Abstract
Background
Androgenetic alopecia (AGA) is the most commonly encountered baldness pattern in men. Epicardial fat tissue is found on the cardiac surface between the myocardium and visceral pericardium. Both AGA and epicardial fat thickness (EFT) are related to coronary artery disease, which is also reflected by an increase in carotid intima media thickness (CIMT).
Objective
The purpose of this study was to investigate the relation of AGA severity with EFT.
Methods
One hundred twenty-six male patients with AGA aged 18 to 55 years without histories of chronic disease were enrolled. Subjects were divided into three groups (mild, moderate, and severe) on the basis of the Hamilton baldness scale as modified by Norwood. Maximum EFT was measured at end-systole on the midventricular free wall of the right ventricle. CIMT was also recorded for all patients.
Results
The groups did not have statistically significant differences with respect to age, height, weight, body mass index, left ventricular ejection fraction, or left atrial diameter (p>0.05 for all comparisons), but the severe group had a higher EFT compared with the moderate (p<0.001; z score, -7.040) and mild groups (p<0.001; z score, -6.667). The moderate group also had higher EFT than the mild group (p<0.001; z score, -5.931). Mean CIMT value in the severe group was significantly higher compared with the value in the other groups.
Conclusion
The study showed that subjects in advanced stages of AGA had increased EFT, which was measured via echocardiography.
Keywords: Androgenetic alopecia, Coronary artery disease, Epicardial fat thickness
INTRODUCTION
Androgenetic alopecia (AGA), the most commonly encountered baldness pattern in men, is a hereditary loss of frontal and vertex scalp hair induced by the effect of androgenic hormones in an individual with the appropriate genetic tendency1. The condition develops from the third decade onward. The prevalence of any degree of AGA can be up to 80% in men during their lifetimes2. Although the pathophysiology has not been clearly documented, androgens particularly dihydrotestosterone (DHT) play a pivotal role in the development of male-pattern baldness. DHT is the strongest androgen in human blood and is also a main testosterone metabolite found in the skin.
AGA is inherited through a complex polygenic pattern3. In AGA, the hormonal and genetic background alters the growth cycle of the follicle and leads to a decrease in follicle diameter, with patchy inflammation around the vessel and the follicle4. There is growing evidence that indicates an association between male-type baldness and coronary artery disease (CAD)5. Although the mechanism in this relation has not been clarified totally, an increased serum DHT/testosterone ratio rather than elevated serum testosterone level has been implicated in the pathophysiology6,7,8,9.
Physiological doses of testosterone replacement have been shown to be beneficial by reducing adverse cardiovascular events in the case of testosterone deficiency, whereas supraphysiological doses are reported to induce adverse cardiovascular outcomes6,7. However, some of the aspects of the relation are still controversial10. In a study by Rebora8, patients with early-onset AGA before the age of 40 years had higher cardiovascular burdens and higher DHT/testosterone ratios than others had. Further studies are therefore needed to clarify that relation.
Epicardial fat tissue is found on the cardiac surface between the myocardium and the visceral pericardium11. Particular properties of adipocytes in epicardial fat tissue differ from those of adipocytes located in cutaneous and other visceral organs11,12. Paracrine and vasocrine release of adipokines and cytokines modulates the myocardium and coronary arteries and induces atherogenesis11,12,13. Epicardial fat thickness (EFT) can be measured by using echocardiography, computed tomography, or magnetic resonance imaging. Echocardiography is a simple, time-saving method with no adverse effects. Correlation between EFT and visceral obesity, metabolic syndrome, diabetes mellitus, and CAD is well established. Measurement of EFT is thus a practical tool for eliciting information about a subject's cardiovascular status14.
In terms of better understanding of clinical outcomes in terms of AGA, measurement of EFT in patients with different degrees of male-type baldness can be significant because both AGA and EFT more or less reflect cardiovascular status. This study was therefore designed to demonstrate the relation between AGA and EFT, which reflects a subject's cardiovascular risk.
