Abstract
Background and Purpose
Brain lesions involving the cerebral cortex are rarely described in patients with neuromyelitis optica spectrum disorder (NMOSD), in contrast to multiple sclerosis. We investigated cerebral cortex involvement using conventional brain magnetic resonance imaging (MRI) in anti-aquaporin-4 (AQP4)-antibody-positive NMOSD patients.
Methods
The study enrolled 215 NMOSD patients who were seropositive for the anti-AQP4 antibody from 5 referral hospitals, and retrospectively analyzed their demographic, clinical, and MRI findings. Abnormal cerebral cortex lesions on brain MRI were identified by a neuroradiologist and two neurologists using consensus.
Results
Most of the 215 enrolled patients (87%) were female. The median age at onset was 22.5 years (range: 15–36 years) and the mean follow-up duration was 123 months. Brain lesions were found in 143 of 194 patients (74%) in whom MRI was performed during follow-up. Brain lesions involving the cerebral cortex were identified in 6 of these 194 patients (3.1%). Five of the patients were female, and the six patients together had a median age of 29 years (range: 15–36 years) at the time of lesion presentation. Three of them showed leptomeningeal enhancement in the lesions. At presentation of the cortex-involving lesions, five of these patients were not being treated at the time of presentation, while the sixth was being treated with interferon-beta.
Conclusions
Although rare, cortical involvement occurs in NMOSD and is commonly combined with leptomeningeal enhancement. We speculate that this occurs only in patients who are not treated appropriately with immunosuppressant drugs.
Keywords: neuromyelitis optica, neuromyelitis optica spectrum disorder, magnetic resonance imaging, cerebral cortex
INTRODUCTION
Neuromyelitis optica (NMO) is an inflammatory disorder of the central nervous system (CNS) that is distinct from multiple sclerosis (MS).1 The discovery of a specific antibody against aquaporin-4 (AQP4) in the sera of NMO patients resulted in the concept of NMO being changed.2 The broadened term "neuromyelitis optica spectrum disorder" (NMOSD) includes anti-AQP4-antibody-positive patients with limited or inaugural forms of NMO, and also the cerebral, diencephalic, and brainstem lesions, as well as anti-AQP4-antibody-positive patients with coexisting autoimmune disorders [e.g., systemic lupus erythematosus (SLE) or Sjögren syndrome (SS)].1
The presence of brain lesions with characteristic locations and configurations is helpful in the diagnosis of NMOSD.3 The 2015 diagnostic criteria for NMOSD classified typical NMOSD brain lesion patterns as follows: 1) lesions involving the dorsal medulla, especially the area postrema, either small and localized, often bilateral, or contiguous with an upper cervical spinal cord lesion; 2) lesions involving the periependymal surfaces of the fourth ventricle in the brainstem/cerebellum; 3) lesions involving the hypothalamus, thalamus, or periependymal surfaces of the third ventricle; 4) large, confluent, unilateral, or bilateral subcortical or deep white-matter lesions; 5) long (at least half the length of the corpus callosum), diffuse, heterogeneous, or edematous corpus callosum lesions; 6) long corticospinaltract lesions, unilateral or bilateral, contiguously involving the internal capsule and cerebral peduncle; and 7) extensive periependymal brain lesions, often with gadolinium enhancement.1
Cortical lesions have been considered as "red flags" against the diagnosis of NMOSD.1 However, brain lesions involving the cerebral cortex are found in clinical practice, albeit very rarely. Lesions involving the cerebral cortex in patients with NMOSD have also been reported.4,5
We examined the involvement of the cerebral cortex using conventional brain magnetic resonance imaging (MRI) in NMOSD patients who were seropositive for the anti-AQP4 antibody, and described their imaging and clinical characteristics.
