neck (69 cases, 33.5%), followed by the trunk (67 cases, 32.5%), scalp (43 cases, 20.9%), lower extremities (23 cases, 11.2%), and upper extremities (4 cases, 1.9%; Table 2B). Of these lesion sites, 56.3% were categorized as sun-exposed and 43.7% as nonexposed.
3) Distribution of subtypes among biopsy-proven SKs
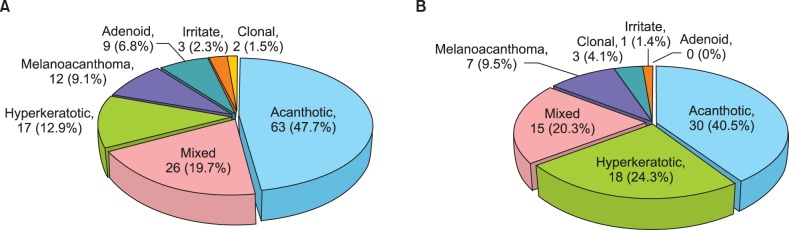
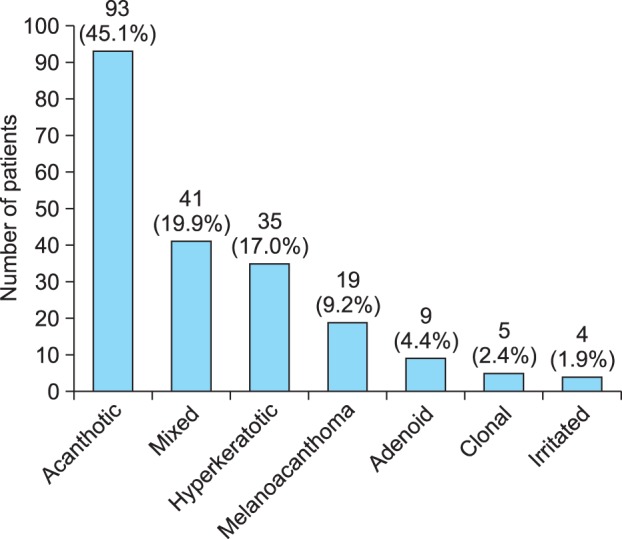
A thorough evaluation revealed that the most frequent histopathological subtype was the acanthotic type (93 cases, 45.1%; Fig. 2), followed by mixed (41 cases, 19.9%), hyperkeratotic (35 cases, 17.0%), melanoacanthoma (19 cases, 9.2%), adenoid (9 cases, 4.4%; Fig. 3), clonal (5 cases, 2.4%), and irritated (4 cases, 1.9%) types. The relative frequency of each subtype is given in Fig. 4.
Fig. 2. An acanthotic type of seborrheic keratosis, taken from the left postauricular region of an 86-year-old woman. The epidermis is greatly thickened. Horny invaginations appear as pseudohorn cysts (H&E, ×40).
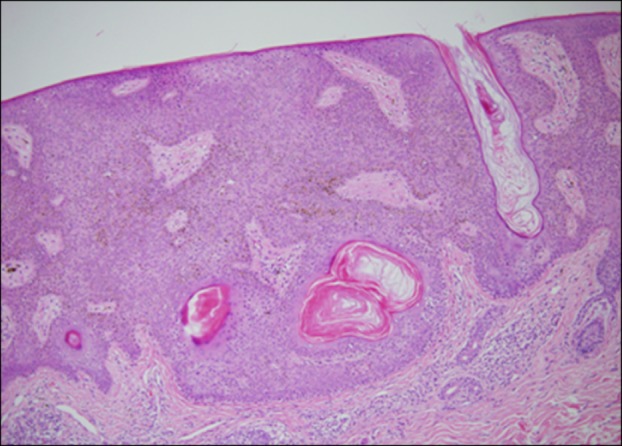
Fig. 3. An adenoid-type seborrheic keratosis taken from the forehead of a 73-year-old man. Numerous thin tracts of epidermal cells extend from the epidermis and branch and interweave within the dermis. Many of the tracts are composed of only a double row of basaloid cells (H&E, ×40).