MATERIALS AND METHODS
Study population
In this prospective study, subjects were enrolled from among male patients referred to the department of dermatology due to hair loss. One hundred and twenty six consecutive patients were eligible for inclusion in accordance with our predefined exclusion criteria of previously diagnosed coronary, cerebral, or peripheral arterial disease; presence of diabetes mellitus, hypertension, chronic kidney disease, congestive heart failure, left ventricular ejection fraction (LVEF) of less than 50%, or thyroid, hypophysis, or adrenal disorders; current or previous alopecia, and use of such drugs as androgen, antiandrogen, weight loss, or insulin-sensitizing drugs or glucocorticoids within the previous 3 months. Metabolic profile, age, height, and weight of each subject were recorded. Body mass index (BMI) was calculated for each subject. Ethical approval and informed consent from each patient were obtained for the study. The research was performed in accordance with the principles outlined in the Declaration of Helsinki and approved by the institutional ethics committee of Bozok University Medical School (IRB No: 05.05.2014/71).
Assessment of androgenetic alopecia
AGA was classified according to the Hamilton baldness scale as modified by Norwood15. Two trained physicians blinded to the echocardiographic measurements separately observed each subject's head from two perspectives (side and top), compared the subject's hair pattern with the Hamilton-Norwood baldness scale, and selected the best matching stage of the scale through consensus. Subjects were then classified into one of three groups to facilitate analysis. The mild group included subjects with AGA stages II and IIa; the moderate group, AGA stages III, IIIa, III vertex, IV, IVa, V, and Va; and the severe group, AGA stages VI and VII, according to the Hamilton-Norwood scale.
Measurement of epicardial fat thickness and carotid intima media thickness
Echocardiography examinations were performed on an ultrasound machine (ProSound Alpha 7, IPF-1701 model, 2009; Hitachi Aloka Medical, Ltd., Tokyo, Japan) with a 2.5-MHz transducer by a cardiologist blinded to the study. Standard two-dimensional measurements (left atrial [LA] diameter, LVEF) were obtained as recommended by the American Society of Echocardiography16.
Epicardial fat tissue was defined as a relatively echo-free space between the right ventricle and the visceral pericardium. Maximum EFT was measured at end systole on the midventricular free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the aortic annulus, used as an anatomic reference point for this aspect17. The means of three cardiac cycles were averaged for the analysis. EFT measurements were performed by a cardiologist blinded to the study. A thickness of 0.7 cm or more was used to define an abnormal level of EFT18.
To evaluate interobserver variability, 20 patients were randomly selected for echocardiographic examination, and EFT measurements were repeated after 1 week. Reproducibility of the measurement was highly significant for interobserver variability (intraclass correlation coefficient [ICC]=0.918, p<0.001).
Carotid Doppler ultrasound was performed with an Aloka ProSound Alpha 6 (Hitachi Aloka Medical America, Wallingford, CT, USA) device equipped with a 7.5-MHz linear array imaging probe. All measurements were performed by the same radiologist blinded to the patient's clinical status, with the patient lying supine, the head turned away from the side of interest, and the neck extended slightly. To maximize the lumen diameter, the transducer was located in the longitudinal plane. At a location of 1 cm proximal to the carotid bifurcation, the images were magnified to achieve higher resolution of detail. Carotid intima media thickness of the far wall was evaluated as the distance between the lumen-intima interface and the media-adventitia interface. Measurements were obtained from five contiguous sites at 1-mm intervals bilaterally, and the mean of all of a patient's measurements was used for statistical analysis. CIMT values greater than 1 mm were regarded as abnormal.
Statistical analyses
All statistical analyses were performed using SPSS ver. 15.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean±standard deviation, and categorical variables as frequencies (%). Except for height, hemoglobin, creatinine, total cholesterol, and low-density-lipoprotein cholesterol (LDL-C), the other continuous variables did not exhibit normal distribution according to the Kolmogorov-Smirnov test. Categorical variables were compared using the chi-square test. Pearson or Spearman simple correlation analyses were performed to determine associations between continuous parameters. Student's t-test, the Mann-Whitney U test, and the Kruskal-Wallis test were used to compare groups. A p-value of less than 0.05 was regarded as statistically significant.
