Abstract
Cellular and/or tissue-based products (CTPs) are emerging treatment options for chronic non-healing wounds. Dehydrated amniotic membrane allograft (DAMA) was used in 7 patients whose wounds had not responded adequately to standard and adjuvant therapies; four VLUs, 2 surgical wounds, and 1 DFU. Patients had multiple comorbidities, including 2 with autoimmune disorders (CREST syndrome and systemic lupus erythematosus). Patients received 3–8 applications of DAMA at weekly to biweekly intervals (average, 5.4 applications). Complete wound healing was observed in 6 of 7 patients during study period, with an average time to closure of 7.9 weeks. Closure was achieved in 3 of 7 patients after 3 DAMA applications. In the patient with CREST syndrome who did not completely close, DAMA reduced the area and volume by nearly 50% and later went on to closure. These cases suggest that DAMA is a viable option for recalcitrant DFUs, VLUs, and surgical wounds.
Keywords: Allograft, Amniotic membrane, Biologic tissue-based products, Leg ulcer, Wound healing
Introduction
The prevalence of chronic skin ulcers of the lower limbs is approximately 1% in industrialized nations.1 Motor, sensory, and autonomic neuropathy can contribute to ulcer formation, and advanced age, underlying disease, presence of comorbidities, poor healing capacity, and poor treatment compliance can interfere with wound healing. Chronic leg ulcers can persist for years and tend to recur. A survey of 600 patients with chronic leg ulcers showed that 20% were not healed after 2 years despite treatment, and two thirds of patients had experienced recurring episodes of ulceration.2 Leg ulcers associated with underlying disease include those related to poorly controlled diabetes (diabetic foot ulcers [DFUs]) and those resulting from advanced chronic venous insufficiency (venous leg ulcers [VLUs]). Chronic leg wounds can also result from surgical procedures, most typically in the setting of underlying medical conditions such as diabetes or peripheral vascular disease that predispose patients to poor healing. Comorbidities that may contribute to poor wound healing include hypertension, obesity, diabetes, and dyslipidemia.3, 4
Non-healing DFUs may be associated with serious medical consequences such as infection, limb amputation, increased risk of myocardial infarction, and increased risk of all-cause mortality.5, 6 Chronic VLUs are susceptible to microbial infections, which may delay healing and result in complications such as cellulitis and sepsis.7 Patients with chronic ulcers report effects on quality of life including social isolation, depression, negative self-image, and decreased mobility and productivity.8 Treatment of ulcers is costly, especially when healing is prolonged and comorbidities exist.9 Therefore, advanced modalities that may shorten the time necessary for healing are needed.
Standard therapy for VLUs includes compression therapy, control of bioburden, debridement, and wound moisture balance,10, 11 while DFU treatment includes offloading, infection control, debridement, dressings, and often surgery.12 If ulcers do not heal with standard treatment, “advanced wound care” therapies including hyperbaric oxygen therapy (HBOT), negative-pressure wound therapy (NPWT), topical agents, and cellular and/or tissue-based products (CTPs) may be used.11, 12
Human amniotic CTPs are non-immunogenic and have anti-inflammatory effects, with beneficial properties in wound healing related to the release of growth factors and cytokines that stimulate ulcer healing in the wound bed.13, 14 Recent advances in human amniotic membrane tissue sterilization and product stability have led to the use of amniotic CTPs for treating chronic wounds. Dehydrated amniotic membrane allograft (DAMA; Amnioexcel®; Derma Sciences, Inc., Princeton, NJ) is a CTP with an intact extracellular matrix to support skin repair, reconstruction, and replacement.15 It is procured from consenting women during live births via planned cesarean section and undergoes sterilization and proprietary DryFlex® processing (BioD LLC, Memphis, TN) to maintain inherent extracellular matrix components, growth factors such as fibroblast growth factor, insulin-like growth factor and vascular endothelial growth factor, and cytokines. DAMA is minimally manipulated during processing to retain the structure and components of the tissue.16 This retrospective case series illustrates the use of DAMA in patients with chronic leg wounds and multiple severe comorbidities.
