Abstract
Developmental genes are essential in the formation and function of adipose tissue and muscle. In this issue of Cell, Teperino et al. demonstrate that non-canonical Hedgehog signaling increases glucose uptake into brown fat and muscle. Modulation of developmental pathways may serve as potential targets for new treatments of diabetes and other metabolic disorders.
Obesity results from an imbalance between energy intake and energy expenditure. In obesity, the excess energy is stored as triglyceride in white adipose tissue (WAT). Mammals, including humans, also have active brown adipose tissue (BAT), which is specialized for energy expenditure. BAT has also been shown to share some common developmental origins with skeletal muscle, the other major tissue involved in thermogenesis (Kajimura et al., 2010).
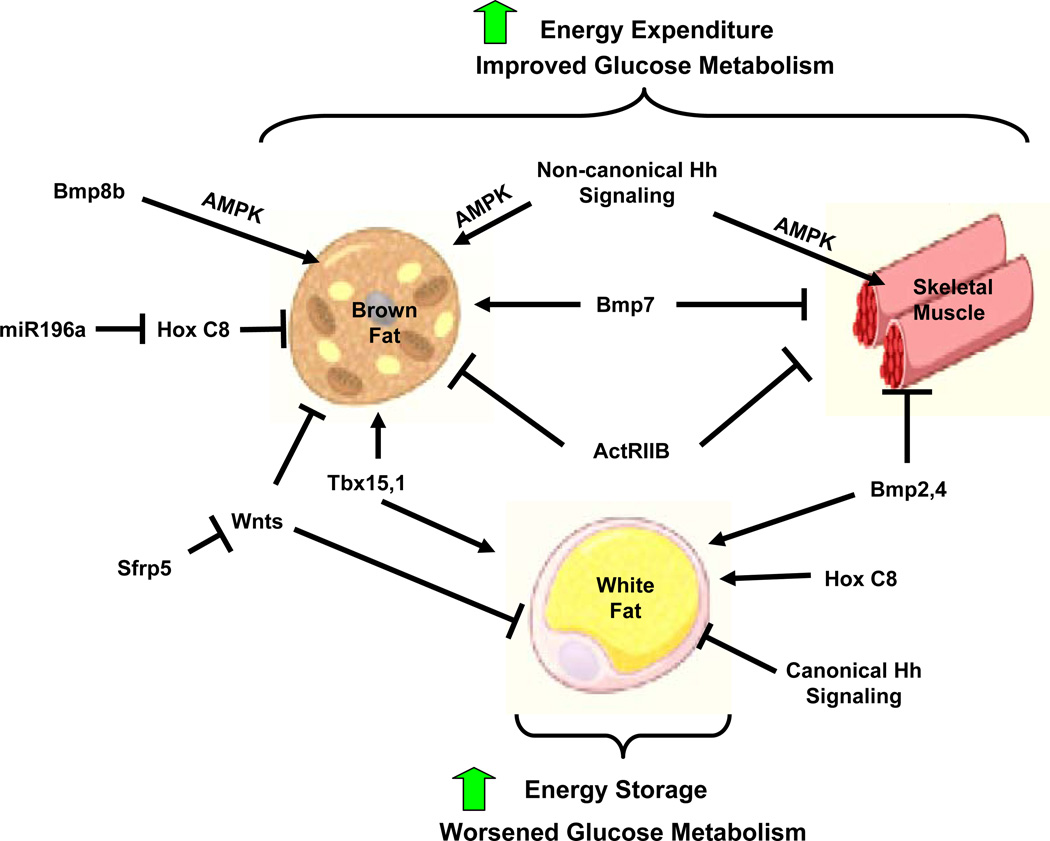
Over the past two decades, the mechanisms underlying the differentiation of WAT and BAT have been elucidated and shown to involve a transcriptional cascade beginning with C/EBPβ, C/EBPδ and Krox20, which induce C/EBPα and PPARγ, the major transcriptional regulators of adipose differentiation. In the case of brown fat, additional co-activators are involved, including PGC1α and PRDM16 (Kajimura et al., 2010). These pathways are regulated positively by a number of growth factors and hormones, especially insulin, IGF-1, and the BMPs, and negatively by the Wnt pathway (Tseng et al., 2008; Fournier et al., 2012; Christodoulides et al., 2009). Recently, fundamental developmental genes, including several Hox and T-box genes, have also been shown to be involved in programming adipose development and function, and contribute to differences in WAT and BAT, as well as differences between WAT in different anatomical depots (Gesta et al., 2011; Mori et al., 2012) (Figure 1).
Figure 1.
The Signaling Pathways Linking Hedgehog Signaling to Energy Storage and Expenditure
In the current issue of Cell, Teperino, et al, demonstrate a novel, non-canonical Hedgehog signaling pathway, a pathway in which Smoothened (Smo) activates the AMP kinase (AMPK) pathway in brown fat and muscle, leading to an increase in insulin-independent glucose uptake and energy expenditure (Teperino et al., 2012). This extends previous studies by this group which showed that fat-specific disruption of Suppressor of Fused, which normally activates the canonical Hedgehog signaling pathway, leads to loss of white, but not brown, fat mass by blocking differentiation of white adipocytes through repression of early adipogenic factors (Kajimura et al., 2010)‥ This also adds to studies which have shown that Hedgehog signaling can inhibit adipogenesis of WAT and that repression of this pathway can lead to an increase fat development. In white fat, this involves the canonical Hedgehog pathway and is dependent upon Gli transactivation of target genes, whereas the current paper shows activation of a Smo-Ca2+-AMPK pathway in brown fat and muscle. The uncoupling of canonical and non-canonical Hedgehog signaling represents a paradigm shift that has several important clinical implications. On one hand, activation of the Smo pathway can explain many of the adverse side effects, including muscle spasms and weight loss, observed during cancer treatment with hedgehog “inhibitors”. On the other hand, this study suggests that the development of selective agonists of this alternative pathway may hold therapeutic promise for the treatment of obesity and diabetes.