METHODS
This study enrolled 215 consecutive NMOSD patients who were seropositive for the anti-AQP4 antibody from 5 referral hospitals, from May 2005 to April 2014. The diagnosis of NMOSD was based on anti-AQP4-antibody seropositivity and the 2007 NMOSD description.6 The presence of NMO-IgG or the anti-AQP4 antibody was tested according to a previously reported tissue-based indirect immunofluorescence assay,7 cell-based indirect immunofluorescence assay,8 and enzyme-linked immunosorbent assay.9 We retrospectively reviewed the demographic, clinical, and MRI findings of the enrolled patients, including age, sex, dates of the first neurological symptom presentation and last follow-up, attack history, and dates of MRI scans. All MRI scans were obtained using either a 1.5- or 3.0-T machine. Abnormal lesions involving the cerebral cortex on brain MRI were identified by three experienced observers using consensus: a neuroradiologist (S.H. Lee) and two neurologists (W. Kim and H.J. Kim).
We collected more-detailed clinical information on patients in whom cortical lesions were identified, including the relapse history of optic neuritis, myelitis, and brain symptoms, and treatments received for NMOSD. Cerebrospinal fluid (CSF) findings at the time of presentation of the cortical lesions were reviewed. The presence of comorbid systemic autoimmune diseases was reviewed, including SLE, SS, psoriasis, and autoimmune thyroiditis.
This study was approved by the Institutional Review Board of the National Cancer Center, Korea.
RESULTS
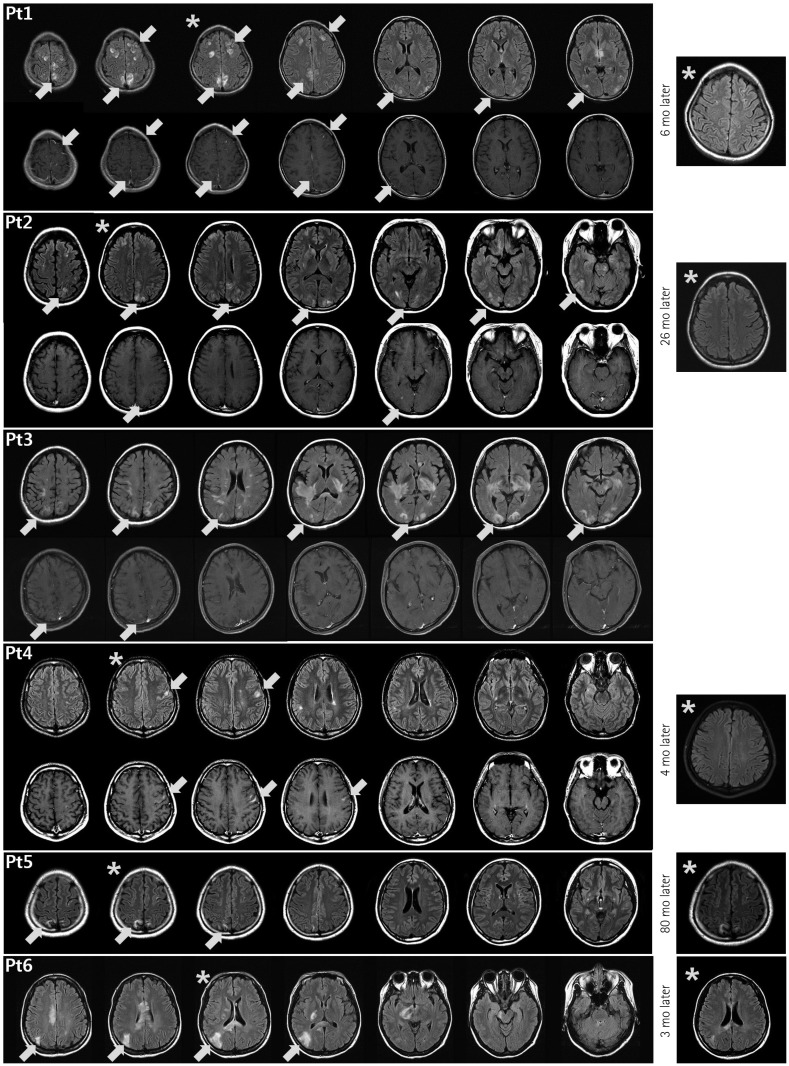
Most of the 215 enrolled patients (87%) were female. The median age at onset was 22.5 years (range: 15–36 years) and the mean follow-up duration was 123 months. Brain lesions were identified in 143 of 194 patients (74%) in whom MRI was performed during follow-up. Brain lesions involving the cerebral cortex were identified in 6 of these 194 patients (3.1%) (Fig. 1). All of the MRI scans were obtained during the acute stage of the correlated neurological symptoms. During the follow-up period, all six patients presented more than one of the "core clinical characteristics" of NMOSD as defined by Wingerchuk et al.1 in recently published international consensus diagnostic criteria, such as optic neuritis, acute myelitis, area postrema syndrome, acute brainstem syndrome, symptoms correlated with NMOSD-typical diencephalic MRI lesions, or symptomatic cerebral syndrome with NMOSDtypical brain lesions. None of these patients had a history of vaccination during the few months preceding when the cortex- involving lesions first appeared.