Materials and Methods
Treatment Setting and Patient Selection
Patients with chronic wounds of the lower extremity were treated with DAMA at the Mercy Hospital Outpatient Wound Care and Hyperbaric Medicine Center, Oklahoma City, OK, between May and October 2014. Criteria for DAMA application included lack of progress toward wound healing despite standard treatments and adjuvant therapies as judged by the physician based on clinical characteristics such as wound bed appearance, wound margin status, changes in wound size, and response to previous treatment modalities. A minimum of 4 weeks of unsatisfactory progress was utilized in each case. The case series protocol was approved by the institutional review board committee and written consent was obtained from patients permitting the use of health information and photographs.
Study Procedures and Analyses
Baseline demographics, history, and wound characteristics were obtained from patients' charts. Medical history included wound etiology and duration, comorbidities, and prior treatment. Preferred method of wound bed preparation prior to DAMA application was sharp debridement where indicated and confirmation that wounds were free of clinical signs of infection. Under sterile conditions, the DAMA was removed from its packaging, trimmed to fit the wound with an approximate 1-mm overlap of wound margins, placed in the wound, and allowed to self-adhere. A saline-moistened cotton swab was then used to remove any air bubbles underlying the DAMA and to ensure close contact with the wound bed; the DAMA was covered with a non-adherent silicone dressing and secured with retention tape. A bolster dressing was then applied to secure the DAMA into place. Patients were given instructions for wound management in accordance with standard of care (SOC), including offloading for diabetic wounds. Methods of offloading varied. For patients with VLU, compression bandages were used throughout the course of therapy with no change in compression level.
Follow-up visits occurred weekly. Patients were evaluated regarding the need for additional DAMA applications based on the investigator's judgment of benefit. If deemed medically necessary, the DAMA was reapplied either weekly or biweekly and wound dressings were changed. In those instances where biweekly application were chosen, standard of care dressings (i.e., compression dressings with moisture control for VLUs and dry, sterile dressings with appropriate offloading for DFUs) were applied and the wound observed for continued response. Patients were evaluated and photographed every week after the first DAMA application until wound closure. Overall wound appearance, wound area (calculated as length × width), wound volume (calculated as length × width × depth), presence and degree of granulation, and need for any additional wound care strategies were documented at each visit. Wound closure was defined as an area and volume of zero. Standard descriptive statistics, including means, percentages, and ranges were calculated for pooled data.
Results
Among the 7 patients included in this series, mean age was 64 years (range, 55–97) (Table 1). Two patients had surgical wounds, 4 patients had VLUs, and 1 patient had a DFU. All patients presented with multiple comorbidities. Four patients had hypertension and 6 had dyslipidemia. Autoimmune disease was present in 2 patients and included systemic lupus erythematosus (SLE; n = 1) and CREST syndrome (n = 1). All patients received prior treatment, including debridement with topical collagenase (n = 3), NPWT (n = 2), offloading and topical antibiotics (n = 1), and multiple applications of another CTP along with multiple rounds of HBOT (n = 1). Mean and median wound durations prior to DAMA application were 27.3 weeks and 19.4 weeks, respectively.
Table 1.
Patient Characteristics and Baseline Wound Characteristics.
| Characteristic | |
|---|---|
| No. of patients | 7 |
| Male, n | 3 |
| Age, mean (range), years | 64.2 (55–97) |
| Wound type, n | |
| VLU | 4 |
| Surgical | 2 |
| DFU | 1 |
| Mean (range) baseline wound size | |
| Area, cm2 | 7.6 (0.9–19.2) |
| Volume, cm3 | 0.74 (0.1–1.9) |
| Mean (range) wound duration, weeks | 27.3 (5.3–104.0) |
DFU, diabetic foot ulcer; VLU, venous leg ulcer.
Patients received 3–8 applications of DAMA at weekly to biweekly intervals (average, 5.4 applications). Mean and median times between applications were 8.0 and 6.5 days, respectively. All but 1 patient showed wound size reduction after 1 DAMA treatment. Six of the 7 patients experienced wound closure (86%), with 3 patients achieving closure after 3 DAMA applications (Table 2). Among the 6 patients who experienced wound closure, the mean time to closure was 7.9 weeks (range: 4.4–15.4). For the patient who did not experience wound closure, the area and volume of the wound were reduced from baseline by 44.6% and 44.3%, respectively, after 8 DAMA treatments and a total of 14 weeks following the initial application.