RESULTS
One hundred twenty-six male patients were enrolled in the study. The mild group, with a mean age of 41±6 years, was composed of 31 subjects; the moderate group, with a mean age of 41±7 years, 65 subjects; and the severe group, with a mean age of 43±6 years, 30 subjects. The groups did not have statistically significant differences with respect to age, height, weight, BMI, LVEF, or LA diameter (p>0.05 for all comparisons), but the severe group had higher EFT values compared with the moderate (p<0.001; z score, -7.040) and mild (p<0.001; z score, -6.667) groups (Fig. 1). The moderate group also had a higher EFT than the mild group (p<0.001; z score, –5.931). Similarly, abnormal EFT was more prevalent in the severe group compared with the other groups (p<0.001). Other clinical and echocardiographic data are shown in Table 1. Correlation analysis revealed that EFT was statistically correlated with LA diameter (p<0.005, r=0.252), whereas there was no correlation with age, BMI or LVEF (p>0.05 for all; r values 0.105, 0.117, and -0.138, respectively).
Fig. 1. Comparison of the groups in terms of epicardial fat thickness. AGA: androgenetic alopecia.
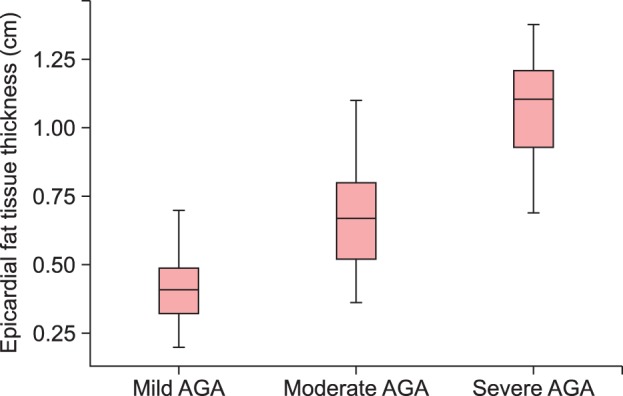
Table 1. Clinical and echocardiographic data for the groups.
| Variable | Mild group (n=31) | Moderate group (n=65) | Severe group (n=30) | p-value |
|---|---|---|---|---|
| Age (yr) | 42±6 | 41±7 | 43±6 | 0.302 |
| Height (cm) | 172±6 | 172±7 | 172±4 | 0.788 |
| Weight (kg) | 86±12 | 85±9 | 86±10 | 0.960 |
| BMI (kg/m2) | 29.2±4.2 | 28.6±2.8 | 29.3±3.1 | 0.746 |
| LVEF (%) | 64±2 | 63±3 | 63±3 | 0.088 |
| LA diameter (mm) | 36±1 | 36±2 | 37±2 | 0.060 |
| EFT thickness (cm) | 0.43±0.13 | 0.68±0.16 | 1.08±0.18 | <0.001 |
| EFT thickness ≥0.7 cm (%) | 2 (6.5) | 29 (44.6) | 29 (96.7) | <0.001 |
| CIMT (mm) | 0.60±0.13 | 0.75±0.15 | 1.02±0.13 | <0.001 |
| CIMT >1 mm | 0 (0) | 5 (7.7) | 16 (53.3) | <0.001 |
Values are presented as mean±standard deviation or number (%).
BMI: body mass index, LVEF: left ventricular ejection fraction, LA: left atrial, EFT: epicardial fat tissue, CIMT: carotid intima media thickness.
We determined no significant difference between the groups in terms of laboratory findings (Table 2). Parallel to those findings, EFT did not correlate significantly with fasting blood glucose, creatinine, total cholesterol, LDL-C, high-density-lipoprotein cholesterol, triglyceride, or thyroid-stimulating hormone (p>0.05 for all comparisons). The mean CIMT value for the severe group was significantly higher than for the mild (z score, -6.547; p<0.001) and moderate groups (z score, -6.554; p<0.001). Although we determined no significant difference between the mild and moderate groups with respect to frequency of abnormal CIMT (p=0.171), the severe group had a higher frequency of abnormal CIMT than both the mild (p<0.001) and moderate (p<0.001) groups. There was a significant correlation between EFT and CIMT values among all subjects with AGA (r=0.602; p<0.001).