Fig. 1. Brain abnormalities in neuromyelitis optica spectrum disorder (NMOSD) patients on fluid-attenuated inversion recovery (FLAIR) (upper rows) and T1-weighted enhanced (lower rows) magnetic resonance imaging (MRI) scans obtained at the time of cortical lesion presentation. The brain MRI scans of Patient 2 have been described previously.15 The lesions involving the cerebral cortex are accompanied by leptomeningeal (Patients 1, 2, and 3) or cortical (Patient 4) enhancement, or no enhancement (Patients 5 and 6). In addition to the cortex-involving lesions, other characteristic brain lesions of NMOSD are evident. In representative follow-up FLAIR images (Patients 1, 2, 4, 5, and 6), obtained 3 to 80 months after the presentation of the cortex-involving lesions, most of the lesions had disappeared or become faint—compared to the correlated slices in the previous images (*)—in all patients except Patient 5. mo: months.
Five of the six patients (83.3%) exhibiting cortex-involving brain lesions were female, and the six patients together had a median age of 29 years (range: 15–36 years) at the time of lesion presentation (Table 1). Three of them (50%) had those lesions at the first presentation of their CNS inflammatory symptoms. At the time of presentation of the cortex-involving lesions, five of the six patients were not being treated, while the sixth was being treated with interferon-beta-1b. Three patients (Patients 1, 2, and 3) presented with encephalopathy, including seizures, confusion, decreased mental status, or hypersomnolence. One (Patient 4) experienced an intermittent myoclonic seizure of the contralateral arm two to five times a day, as confirmed by electroencephalography, and two (Patients 5 and 6) did not show obvious symptoms that were correlated with the cortical lesions. Patient 6 presented hemiparesis on her left limbs that was correlated with lesions involving the right corticospinal tract.
Table 1. Clinical characteristics of neuromyelitis optica spectrum disorder patients with involvement of cerebral cortex in brain MRI.
| Pt. | Sex | Onset age | Age & disease duration at the presentation of cortical lesion(s) | NMO-IgG or anti-aquaporin 4 antibody test | Enhancement | Symptoms at the presentation of the cortical lesion(s) | CSF findings at the presentation of the cortical lesion(s) | NMOSD symptoms presented before the presentation of cortical lesions | NMOSD symptoms presented during disease duration | Treatment proceeding the presentation of cortical lesion(s) | Treatment after presentation of cortical lesion(s) | Comorbid systemic autoimmune disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 15 | 15 (onset) | TBA(+) CBA(+) ELISA(+) |
LM | Seizure, confusion, dysarthria, decreased mental status Myelitis (confirmed) |
WBC 6, Protein 141, Glucose 59 | - | ON Myelitis Brain Sx |
No | RT | Sjögren syndrome |
| 2 | F | 36 | 36 (onset) | TBA(+) CBA(+) ELISA(+) |
LM | Headache, confusion, hypersomnolence | N/A | - | Myelitis Brain Sx |
No | RT | Sjögren syndrome |
| 3 | F | 24 | 29 (5 years) | TBA(+) CBA(+) ELISA(+) |
LM | Fever, headache, confusion, decreased mental status, paraparesis | WBC 45, Protein 178, Glucose 35 | ON | ON Brain Sx |
No | RT | - |
| 4 | M | 21 | 21 (onset) | CBA(+) ELISA(+) |
Cortical | Dysarthria, facial numbness, myoclonic seizure of the Rt. arm | WBC 1, Protein 25.4, Glucose 61 | - | ON Brain Sx |
No | AZA → MMF | - |
| 5 | F | 19 | 29 (10 years) | TBA(+) CBA(+) ELISA(-) |
No | Dizziness | N/A | ON Myelitis Brain Sx |
ON Myelitis Brain Sx |
No | RT | - |
| 6 | F | 34 | 34 (6 months) | TBA(+) CBA(+) ELISA(+) |