Table 2.
Summary of Cases.
| Case no. | Age (years)/gender | Comorbidities | Wound type/location | Baselinea wound area (cm2)/volume (cm3) | Wound duration prior to DAMA (weeks) | No. of DAMA applications | Time to closure (weeks) |
|---|---|---|---|---|---|---|---|
| Included as case vignettes | |||||||
| 1 | 97/F | AFib, dyslipidemia, HTN, CVI, hypothyroidism, CAD, PVD, skin cancer | VLU/anterior lower leg | 3.8/1.1 | 7.5 | 3 | 6.7 |
| 2 | 56/M | Dyslipidemia, HTN | VLU/medial lower left leg | 16.8/1.7 | 19.4 | 8 | 15.4 |
| 3 | 57/F | CREST syndrome, RA | Surgical/lateral over distal fibula, right ankle | 3.5/0.4 | 25.1 | 8 | NA |
| 4 | 56/F | SLE, dyslipidemia, PUD, osteoporosis | Surgical/anterior right knee | 1.7/0.2 | 24.5 | 3 | 4.4 |
| Not included as case vignettes | |||||||
| 5 | 55/M | Diabetes (poorly controlled), HTN, dyslipidemia | VLU/lateral left leg | 19.2/1.9 | 5.3 | 3 | 5.0 |
| 6 | 63/M | Diabetes, chronic kidney disease (stage 3), dyslipidemia | DFU/left great toe | 0.9/0.1 | 5.3 | 6 | 6.3 |
| 7 | 66/F | Raynaud's disease, HTN, dyslipidemia | VLU/lateral, distal right leg | 7.4/0.7 | 104 | 7 | 9.9 |
AFib, atrial fibrillation; CAD, coronary artery disease; CVI, chronic venous insufficiency; DFU, diabetic foot ulcer; HTN, hypertension; NA, not applicable; PUD, peptic ulcer disease; PVD, peripheral vascular disease; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; VLU, venous leg ulcer.
Baseline was at first application of DAMA.
Representative Case Studies
Detailed information is provided on the following 4 cases of interest.
Case 1
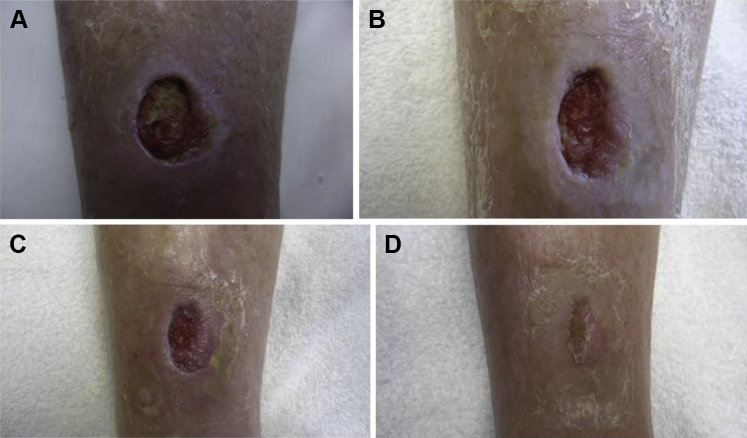
Patient 1, a 97-year-old female, presented in April 2014 with a VLU on the lower anterior aspect of the right leg that developed following hematoma from a traumatic injury. At initial presentation and following hematoma evacuation, the wound area and volume were 5.75 cm2 and 2.88 cm3, respectively. Comorbidities included dyslipidemia, hypertension, chronic venous insufficiency, and peripheral vascular disease as confirmed by arterial Doppler ultrasound. Previous treatment included periodic sharp debridement, collagenase ointment, and level-2 compression. After – multiple follow-up visits over a 2 month period and initial improvement, the wound area and volume stalled at 3.75 cm2 and 1.1 cm3, respectively. DAMA treatment was initiated and was reapplied weekly. After a small improvement in the first week of treatment, substantial reductions from baseline in area (39%) and volume (59%) were observed after the second DAMA application. After the third DAMA application, the patient was followed until closure, which occurred 6.7 weeks after the first DAMA application. Compression was continued throughout treatment (see Fig. 1).