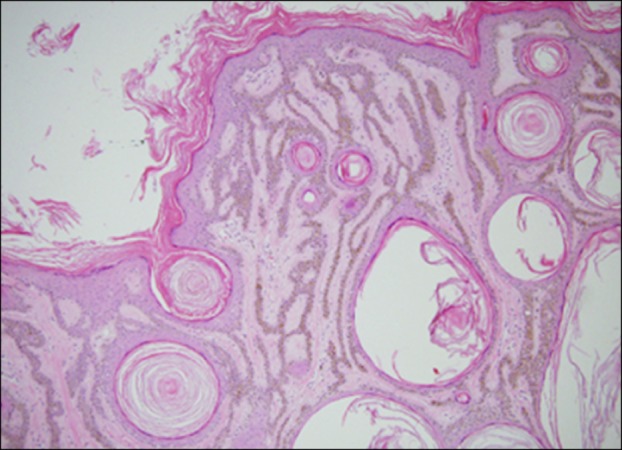
Fig. 4. Distribution of histopathological subtypes in the 206 biopsy-proven cases of seborrheic keratosis.
4) Distribution of subtypes according to sun exposure
The most common subtype of sun-exposed body sites was the acanthotic type, with 63 cases (47.7%), followed by the mixed type (26 cases, 19.7%), the hyperkeratotic type (17 cases, 12.9%), melanoacanthoma (12 cases, 9.1%), the adenoid type (9 cases; 6.8%), the irritated type (3 cases, 2.3%), and the clonal type (2 cases, 1.5%; Fig. 5A). The most common subtype of nonexposed body sites was the acanthotic type, with 30 cases (40.5%), followed by the hyperkeratotic type (18 cases; 24.3%), the mixed type (15 cases, 20.3%), melanoacanthoma (7 cases, 9.5%), the clonal type (3 cases, 4.1%), and the irritated type (1 case, 1.4%). The adenoid type was not observed in nonexposed areas (Fig. 5B). The adenoid type was significantly more frequent in sun-exposed than in nonexposed areas (p=0.028), whereas the hyperkeratotic type was significantly more frequent in nonexposed than in sun-exposed areas (p=0.036). The distributions of the other SK types did not differ significantly between sun-exposed and nonexposed areas (p>0.05).
Fig. 5. Distribution of histopathological subtypes in the (A) sun-exposed and (B) nonexposed sites.
5) Clinicopathological mismatch among biopsy-proven SKs
There were 26 clinicopathological mismatch cases among biopsy-proven SKs. The most common diagnosis that was mistaken for SK was the common wart (verucca vulgaris; 7 cases, 26.9%), followed by flat wart (verruca plana; 4 cases, 15.4%), basal cell carcinoma (3 cases, 11.5%), squamous cell carcinoma (2 cases, 7.7%), dysplastic nevus (2 cases, 7.7%), compound nevus (2 cases, 7.7%), actinic keratosis (1 case, 3.8%), Bowen disease (1 case, 3.8%), melanoma (1 case, 3.8%), condyloma acuminatum (1 case, 3.8%), intradermal nevus (1 case, 3.8%), and soft fibroma (1 case, 3.8%). Premalignant and malignant entities represented 23.1% and 7.6% of the mismatch cases, respectively (Table 4).
Table 4. Cases clinically mistaken for a lesion other than SK among biopsy-proven SK.
| Diagnosis | Frequency (n=26) |
|---|---|
| Verruca vulgaris/warts | 7 (26.9) |
| Verruca plana | 4 (15.4) |
| Basal cell carcinoma | 3 (11.5) |
| Squamous cell carcinoma | 2 (7.7) |
| Dysplastic nevi | 2 (7.7) |
| Compound nevi | 2 (7.7) |
| Miscellaneous* | 6 (23.1) |
Values are presented as frequency (%). *Miscellaneous includes various other cases (actinic keratosis, Bowen disease, melanoma, condyloma acuminatum, intradermal nevus, soft fibroma). SK: seborrheic keratosis.
DISCUSSION
This retrospective study analyzed clinical and histopathological features of 271 skin biopsy cases clinically diagnosed as SKs and subclassified 206 skin biopsy cases confirmed as SKs according to their histopathological characteristics.
The findings of this study show that clinically diagnosed and biopsy-proven SKs affect men and women with almost equal frequencies, in agreement with the findings of other studies3,9,10. The most affected decade of age, also as in other studies1,9, was the 60s, with both clinically diagnosed SKs and biopsy-proven SKs. When comparing affected ages among the studies1,9,11, it appears that the mean affected age has increased over time—52.2 years in the study of Ahn et al. (1980s)11 vs. 60.9 years in the present study. Although the etiology of SK is currently unclear, it has been suggested that exposure to sunlight leads to the development of SKs3,4. Thus, it may be that this apparent increase in SK-affected age over the past three decades is attributable to a reduction in the exposure to ultraviolet (UV) light due to the average amount of time spent indoors having also increased during this period. Additionally, longer life expectancies and more frequent hospital visits by older people than in the past may explain the increase in affected age.