No | Left hemiparesis, dizziness | N/A | ON Myelitis |
ON Myelitis Brain Sx |
Interferon-beta 1b | RT | - |
AZA: azathioprine, CBA: cell-based indirect immunofluorescence assay, ELISA: enzyme-linked immunosorbent assay, LM: leptomeningeal, MMF: mycophenolate mofetil, MT: mitoxantrone, N/A: not available, NMO: neuromyelitis optica, NMO-IgG: neuromyelitis optica-immunoglobulin G, NMOSD: neuromyelitis optica spectrum disorder, ON: optic neuritis, RT: rituximab, Sx: symptoms, TBA: tissue-based indirect immunofluorescence assay, WBC: white blood cells.
The cortex-involving lesions were located most commonly in the frontal, posterior parietal, and occipital lobes (Fig. 1). In fluid-attenuated inversion recovery (FLAIR) images, many of the cortex-involving lesions showed heterogeneous signal intensities and blurred margins, and simultaneously involved the cortex and subcortex. At the time of presentation of the cortex-involving lesions, various other lesions were seen in the brain. Some were characteristic lesions of NMOSD, such as periependymal lesions of the third (Patient 1) and fourth (Patient 6) ventricles, large confluent subcortical and deep white-matter lesions (Patients 3 and 6), long corpus callosum lesions (Patients 3 and 6), corticospinal-tract lesions (Patient 2, 3, and 6), and extensive periependymal brain lesions (Patient 3). Three of the lesions showed leptomeningeal cortical enhancement (Patients 1, 2, and 3) and one showed cortical enhancement (Patient 4).
Follow-up brain MRI scans were available in five patients (Patients 1, 2, 4, 5, and 6). In the follow-up FLAIR images, obtained 3 to 80 months after the presentation of the cortex-involving lesions, most of the lesions had disappeared or become faint in four of these five patients (Patients 1, 2, 4, and 6) (Fig. 1).
DISCUSSION
This study found that in very rare cases cortical involvement occurs in NMOSD patients and is commonly combined with leptomeningeal enhancement. We speculate that this rare finding occurs only in patients who are not treated appropriately with immunosuppressant drugs.
Several studies using advanced techniques, such as double inversion recovery sequences10 or 7.0-T MRI,11 did not observe cortical lesions in NMOSD; however, those studies involved relatively small numbers of patients (30 and 10 NMO/NMOSD patients, respectively), and imaging was not performed at the acute stage of relapses. The absence of cortical demyelination in NMO was also found in a study of the pathology of the lesions that performed immunohistochemical analyses of 19 NMO cases.12 This led to a lack of cortical demyelination in NMOSD being suggested as one of the features that could help to differentiate it from MS.13 However, our results suggest that brain lesions involving the cerebral cortex do not absolutely rule out NMOSD. It is noteworthy that the MRI characteristics of the lesions involving the cerebral cortex observed in our patients are quite different from the typical cortical lesions described in MS.10,11,14
Two of our patients (Patients 1 and 2) have been described in our previous report on NMOSD patients who showed brain abnormalities as an initial manifestation.15 However, the present study had a totally different focus: we examined brain MRI scans focusing on cortical involvement in this study, whereas our previous report merely described the brain lesions of Patient 2 as "diffuse subcortical lesions."15 In fact, the brain lesions of that patient involved mainly the subcortex, but we also found some lesions simultaneously involving the cerebral cortex since we concentrated on the cortical area.