Figure 1.
A 97-year-old female with a right anterior lower leg venous leg ulcer (Patient 1). (A) Prior to first dehydrated amniotic membrane allograft (DAMA) application. (B) 2 weeks after first DAMA application. (C) 4 weeks after first DAMA application. (D) 7 weeks after first DAMA application (closure).
Case 2
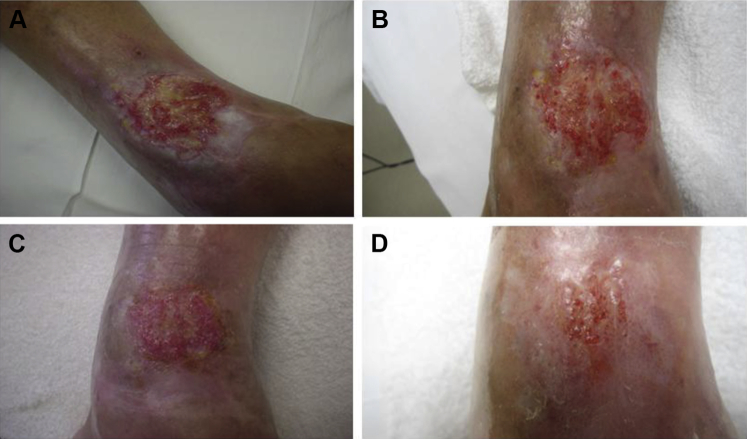
Patient 2, a 56-year-old male, presented in February 2014 with a traumatic VLU involving the medial lower aspect of the left leg of greater than 3 months duration and an initial area and volume of 11.55 cm2 and 1.16 cm3, respectively. The VLU developed due to new trauma at a site of scarring and chronic venous insufficiency following a motorcycle accident as a youth. Comorbidities included dyslipidemia and hypertension. Previous treatment included topical antibiotic ointment. A multilevel transcutaneous oxygen tension study confirmed moderate local tissue hypoxia of the periwound area due to scarring. A wound culture was positive for colonization with Pseudomonas aeruginosa and Morganella morganii. Treatment with appropriate culture-guided antibiotics was initiated, and repeat wound culture performed 2 weeks later confirmed eradication. The wound had not improved after 8 weeks of standard of care treatment including collagenase, class I compression, and eradication of bioburden. DAMA was initiated 8 weeks after initial presentation. The wound area and volume were 16.8 cm2 and 1.7 cm3, respectively at the first DAMA application. The wound immediately began to show signs of proliferation after the first DAMA application. Thereafter progress toward healing plateaued multiple times following periods of improvement. DAMA was applied 8 times at weekly intervals. At the final DAMA application, wound area and volume had decreased to 2.3 cm2 and 0.2 cm3, respectively. Foam dressings and level-2 compression were resumed. The wound closed 6.5 weeks thereafter (15.5 weeks from initial DAMA application) (see Fig. 2).
Figure 2.
A 56-year-old male with a left medial lower leg venous leg ulcer (Patient 2). (A) Prior to first dehydrated amniotic membrane allograft (DAMA) application. (B) 2 weeks after first DAMA application. (C) 4 weeks after first DAMA application. (D) 15 weeks after first DAMA application (closure).