As to the location of biopsy-proven SKs, sun-exposed body sites (i.e., face, scalp, neck, and upper extremities) were more frequently affected than nonexposed sites (i.e., trunk and lower extremities; 56.3% vs. 43.7%). Kwon et al.4 analyzed 303 male patients with SKs and reported that SKs were more prevalent on exposed sites than on partly exposed sites and that the mean number of SKs was higher on exposed areas than on partly exposed areas. However, Gill et al.10 investigated 170 Australian SK patients aged 15 to 30 years and found that SKs appeared more frequently on the trunk than on the limbs, head, and neck. They explained this contrasting finding as a reflection of the changing sunlight-exposure patterns of the younger community; truncal exposure is now almost as great as for traditional light-exposed areas10.
In the present study of 206 biopsy-proven SK patients, the most common subtype was the acanthotic type (45.1%), in agreement with most previous studies1,11. However, the unexpectedly high percentage (9.2%) of the melanoacanthoma variant differs from that reported in previous literature1,11. To our knowledge, the exact incidence or proportion of melanoacanthoma among the histopathological subtypes is unclear, at least according to large-scale studies. Only scattered reports exist on melanoacanthoma of the external ear and oral cavity12,13,14,15,16. In agreement with the present study, the findings of Simón et al.17 refuted claims that melanoacanthoma is extremely rare; the group used Fontana-Masson silver impregnation to stain 189 samples from cases of SK and observed that 28% (53 cases) fulfilled the diagnostic requirements for melanoacanthoma. Hence, it seems that salient proliferation of dendritic melanocytes is not rare in SK.
To the best of our knowledge, no previous studies have analyzed the histopathological subtypes of SK according to disease location. In our study, we analyzed histopathological subtype according to location, and the adenoid type was found to occur more commonly in sun-exposed sites. Thus, it appears that UV light plays a definite role in the development of at least the adenoid type of SK. Hafner et al.18 reported that UV-light-induced mutations of the gene encoding FGFR3 may contribute to the formation of SKs, particularly the adenoid subtype.
The association between frequency of clinicopathological mismatch among clinically diagnosed SK patients and SK lesion location was also evaluated in the present study. Premalignant and malignant entities together represented almost one quarter (24.2%) of the clinicopathological mismatch cases. The frequency of such mismatches was significantly higher for sun-exposed lesions than for nonexposed lesions (p=0.043); this finding is consistent with that of Park et al.9 Moreover, Maize and Snider19 investigated nonmelanoma skin cancers in association with SK and reported that all of the malignant lesions found in their series were associated with SKs situated on sun-exposed skin and were of the adenoid type. Therefore, we suggest that SKs situated on sun-exposed areas should be biopsied and thoroughly analyzed to exclude premalignant or malignant possibilities.
The main limitation of this retrospective study was that biopsy samples were not taken from all suspected SK lesions. Thus, exact information regarding the distribution of subtypes cannot be provided. Furthermore, this could represent a bias with respect to our report of the prevalent location of the disease because it is possible that more biopsy samples were taken from exposed areas than from nonexposed areas to rule out sunlight-associated skin cancer. Thus, larger-scale studies with more detailed analysis are needed.
In conclusion, the most frequent SK histopathological subtype found in this study was the acanthotic type, and melanoacanthoma was not an infrequent subtype. The adenoid type was more common in sun-exposed sites than in nonexposed sites. In addition, a high frequency of clinicopathological diagnosis mismatches was associated with SKs situated on sun-exposed areas. However, patients with SKs usually visit outpatient dermatology departments to demand instant removal of the lesions, often without biopsy. Thus, biopsy sampling of suspected SKs with a thorough histopathological examination of the entire specimen should be conducted, particularly for lesions situated on sun-exposed areas, to exclude other premalignant or malignant diseases.
ACKNOWLEDGMENT
This paper was supported by Konkuk University in 2016.