Table 2. Laboratory findings for the groups.
| Variable | Mild group (n=31) | Moderate group (n=65) | Severe group (n=30) | p-value |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 15.4±1.1 | 15.4±1.3 | 14.9±1.5 | 0.228 |
| Glucose (mg/dl) | 102±10 | 101±16 | 102±13 | 0.455 |
| Creatinine (mg/dl) | 0.94±0.13 | 0.96±0.12 | 0.99±0.14 | 0.499 |
| Total cholesterol (mg/dl) | 196±37 | 205±44 | 206±38 | 0.498 |
| Triglyceride (mg/dl) | 193±72 | 204±108 | 201±77 | 0.842 |
| HDL-C (mg/dl) | 36±6 | 40±8 | 40±8 | 0.245 |
| LDL-C (mg/dl) | 129±35 | 155±31 | 131±28 | 0.593 |
| TSH (µIU/ml) | 1.48±1.15 | 1.36±0.73 | 1.28±0.86 | 0.775 |
Values are presented as mean±standard deviation.
HDL-C: high-density-lipoprotein cholesterol, LDL-C: low-density-lipoprotein cholesterol, TSH: thyroid-stimulating hormone.
DISCUSSION
AGA is defined as recession of the frontal hairline leading to balding of the scalp cortex. AGA is classified according to the Hamilton baldness scale as modified by Norwood15. There is growing evidence that indicates an association between male-type baldness and CAD5. Although the mechanism involved has not been completely clarified, an increased serum DHT/testosterone ratio rather than an elevated serum testosterone level is important in the pathophysiology6,7,8,9. Patients with early-onset AGA before the age of 40 years are reported to have a higher risk of adverse cardiovascular events8. Measurements of EFT, arterial stiffness, or carotid intima media thickness can thus be used along with other diagnostic tools to identify such high-risk patients who have AGA. In this study, we used echocardiographically measured EFT to assess the possible relation between AGA and asymptomatic atherosclerosis. Epicardial fat tissue is not a simple visceral fat; it has both protective and harmful effects on the heart. It protects vascular health via secretion of adiponectin and adrenomedullin while at the same time it leads to coronary atherosclerosis by acting as a source of several proinflammatory and proatherogenic cytokines12,17. The balance becomes impaired in such clinical states as obesity, diabetes, and insulin resistivity, which induce increments in EFT19,20. A similar two-way mechanism also applies in androgenetic activity, which modulates AGA6,7,8,9. There is insufficient information in the literature about a possible relationship between EFT and AGA. The aim of this study was therefore to investigate the relationship between EFT and AGA, which have similar cardiovascular outcomes.
Studies involving AGA usually classify subjects as either with or without AGA for the sake of easier statistical analysis while overlooking earlier stages of AGA. In our study, we graded subjects into three categories mild, moderate, or severe according to the Hamilton-Norwood scale. The categorizations also provided us with data on subjects in the early stages of AGA. The severe group had a higher EFT compared with the moderate (p<0.001) and mild (p<0.001) groups, and the moderate group had a statistically higher EFT than the mild group (p<0.001). Lipid profile, age, and BMI values were similar across the groups. Those findings indicate a strong correlation between AGA status and EFT.
EFT of 0.7 cm or more is considered abnormal and indicates subclinical atherosclerosis18. In our study, the severe group had a higher prevalence of abnormal EFT than the moderate group (97% vs. 47%, p<0.001) and the mild group (97% vs. 7%, p<0.001), which implies that subjects with severe AGA have a tendency toward abnormal EFT. Therefore, subjects with severe AGA should be evaluated for possible cardiovascular disease due to atherosclerosis.
EFT measurement via echocardiography is practical and reproducible. Echocardiographic measurement of EFT on the right ventricle free wall was well correlated with EFT values measured via magnetic resonance imaging (p<0.001). Excellent interobserver agreement on EFT measurement has been reported, with ICCs of 0.93 to 0.9817. Twenty subjects in our study were randomly selected, and repeat echocardiographic measurements of EFT were performed. ICC was 0.94 (p<0.001). The result was consistent with good reliability.
