Platelet-rich plasma (PRP) is an autologous product that concentrates a large number of platelets in a small volume of plasma [1,2]. PRP has long been used in hospitals to accelerate the body's own healing process, but it is only fairly recently that advances in technology have allowed this same technique to be used in the clinics as well. The blood platelets perform several essential functions in the body, including blood clot formation and the release of growth factors that help to heal wounds. These growth factors stimulate the stem cells to produce new host tissue as quickly as possible, which explains PRP is so effective in the post-treatment healing process. This unique property of PRP speeds up the healing process at the cellular level and there is virtually no risk for allergic reaction or rejection because patient's own blood is used.
Mechanism of action of PRP
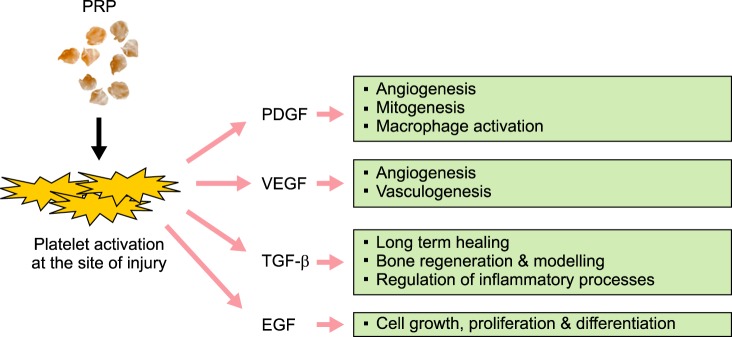
PRP has 7 fundamental proteins: platelet derived growth factors (PDGF), transforming growth factor–β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and adhesive proteins – fibrin, fibronectin, and vitronectin [2].
PDGF is a glycoprotein, which emerges from degranulation of platelets at the site of injury. It activates cell membrane receptors on target cell which in turn develop high energy phosphate bonds, which activates the signal proteins to initiate specific activity of target cells. These specific activities include mitogenesis, angiogenesis and macrophage activation (Fig. 1). TGF-β is secreted by platelets, and also by macrophages, and acts as an antiproliferative factor in normal epithelial cells. It acts as paracrine and autocrine in nature [2]. The target cells for TGF-β are fibroblasts, marrow stem cells and pre-osteoblasts. They represent long term healing, bone regeneration and bone modelling. TGF-β also inhibits osteoclast formation. VEGF, originally known as vascular permeability factor, is a signal protein produced by cells that stimulates vasculogenesis and angiogenesis [3]. EGF is a growth factor that stimulates cell growth, proliferation, and differentiation by binding to its receptor EGFR [4].
Fig. 1. Mechanism of action of PRP. Wound healing and tissue regeneration can be accelerated at the site of tissue injury by various growth factors produced from activated platelets. Abbreviations: PRP, platelet-rich plasma; PDGF, platelet derived growth factor; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor.
Clinical applications
The use of PRP has become a very important clinical tool as an alternative source of growth factors for several types of medical treatments. In total knee arthroplasty PRP was applied to cut surfaces, synovium and lining of the wound closure and it was found that there was decrease in inflammation and blood loss [5]. In various facelift surgeries, it was observed clinically that there was significant decrease in edema of face [6,7]. PRP has recently been shown to reduce the incidence of sternal infections after cardiac surgery [7]. PRP has also found popular and effective applications in sports and sports medicine in mending injured ligaments and tendons without surgeries. Utilizing the methods of enhancement of bone and soft tissues regeneration with the use of PRP is becoming more evident in many areas of dentistry as well. These procedures are applicable for improving the contour of alveolar ridge in relation to ideal pontic, papilla esthetics for fixed partial prosthesis, healthy dento-alveolar complex for periodontal attachment and bone for dental implant placement.
In addition of the benefits at the wound or at the graft site, threefold or greater concentration of platelets at the wound site can be expected to have profound effect on wound healing enhancement and bone regeneration. It was found that there is a seven–fold increase in TGF-β and a thirty-fold increase in PDGF and a ten–fold increase in EGF [8].
In the field of oral and maxillofacial surgery, it is used in onlay grafts, particulate grafts, alveolar cleft palate repair, oral/nasal fistula repair, postoperative hemostasis of bone graft donor sites, continuity defects of the mandible and hemophiliacs undergoing surgery.
Risk factors and contraindications
Most current methods of PRP preparation use calcium and bovine thrombin to initiate formation of PRP gel. By the use of bovine thrombin, very rare cases of bleeding were reported. The use of bovine thrombin has unfortunately been associated with the development of antibodies to human clotting factors V, XI, and thrombin, resulting in a risk of potentially life-threatening coagulopathies. Alternative agents for activation of PRP, such as autologous human thrombin or synthetic peptides such as thrombin receptor agonist peptide-6 are now available to contradict this side-effect [9]. Treatment with autologous PRP is generally considered safe in appropriately selected patients. Potential candidates for treatment with PRP should undergo a pretreatment hematologic evaluation to rule out potential coagulopathies and disorders of platelet function. Patients who are anemic and those with thrombocytopenia may be unsuitable candidates for treatment with PRP. Other potential contraindications include hemodynamic instability, severe hypovolemia, unstable angina, sepsis, and anticoagulant or fibrinolytic drug therapy.
Perspectives
PRP is obtained from a small sample of the patient's own blood. It is centrifuged to separate platelet growth factors from red blood cells. The concentration of platelets triggers rapid growth of new bone and soft tissue and there is very little risk because the natural healing process is being accelerated by the PRP. Autologous PRP is a relatively new biotechnology that has shown promise in the stimulation and acceleration of soft-tissue and bone healing. The efficacy of this treatment lies in the local delivery of a wide range of growth factors and proteins, mimicking and supporting physiologic wound healing and reparative tissue processes. Accelerated healing is a goal we have been seeking in medical field and we now have treatment that activates the natural healing process. PRP is a very promising development and can be considered that PRP "jump starts" the cascade of regenerative events.
Footnotes
Author's Diclosure of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 2.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 4.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(Suppl 2):21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–159e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 7.Serraino GF, Dominijanni A, Jiritano F, et al. Platelet-rich plasma inside the sternotomy wound reduces the incidence of sternal wound infections. Int Wound J. 2015;12:260–264. doi: 10.1111/iwj.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 9.Landesberg R, Burke A, Pinsky D, et al. Activation of platelet-rich plasma using thrombin receptor agonist peptide. J Oral Maxillofac Surg. 2005;63:529–535. doi: 10.1016/j.joms.2004.12.007. [DOI] [PubMed] [Google Scholar]