References
- 1.Lee GS, Ahn KJ, Kim JM, Lee ES. A histopathologic study of the seborrheic keratosis. Korean J Dermatol. 1992;30:76–80. [Google Scholar]
- 2.Elder DE, Elenitsas R, Johnson BL, Jr, Murphy GF, Xu X. Lever's histopathology of the skin. 10th ed. Philadelphia: Lippincott-Raven; 2009. pp. 795–798. [Google Scholar]
- 3.Yeatman JM, Kilkenny M, Marks R. The prevalence of seborrhoeic keratoses in an Australian population: does exposure to sunlight play a part in their frequency? Br J Dermatol. 1997;137:411–414. [PubMed] [Google Scholar]
- 4.Kwon OS, Hwang EJ, Bae JH, Park HE, Lee JC, Youn JI, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73–80. doi: 10.1034/j.1600-0781.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 5.Park JW, Kyung MS, Kim KS, Shin DH, Choi JS, Kim KH. An acantholytic variant of sevorrheic keratosis. Korean J Dermatol. 2000;38:705–707. [Google Scholar]
- 6.Kim HY, Kim HS, Cho EB, Park EJ, Kim KH, Kim KJ. A clinicohistopathological study on the lesion resembling seborrheic keratoses of the face. Korean J Dermatol. 2013;51:494–500. [Google Scholar]
- 7.Jeong YI, Lee WJ, Bak HN, Oh SH, Jung HJ, Chang SE, et al. Detection of human papilloma virus DNA in seborrheic keratosis of Korean skin. Ann Dermatol. 2007;19:99–105. [Google Scholar]
- 8.Marks R, Jolley D, McCormack C, Dorevitch AP. Who removes pigmented skin lesions? J Am Acad Dermatol. 1997;36:721–726. doi: 10.1016/s0190-9622(97)80324-6. [DOI] [PubMed] [Google Scholar]
- 9.Park S, Park H, Cho K. Clinical and histopathologic study of seborrheic keratosis. Korean J Dermatol. 2011;49:12–19. [Google Scholar]
- 10.Gill D, Dorevitch A, Marks R. The prevalence of seborrheic keratoses in people aged 15 to 30 years: is the term senile keratosis redundant? Arch Dermatol. 2000;136:759–762. doi: 10.1001/archderm.136.6.759. [DOI] [PubMed] [Google Scholar]
- 11.Ahn SK, Shin DH, Lee KG, Choi IJ. Seborrheic keratosis: A clinical and histopathological study. Korean J Pathol. 1986;20:484–490. [Google Scholar]
- 12.Cheng AG, Deubner H, Whipple ME. Melanoacanthoma of the external auditory canal: a case report and review of the literature. Am J Otolaryngol. 2007;28:433–435. doi: 10.1016/j.amjoto.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Carlos-Bregni R, Contreras E, Netto AC, Mosqueda-Taylor A, Vargas PA, Jorge J, et al. Oral melanoacanthoma and oral melanotic macule: a report of 8 cases, review of the literature, and immunohistochemical analysis. Med Oral Patol Oral Cir Bucal. 2007;12:E374–E379. [PubMed] [Google Scholar]
- 14.Andrews BT, Trask DK. Oral melanoacanthoma: a case report, a review of the literature, and a new treatment option. Ann Otol Rhinol Laryngol. 2005;114:677–680. doi: 10.1177/000348940511400904. [DOI] [PubMed] [Google Scholar]
- 15.Kihiczak GG, Centurión SA, Schwartz RA, Lambert WC. Giant cutaneous melanoacanthoma. Int J Dermatol. 2004;43:936–937. doi: 10.1111/j.1365-4632.2004.01848.x. [DOI] [PubMed] [Google Scholar]
- 16.Fornatora ML, Reich RF, Haber S, Solomon F, Freedman PD. Oral melanoacanthoma: a report of 10 cases, review of the literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25:12–15. doi: 10.1097/00000372-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Simón P, Requena L, Sánchez Yus E. How rare is melanoacanthoma? Arch Dermatol. 1991;127:583–584. [PubMed] [Google Scholar]
- 18.Hafner C, van Oers JM, Hartmann A, Landthaler M, Stoehr R, Blaszyk H, et al. High frequency of FGFR3 mutations in adenoid seborrheic keratoses. J Invest Dermatol. 2006;126:2404–2407. doi: 10.1038/sj.jid.5700422. [DOI] [PubMed] [Google Scholar]
- 19.Maize JC, Snider RL. Nonmelanoma skin cancers in association with seborrheic keratoses. Clinicopathologic correlations. Dermatol Surg. 1995;21:960–962. doi: 10.1111/j.1524-4725.1995.tb00533.x. [DOI] [PubMed] [Google Scholar]