Case 3
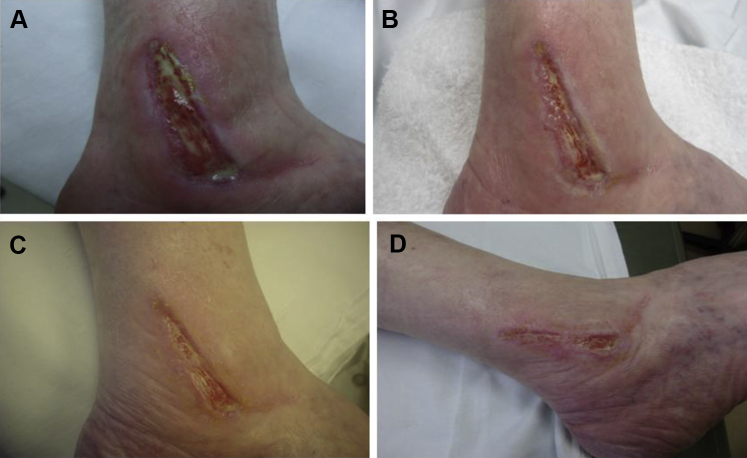
Patient 3, a 57-year-old female, presented in January 2014 with a surgical wound on the lateral aspect of the right ankle over the distal fibula with exposed tendon. The patient had undergone repair of the peroneal brevis tendon, peroneus longus tendon, and primary repair of the anterior talofibular ligament of the right ankle earlier that month. At initial presentation, the area and volume of the wound were 27.0 cm2 and 2.7 cm3, respectively. The patient had comorbidities of CREST syndrome and rheumatoid arthritis (RA) and was receiving rituximab IV injections. She had received 8 dehydrated human amnion/chorion CTP treatments and 40 HBOT treatments under the care of a podiatrist prior to being referred for further treatment 5 months after surgery. Despite substantial initial progress, wound healing again stalled despite standard of care treatment with foam dressings. DAMA was initiated approximately 8 weeks after the final previous CTP placement and 6 months after initial presentation; the wound area and volume were 3.5 cm2 and 0.4 cm3, respectively at first DAMA application. After 8 weekly DAMA treatments, the wound had not closed completely; however, the wound area had decreased by 45%. Wound healing continued with ongoing evidence of proliferation over the ensuing month with SOC. The wound area and volume were 0.4 cm2 and 0.04 cm3, respectively, by the end of the reporting period, 14 weeks after the initial DAMA treatment. The patient went on to achieve successful wound resolution within 6 weeks of the conclusion of the study (see Fig. 3).
Figure 3.
A 57-year-old female with a right lateral ankle surgical wound over the distal fibula (Patient 3). (A) Prior to first dehydrated amniotic membrane allograft (DAMA) application. (B) 4 weeks after first DAMA application. (C) 6 weeks after first DAMA application. (D) 8 weeks after first DAMA application.
Case 4
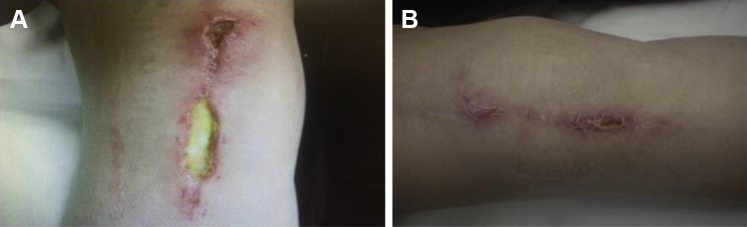
Patient 4, a 56-year-old female, presented in April 2014 with a non-healing surgical wound on the anterior aspect of the right knee following a total knee replacement. Three weeks after undergoing surgery, the patient fell on the incision site, exposing the patellar tendon and exacerbating the severity of the wound. The wound area and volume at initial presentation were 5.2 cm2 and 1.0 cm3, respectively, with areas of tendon still exposed. The patient had multiple comorbidities, including dyslipidemia and SLE. She was receiving chronic steroid treatment, methotrexate and adalimumab for SLE. Initial wound treatments, including NPWT and foam dressing containing silver, resulted in granulation of the wound bed over the tendon and decrease in wound area by greater than 60%. However, after 2 months of continuing these treatments, no additional progress was observed. DAMA treatment was begun, at which time the wound area and volume were 1.7 cm2 and 0.2 cm3, respectively. Secondary collagen dressings, adhesive dressings, Steri-Strips® (3M, Oakdale, MN), and gauze/Hypafix® tape (Smith & Nephew, London, UK) were used with the first and second DAMA applications. With weekly DAMA applications, consistent reductions in wound area and volume were observed. After the second DAMA application, area and volume of the wound had improved by 40% and 41%, respectively. After the third weekly DAMA application, the wound continued to improve, with closure achieved 4.4 weeks after the initial DAMA treatment (see Fig. 4).
Figure 4.
A 56-year-old female with an anterior right knee surgical wound (Patient 4). (A) Prior to first dehydrated amniotic membrane allograft (DAMA) application. (B) 4 weeks after first DAMA application (closure).