Like Hedgehog, the activation of the Wnt signaling pathway stimulates myogenesis and largely inhibits adipocyte differentiation, while expression of negative regulators of Wnt signaling such as secreted frizzled-related proteins (Sfrps) stimulate adipogenesis (Christodoulides et al., 2009). Interestingly, activation of Wnt signaling in mature brown adipocytes drives their conversion into white adipocytes. Likewise, whereas TGF-β itself inhibits adipogenesis, the closely related bone morphogenetic proteins (BMPs) are positive regulators of this process. Both BMP2 and BMP4 have been shown to promote adipogenesis of WAT while inhibiting myogenesis, whereas BMP7 promotes differentiation of brown preadipocytes (Tseng et al., 2008). Inhibition of the Activin receptor IIb also results in an increase in the mass of the interscapular BAT and skeletal muscle without affecting WAT (Fournier et al., 2012).
Several T-box (Tbx) and Hox genes have been shown to control adipose and muscle function. Tbx15 is differentially expressed between different WAT depots, and regulates oxidative metabolism (Gesta et al., 2011), while Tbx1 has been shown to be preferentially expressed in the inducible pool of brown adipocytes present in white fat (Wu et al., 2012). Hox gene expression clearly demarcates brown and white adipose tissue, and decreasing HoxC8 expression by over-expressing its targeting microRNA, miR-196a, leads to an increase brown adipocytes within white adipose tissue (Mori et al., 2012).
Numerous potential mechanisms could link developmental genes to control of adipogenesis. microRNAs, pocket proteins (necdin and Rb protein), phosphatases and matrix proteins have all been shown to have important roles in adipose tissue biology. The study of Teperino et al (Teperino et al., 2012) demonstrates that Hedgehog acts via a cilia-dependent, non-canonical pathway which leads to activation of AMPK and glucose uptake. Cyclopamine, a classical Hedgehog antagonist, activates this pathway, increasing glucose uptake in an insulin-independent manner in brown adipose tissue and muscle. Other regulators of adipose and muscle function also converge on the AMPK pathway. Alterations in Wnt signaling have been shown to alter AMPK activity and glucose metabolism in muscle. Furthermore, expression of constitutively active β-catenin in preadipocytes can leads to the generation of an uncharacterized mediator that active AMPK and enhance glucose uptake in an insulin-independent manner in both brown fat and skeletal muscle (Zeve et al., 2012). Bmp8b treatment of brown adipocytes has also been shown to lead to an increase in AMPK activation (Whittle et al., 2012). While pharmacological activators of AMPK, especially metformin, are already in clinical use, activators of the non-canonical Hedgehog pathway and modulators of these other developmental gene pathways may serve as targets for new therapeutics for diabetes and obesity.
The current study from Teperino et al adds to the burgeoning body of data that developmental genes and signaling pathways play integral roles not only in the formation, but also the function, of brown fat, white fat and muscle. Thus, these pathways not only regulate development of these tissues, but also the metabolism of the fully differentiated cells. These findings suggest that modulation of these developmental genes and their signaling pathways has therapeutic implications across a wide array of disease states, including cancer and metabolic disorders.
Reference List
- Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009;20:16–24. doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier B, Murray B, Gutzwiller S, Marcaletti S, Marcellin D, Bergling S, Brachat S, Persohn E, Pierrel E, Bombard F, Hatakeyama S, Trendelenburg AU, Morvan F, Richardson B, Glass DJ, Lach-Trifilieff E, Feige JN. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol. Cell Biol. 2012;32:2871–2879. doi: 10.1128/MCB.06575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Bezy O, Mori MA, Macotela Y, Lee KY, Kahn CR. Mesodermal developmental gene Tbx15 impairs adipocyte differentiation and mitochondrial respiration. Proc Natl Acad Sci U S. A. 2011;108:2771–2776. doi: 10.1073/pnas.1019704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS. Biol. 2012;10:e1001314. doi: 10.1371/journal.pbio.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G, Dalgaard K, Selvaraj M, Reiter J, Gaster M, Lee-Young RS, Febbraio MA, Knauf C, Cani PD, Aberger F, Penninger JM, Pospisilik JA, Esterbauer H. A Smoothened-Ampk Axis Rewires Metabolism. Hedgehog partial agonism drivesWarburg-like metabolism in muscle and brown fat. Cell. 2012 doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, Lopez M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeve D, Seo J, Suh JM, Stenesen D, Tang W, Berglund ED, Wan Y, Williams LJ, Lim A, Martinez MJ, McKay RM, Millay DP, Olson EN, Graff JM. Wnt signaling activation in adipose progenitors promotes insulin-independent muscle glucose uptake. Cell Metab. 2012;15:492–504. doi: 10.1016/j.cmet.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]