Lesions involving the cerebral cortex in patients with NMOSD have been found in previous studies, although only very rarely.4,5 In a study describing brain abnormalities in 17 NMO patients, 11 patients (64.7%) had abnormal MRI findings, and 1 of them (5.8%) had lesions in the cerebral cortex.4 A case with cortical oscillopsia without nystagmus as a manifestation of NMOSD with lesions of the visual cortex has also been reported.5
Most of the cortex-involving lesions in our patients disappeared or became faint on FLAIR images after 3 to 80 months, as has also been reported for other brain lesions.3,13,15,16 Considering that brain lesions in NMOSD can disappear on subsequent imaging MRI, performing MRI evaluations during the acute phase of the clinical symptoms is important to discovering brain lesions in NMOSD.
At least three of the six patients exhibiting cortex-involving brain lesions developed encephalopathy, including seizures, confusion, decreased mental status, or hypersomnolence, and the symptoms and brain MRI features showed reversibility in most of our patients. The clinical and imaging features of these three patients resembled acute disseminated encephalomyelitis (ADEM) or posterior reversible encephalopathy syndrome (PRES). In fact, some of our patients were diagnosed with ADEM or PRES at the time of presentation of encephalopathy, as described in our previous report.15 In NMOSD, the encephalopathy associated with diffuse whitematter lesions may appear similar to ADEM.17 A pediatric case of ADEM was recently reported in which seropositivity for the anti-AQP4 antibody presented with optic neuritis and myelitis with encephalopathy at 14 days after an influenza vaccination.18 Several cases of PRES-type lesions in NMOSD have also been described.13,19 However, none of our patients had a history of vaccination during the few months preceding when the cortex-involving lesions first appeared, and they did also not have any comorbid medical conditions or drug histories typical of PRES.
Three of our patients showed leptomeningeal enhancement in association with cortical lesions. Leptomeningeal enhancement in brain MRI is not commonly observed in NMOSD patients, but this was recently found to be more frequent in patients with NMOSD than in patients with MS.20 Disruption of the blood-brain barrier is normally the main explanation for gadolinium enhancement.20 Consistent with the location of AQP4 in the leptomeninges, which is associated with the integrity of the interface between the brain and blood, and between the CNS and CSF, the anti-AQP4 antibody binds to such sites selectively and readily.7,21 Therefore, the infiltration of inflammatory cells via the damaged BBB into the adjacent cortex is one possible explanation of the cortical involvement—especially with leptomeningeal enhancement—in our patients.
None of our patients had been treated appropriately when they presented with the cortex-involving lesions. Three of them already had the lesions at the first presentation of their disease, while among the others, on presentation of the cortex-involving lesions, two of them had not been treated and one was being treated with interferon-beta-1b. This means that cortex-involving lesions could be present in NMOSD patients when the disease activity is high, given that none of the six patients experienced relapse associated with cortexinvolving lesions after being treated with appropriate immunosuppressive drugs.
This study had some limitations, including its retrospective design, the inclusion of a population with a single ethnicity, the possibility of unintentional selection bias, and the unknown status of the anti-AQP4 antibody at the time of presentation of the cortex-involving lesions. In addition, only conventional 1.5- and 3.0-T MRI machines were used.
In conclusion, cortical involvement occurs in NMOSD patients, although only very rarely. Further evaluations are needed using advanced MRI techniques or pathological investigations to reveal the characteristics of those cortex-involving lesions and leptomeningeal enhancement in NMOSD.