Discussion
This case series of patients presenting with chronic non-healing wounds of various etiologies and various comorbidities for whom previous SOC and other wound treatments were unsuccessful demonstrated that wound closure occurred in 6 of 7 patients after DAMA treatment, with improvement in the patient who did not achieve wound closure. The average time to closure was 7.9 weeks. All patients treated with DAMA experienced reduction in wound size and, for most patients, improvement was noted after only 1 DAMA treatment.
An important aspect of this case series is the inclusion of patients with numerous severe comorbidities. Patients with comorbidities are often excluded from controlled clinical trials to simplify patient selection and interpretation of findings.17 The 7 patients in this case series had multiple comorbidities, of which 2 were autoimmune related. Autoimmune disorders such as CREST syndrome and SLE have been shown to contribute to the delayed healing of ulcers. In a chart review of 340 patients with chronic wounds, 23% of patients presented with autoimmune disease including rheumatoid arthritis, SLE, and CREST syndrome. In these patients, wounds were larger, and though not statistically significant, they appeared to take longer to heal (mean 14.6 vs 10.3 months, P = 0.07), and were less responsive to some treatments compared with patients without autoimmune disorders.18 Chronic use of treatments for autoimmune disease, such as systemic steroids (as seen in case 4), may also contribute to impaired wound healing.19
The results of this case series support findings from 2 previous case studies in which DAMA was used to treat chronic DFUs.20, 21 Similar to the present results, these case studies reported healing times ranging from approximately 3–8 weeks. The results reported herein are also similar to findings from case series of other human amniotic CTPs. A case series of human amniotic CTP to treat various chronic wounds that failed to heal within 4 weeks of SOC showed closure with 1–3 applications in 3–17 weeks.22 In another case series of patients with leg ulcers of multiple etiologies that failed to heal within 4 weeks of SOC treatment, the mean time to wound closure after human amniotic CTP treatment was approximately 8.5 weeks.23 The time to wound closure in our case series was similar despite the presence of multiple comorbidities in our patients.
As with all case series reports, inherent limitations affect interpretation. Data are reported from a single treatment center, which may result in selection bias and limit generalizability to a wider population. Additionally, the lack of a control group limits the ability to draw conclusions about the effectiveness of one treatment compared with another, although the present case series provides evidence of the effectiveness of DAMA treatment in patients for whom conventional therapies were ineffective.
Although direct comparisons between case studies and clinical trials can be problematic, data on the effectiveness and safety of CTPs derived from non-amniotic and amniotic sources may provide additional information for evaluation of the use of these products. CTPs derived from various non-amniotic tissue and cell culture sources have demonstrated efficacy versus SOC alone in treating chronic wounds.24, 25, 26 In these studies, healing rates vary substantially (30%–76% at 12 weeks), possibly due to different study designs, product characteristics, baseline wound size, and duration and frequency of application. In a prospective, single-blind, randomized, controlled study, 245 patients with DFUs of at least 2 weeks' duration were randomized to receive a cultured human dermal fibroblast–derived CTP treatment or conventional therapy.24 In the CTP group, which received up to 8 weekly applications, there was a significantly greater proportion of healed ulcers (defined as full epithelialization with no drainage) compared with the control group (30% vs 18%; P = 0.023). Although details of time to healing were not described, the authors reported that wounds treated with CTP also had a significantly faster time to complete wound closure compared with those in the control group (P = 0.04). Another randomized study investigating a cultured bilayered skin–equivalent CTP compared with SOC alone enrolled 208 patients with DFUs of at least 2 weeks' duration.25 During the first 4 weeks of treatment, the CTP was applied weekly and patients were allowed to receive up to 5 applications. At week 12, 56% of those treated with CTP compared with 38% treated with SOC alone experienced wound closure, defined as full wound epithelialization without drainage (P = 0.0042). Median time to closure was 65 days for CTP compared with 90 days for SOC alone (P = 0.0026). The average number of CTP applications per patient was 3.9. A retrospective study assessed the efficacy of a CTP derived from placental tissue in 66 patients with chronic wounds that had failed to heal after 4 or more weeks.26 After 12 weeks of CTP treatment, 76% of the wounds had healed (68% of VLUs and 85% of DFUs). Average time to closure, defined as 100% re-epithelialization and no evidence of drainage, was 5.8 (±2.5) weeks, and among patients with wounds that healed, the average number of applications was 3.2.