Subclinical atherosclerosis involves all peripheral vasculature and can be detected by measuring CIMT. In our study, to obtain different indicators of subclinical atherosclerosis rather than EFT, a radiologist blinded to patients' data measured CIMT values. Thus, we gained the opportunity to compare subjects' EFT and CIMT values with AGA. In the analysis, there was a significant correlation between EFT and CIMT values among subjects with AGA (r=0.602, p<0.001). The relationship between AGA and an increase in CIMT has been documented in studies, and we also found that subjects with severe AGA had higher CIMT values compared with subjects with moderate or mild AGA21.
In conclusion, this study showed that subjects in advanced stages of AGA had higher echocardiographically measured EFT and higher CIMT values. Because both parameters indicate subclinical atherosclerosis, it may be suggested that subjects in advanced stages of AGA with increased EFT and/or CIMT values should be further evaluated for possible cardiovascular disease.
Footnotes
This study was presented as a poster at the 25th National Dermatology Congress.
References
- 1.Stough D, Stenn K, Haber R, Parsley WM, Vogel JE, Whiting DA, et al. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clin Proc. 2005;80:1316–1322. doi: 10.4065/80.10.1316. [DOI] [PubMed] [Google Scholar]
- 2.Piraccini BM, Alessandrini A. Androgenetic alopecia. G Ital Dermatol Venereol. 2014;149:15–24. [PubMed] [Google Scholar]
- 3.Hogan DJ, Chamberlain M. Male pattern baldness. South Med J. 2000;93:657–662. [PubMed] [Google Scholar]
- 4.Agac MT, Bektas H, Korkmaz L, Cetin M, Erkan H, Gurbak I, et al. Androgenetic alopecia is associated with increased arterial stiffness in asymptomatic young adults. J Eur Acad Dermatol Venereol. 2015;29:26–30. doi: 10.1111/jdv.12424. [DOI] [PubMed] [Google Scholar]
- 5.Sharma KH, Jindal A. Association between androgenetic alopecia and coronary artery disease in young male patients. Int J Trichology. 2014;6:5–7. doi: 10.4103/0974-7753.136747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traish AM. Adverse health effects of testosterone deficiency (TD) in men. Steroids. 2014;88:106–116. doi: 10.1016/j.steroids.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 8.Rebora A. Baldness and coronary artery disease: the dermatologic point of view of a controversial issue. Arch Dermatol. 2001;137:943–947. [PubMed] [Google Scholar]
- 9.Bolduc C, Shapiro J. Management of androgenetic alopecia. Am J Clin Dermatol. 2000;1:151–158. doi: 10.2165/00128071-200001030-00002. [DOI] [PubMed] [Google Scholar]
- 10.Grober ED. Testosterone deficiency and replacement: Myths and realities. Can Urol Assoc J. 2014;8(7-8 Suppl 5):S145–S147. doi: 10.5489/cuaj.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Şengül C, Özveren O. Epicardial adipose tissue: a review of physiology, pathophysiology, and clinical applications. Anadolu Kardiyol Derg. 2013;13:261–265. doi: 10.5152/akd.2013.075. [DOI] [PubMed] [Google Scholar]
- 12.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 13.Yudkin JS, Eringa E, Stehouwer CD. "Vasocrine" signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 14.Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol. 2012;110:534–538. doi: 10.1016/j.amjcard.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975;68:1359–1365. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1319. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Nabati M, Saffar N, Yazdani J, Parsaee MS. Relationship between epicardial fat measured by echocardiography and coronary atherosclerosis: a single-blind historical cohort study. Echocardiography. 2013;30:505–511. doi: 10.1111/echo.12083. [DOI] [PubMed] [Google Scholar]
- 19.Mohar DS, Salcedo J, Hoang KC, Kumar S, Saremi F, Erande AS, et al. Epicardial adipose tissue volume as a marker of coronary artery disease severity in patients with diabetes independent of coronary artery calcium: findings from the CTRAD study. Diabetes Res Clin Pract. 2014;106:228–235. doi: 10.1016/j.diabres.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 21.Dogramaci AC, Balci DD, Balci A, Karazincir S, Savas N, Topaloglu C, et al. Is androgenetic alopecia a risk for atherosclerosis? J Eur Acad Dermatol Venereol. 2009;23:673–677. doi: 10.1111/j.1468-3083.2009.03137.x. [DOI] [PubMed] [Google Scholar]