Footnotes
Conflicts of Interest: Ho Jin Kim has received honoraria for speaking or consulting from Bayer Schering Pharma, Biogen Idec, Genzyme, Merck Serono, Novartis, MedImmune, and Teva-Handok and has received research grants from the Ministry of Science, ICT & Future Planning, Genzyme, Merck Serono, and Kael-GemVax. He serves on a steering committee for MedImmune and serves as an editor of Multiple Sclerosis Journal - Experimental, Translational and Clinical. Woojun Kim, Su-Hyun Kim, So-Young Huh, Jae-Won Hyun, In Hye Jeong, Min-Su Park, Joong Yang Cho, Sang-Hyun Lee, and Kwang Soo Lee have nothing to declare.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim W, Kim SH, Kim HJ. New insights into neuromyelitis optica. J Clin Neurol. 2011;7:115–127. doi: 10.3988/jcn.2011.7.3.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84:1165–1173. doi: 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JE, Kim SM, Ahn SW, Lim BC, Chae JH, Hong YH, et al. Brain abnormalities in neuromyelitis optica. J Neurol Sci. 2011;302:43–48. doi: 10.1016/j.jns.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Kim SM, Kim JS, Heo YE, Yang HR, Park KS. Cortical oscillopsia without nystagmus, an isolated symptom of neuromyelitis optica spectrum disorder with anti-aquaporin 4 antibody. Mult Scler. 2012;18:244–247. doi: 10.1177/1352458511414149. [DOI] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 7.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, et al. Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J Exp Med. 2006;210:307–313. doi: 10.1620/tjem.210.307. [DOI] [PubMed] [Google Scholar]
- 9.Kim W, Lee JE, Li XF, Kim SH, Han BG, Lee BI, et al. Quantitative measurement of anti-aquaporin-4 antibodies by enzyme-linked immunosorbent assay using purified recombinant human aquaporin-4. Mult Scler. 2012;18:578–586. doi: 10.1177/1352458511424590. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese M, Oh MS, Favaretto A, Rinaldi F, Poretto V, Alessio S, et al. No MRI evidence of cortical lesions in neuromyelitis optica. Neurology. 2012;79:1671–1676. doi: 10.1212/WNL.0b013e31826e9a96. [DOI] [PubMed] [Google Scholar]
- 11.Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi: 10.1155/2013/398259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popescu BF, Parisi JE, Cabrera-Gómez JA, Newell K, Mandler RN, Pittock SJ, et al. Absence of cortical demyelination in neuromyelitis optica. Neurology. 2010;75:2103–2109. doi: 10.1212/WNL.0b013e318200d80c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabrera-Gomez JA, Kister I. Conventional brain MRI in neuromyelitis optica. Eur J Neurol. 2012;19:812–819. doi: 10.1111/j.1468-1331.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 14.Puthenparampil M, Poggiali D, Causin F, Rolma G, Rinaldi F, Perini P, et al. Cortical relapses in multiple sclerosis. Mult Scle. 2015 doi: 10.1177/1352458514564483. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler. 2011;17:1107–1112. doi: 10.1177/1352458511404917. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Huh SY, Hyun JW, Jeong IH, Lee SH, Joung A, et al. A longitudinal brain magnetic resonance imaging study of neuromyelitis optica spectrum disorder. PLoS One. 2014;9:e108320. doi: 10.1371/journal.pone.0108320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Weinshenker BG. Acute disseminated encephalomyelitis, transverse myelitis, and neuromyelitis optica. Continuum (Minneap Minn) 2013;19(4 Multiple Sclerosis):944–967. doi: 10.1212/01.CON.0000433289.38339.a2. [DOI] [PubMed] [Google Scholar]
- 18.Okumura A, Nakazawa M, Igarashi A, Abe S, Ikeno M, Nakahara E, et al. Anti-aquaporin 4 antibody-positive acute disseminated encephalomyelitis. Brain Dev. 2015;37:339–343. doi: 10.1016/j.braindev.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Magaña SM, Matiello M, Pittock SJ, McKeon A, Lennon VA, Rabinstein AA, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72:712–717. doi: 10.1212/01.wnl.0000343001.36493.ae. [DOI] [PubMed] [Google Scholar]
- 20.Long Y, Chen M, Zhang B, Gao C, Zheng Y, Xie L, et al. Brain gadolinium enhancement along the ventricular and leptomeningeal regions in patients with aquaporin-4 antibodies in cerebral spinal fluid. J Neuroimmunol. 2014;269:62–67. doi: 10.1016/j.jneuroim.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008;65:78–83. doi: 10.1001/archneurol.2007.17. [DOI] [PubMed] [Google Scholar]