Recent studies of human amniotic tissue-derived CTPs demonstrate efficacy compared with SOC. In a randomized single-center study (N = 25) investigating treatment with human amniotic CTP (applied every 2 weeks; maximum of 6 applications) plus SOC compared with SOC alone in patients with DFUs of at least 4 weeks' duration, wound size was reduced by 97% in the CTP group compared with 32% in patient treated with SOC alone after 4 weeks (P < 0.001).27 At 6 weeks, CTP-treated wounds were reduced in size by 98% compared with a 2% increase in size with SOC alone (P < 0.001). Overall healing rates (defined as complete epithelialization) at 4 and 6 weeks, respectively, were 77% and 92% with CTP compared with 0% and 8% with SOC (P < 0.001). In a retrospective crossover study (N = 11) assessing a dehydrated human amniotic membrane CTP for the treatment of DFUs that failed to heal with SOC for 6 or more weeks, CTP was applied every 2 weeks for up to 6 applications. At week 12, 91% of wounds were healed.28 Mean time to complete healing (defined as complete epithelialization) was 4.2 weeks. After 4 weeks (2 CTP applications), wounds treated with CTP decreased in size by 86% compared with 27% for those treated with SOC (P < 0.001).
Several practical questions regarding the optimal use of DAMA warrant future evaluation through clinical experience and studies. In many CTP clinical trials and case series, wound durations are variable and relatively short; some studies required wound duration of at least 2 weeks while others required wound durations of at least 4 or 6 weeks. On average, wounds in our case series had been present for 2–7 months prior to the first DAMA treatment and had been treated unsuccessfully with various products and treatment modalities. It is unknown whether DAMA would result in faster and more complete wound healing in patients who have wounds of durations shorter than those in this series. The maximum number of CTP applications permitted and the optimal interval of application also vary among clinical studies and case series. Additional study is needed regarding the optimal interval and duration of DAMA treatment. Factors that could impact the number and frequency of DAMA applications include patient and ulcer characteristics and financial considerations. Treatment should be tailored to the patient, particularly when multiple comorbidities are present that may slow the healing process.
Conclusions
The successful outcomes with DAMA treatment in this case series of patients with chronic non-healing wounds and multiple comorbidities are encouraging. The challenging nature of these wounds and the substantial negative impact on quality of life and functioning emphasize the importance of identifying effective treatments that result in timely wound resolution. Although additional data are needed to fully assess effectiveness, these cases suggest that DAMA may be a viable option for treating chronic DFUs, VLUs, and surgical wounds in patients with comorbidities who have not responded to traditional therapy.
Acknowledgments
Medical writing assistance was provided by Michael Pucci, PhD, CMPP, of Peloton Advantage and Emily H. Seidman, MSc, CMPP, and funded by Derma Sciences, Inc., Princeton, NJ.
References
- 1.Graham I.D., Harrison M.B., Nelson E.A., Lorimer K., Fisher A. Prevalence of lower-limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care. 2003;16:305–316. doi: 10.1097/00129334-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Callam M.J., Harper D.R., Dale J.J., Ruckley C.V. Chronic ulcer of the leg: clinical history. Br Med J (Clin Res Ed) 1987;294:1389–1391. doi: 10.1136/bmj.294.6584.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jockenhofer F., Gollnick H., Herberger K. Aetiology, comorbidities and cofactors of chronic leg ulcers: retrospective evaluation of 1000 patients from 10 specialised dermatological wound care centers in Germany. Int Wound J. 2014 doi: 10.1111/iwj.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hostetler S.G., Xiang H., Gupta S., Sen C., Gordillo G.M. Discharge patterns of injury-related hospitalizations with an acute wound in the United States. Wounds. 2006;18:340–351. [Google Scholar]
- 5.Alexiadou K., Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3:4. doi: 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownrigg J.R., Davey J., Holt P.J. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia. 2012;55:2906–2912. doi: 10.1007/s00125-012-2673-3. [DOI] [PubMed] [Google Scholar]
- 7.Tuttle M.S. Association between microbial bioburden and healing outcomes in venous leg ulcers: a review of the evidence. Adv Wound Care (New Rochelle ) 2015;4:1–11. doi: 10.1089/wound.2014.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips T., Stanton B., Provan A., Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol. 1994;31:49–53. doi: 10.1016/s0190-9622(94)70134-2. [DOI] [PubMed] [Google Scholar]
- 9.Olin J.W., Beusterien K.M., Childs M.B., Seavey C., McHugh L., Griffiths R.I. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med. 1999;4:1–7. doi: 10.1177/1358836X9900400101. [DOI] [PubMed] [Google Scholar]
- 10.Association for the Advancement of Wound Care (AAWC) Venous Ulcer Guideline. Association for the Advancement of Wound Care. Available at: http://aawconline.org/wp-content/uploads/2014/02/AAWC-Venous-Ulcer-Guideline-Update+Algorithm-v28-updated-11Feb2014.pdf; Accessed 28.10.15.
- 11.O'Donnell T.F., Jr., Passman M.A., Marston W.A. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery (R) and the American Venous Forum. J Vasc Surg. 2014;60:3S–59S. doi: 10.1016/j.jvs.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Steed D.L., Attinger C., Colaizzi T. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14:680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 13.Ueta M., Kweon M.N., Sano Y. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol. 2002;129:464–470. doi: 10.1046/j.1365-2249.2002.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Y., Ma D.H., Hwang D.G., Kim W.S., Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 15.AMNIOEXCEL [Product Preparation Insert] Derma Sciences, Inc.; Princeton, NJ: 2014. [Google Scholar]
- 16.AmnioExcel monograph. Derma Sciences, Inc. Available at: http://www.dermasciences.com/sites/default/files/brand/product_brochure/DS1405%20AMNIOEXCEL%20sell%20sheet.pdf; Accessed 28.10.15.
- 17.Carter M.J., Fife C.E., Walker D., Thomson B. Estimating the applicability of wound care randomized controlled trials to general wound-care populations by estimating the percentage of individuals excluded from a typical wound-care population in such trials. Adv Skin Wound Care. 2009;22:316–324. doi: 10.1097/01.ASW.0000305486.06358.e0. [DOI] [PubMed] [Google Scholar]
- 18.Shanmugam V.K., Schilling A., Germinario A. Prevalence of immune disease in patients with wounds presenting to a tertiary wound healing centre. Int Wound J. 2012;9:403–411. doi: 10.1111/j.1742-481X.2011.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang A.S., Armstrong E.J., Armstrong A.W. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206:410–417. doi: 10.1016/j.amjsurg.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Lintzeris D., Yarrow K., Johnson L. October 16-18, 2014. Case series demonstrating the impact of dehydrated human amniotic membrane allograft* on would healing in acute and chronic wounds [poster] Presented at: Las Vegas, NV. Fall 2014 Symposium on Advanced Wound Care. [Google Scholar]
- 21.Rosenblum B.I. October 16-18, 2014. Case series demonstrating the healing capability of diabetic foot ulcers using dehydrated amniotic membrane allograft [poster] Presented at: Las Vegas, NV. Fall 2014 Symposium on Advanced Wound Care. [Google Scholar]
- 22.Sheikh E.S., Sheikh E.S., Fetterolf D.E. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J. 2014;11:711–717. doi: 10.1111/iwj.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes J., Fetterolf D.E. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: a case series. J Wound Care. 2012;21:290–296. doi: 10.12968/jowc.2012.21.6.290. [DOI] [PubMed] [Google Scholar]
- 24.Marston W.A., Hanft J., Norwood P., Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701–1705. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 25.Veves A., Falanga V., Armstrong D.G., Sabolinski M.L. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290–295. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 26.Regulski M., Jacobstein D.A., Petranto R.D., Migliori V.J., Nair G., Pfeiffer D. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage. 2013;59:38–43. [PubMed] [Google Scholar]
- 27.Zelen C.M., Serena T.E., Denoziere G., Fetterolf D.E. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10:502–507. doi: 10.1111/iwj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelen C.M. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22:347–351. doi: 10.12968/jowc.2013.22.7.347. [DOI] [PubMed] [Google Scholar]