Practical Implications
Consider checking JC virus titers prior to the initiation of rituximab in patients with myasthenia gravis on multiple immunosuppressants. Recognize the potential risk of progressive multifocal leukoencephalopathy in patients treated with rituximab who have been treated with prior immunosuppression.
Progressive multifocal leukoencephalopathy (PML) is an often fatal opportunistic infection of the JC virus resulting in demyelination of the CNS. PML is well-described in patients with multiple sclerosis (MS) exposed to natalizumab. PML is rare in patients with myasthenia gravis (MG) and is also rare following rituximab exposure. We report PML in a patient with MG after immunosuppression that includes rituximab.
Case report
A 58-year-old woman presented in 2007 with fatigable muscle weakness, dysphagia, dysarthria, and dyspnea. Acetylcholine receptor binding and muscle-specific kinase antibodies were negative. Low-frequency repetitive nerve stimulation studies demonstrated a decremental response. Single-fiber EMG demonstrated increased jitter. She was diagnosed with seronegative MG, further confirmed as her weakness improved with immunomodulation. She required multiple hospitalizations from 2008 to 2014 for MG exacerbations. She was treated with chronic prednisone (up to 40 mg daily), pyridostigmine, and IV immunoglobulin or plasmapheresis. Steroid-sparing medications included mycophenolate mofetil (1,500 mg BID) from July 4, 2008, to August 2009 (stopped due to refractory symptoms), azathioprine (3.63 mg/kg) from August 2009 to July 16, 2011 (stopped due to elevated liver function tests), and 4 rituximab infusions (375 mg/m2 each) on June 14, July 12, August 9, and September 6, 2013. She had normal leukocyte counts with low absolute lymphocyte counts in November 2009, May 2011, and February 2014, with return to normal (1.0–5.0 k/μL) between these dates, last measured at 1.97 k/μL on May 13, 2014.
The patient was cognitively normal in June 2014 after hospitalization for another myasthenia exacerbation. Over the following months, she could not manage her finances due to progressing encephalopathy. On September 22, 2014, she was found on the ground, acutely confused. Head CT demonstrated bilateral frontal hypodensities, so she was admitted.
The patient's initial examination was notable for disorientation, abulia, neck flexion weakness, and ankle clonus. MRI brain on September 24, 2014, revealed bilateral white matter fluid-attenuated inversion recovery hyperintensities, which were hypointense on T1-weighted imaging (figure 1). Based on these characteristics, PML was suspected. Serum stratify JC virus antibody with reflex to inhibition assay on September 24, 2014, was positive (index 3.31), and she was lymphopenic to 0.19 k/μL. CD19 and CD20 markers were undetectable on September 30 (0%, 0%, respectively). CD3, CD4, and CD8 markers were normal (82%, 39%, 43%, respectively). CSF on September 25, 2014, revealed elevated protein to 96 and qualitative CSF JC virus PCR sent to the Mayo Clinic was positive, confirming the diagnosis of PML.
Figure 1. MRI features of a patient with myasthenia gravis with progressive multifocal leukoencephalopathy.
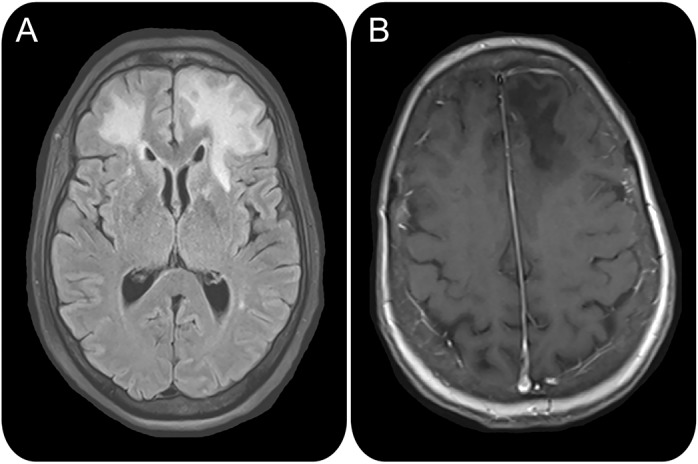
(A) Fluid-attenuated inversion recovery hyperintensities in bilateral frontal subcortical white matter are seen. (B) T1-weighted imaging shows left frontal subcortical white matter hypointensity.
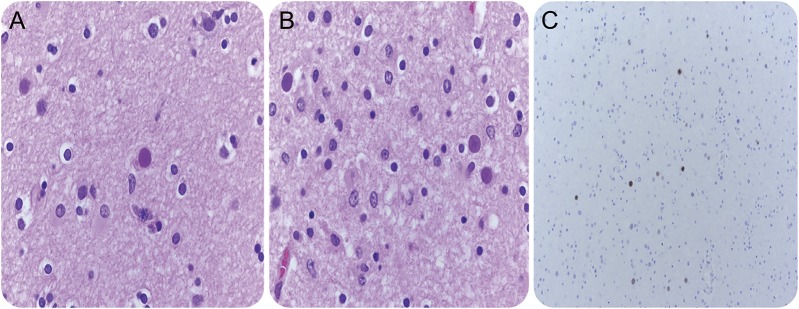
The patient died on October 31, 2014. Postmortem neurohistopathology evaluation on November 3, 2014, revealed demyelination, eosinophilic nuclear inclusions, and positive immunohistochemical testing for JC virus (figure 2).
Figure 2. Neurohistopathology shows findings consistent with PML.
(A, B) High-power view of the edge of a demyelinating lesion shows viral inclusions. (C) Positive immunohistochemical stain for polyomavirus.
DISCUSSION
PML is documented most notably with natalizumab used to treat MS.1 Other immunosuppressant medications, like rituximab, mycophenolate mofetil, and azathioprine, have also been associated with PML.1–4
To our knowledge, only 2 other cases of PML in MG have been reported. The first occurred with combination therapy of prednisone and azathioprine,5 while the other was with prednisolone, IV immunoglobulin, and azathioprine therapy.3 Neither patient received rituximab. Our patient represents PML in a myasthenic patient following rituximab treatment.
Rituximab, an anti-CD20 monoclonal antibody that depletes B cells, is an emerging therapy for treatment of MG.6 CD20 counts recover in 6–9 months, and rituximab is detectable in the plasma for 2–3 months following each infusion.1,7 The reported incidence of PML in rituximab-exposed patients is 1 in 25,000 vs 1 in 1,000 for natalizumab-exposed patients.4 While most patients do not have prolonged immune dysfunction following rituximab, one study documented a notable delay in recovery of B cells, with a mean time to B-cell reconstitution of 23 months.7 These patients were thought predisposed to long-term dysfunction of B cells.7 Furthermore, PML risk increases with prolonged treatment duration with natalizumab, prior immunosuppressive medications, and seropositivity to the JC virus.1 Our patient's absolute lymphocyte count was intermittently low but was normal prior to rituximab. During admission, her CD19 and CD20 markers were undetectable 12 months following her last rituximab infusion and lymphocyte count was low, hence she may have developed PML due to immunosuppression from multiple agents, including rituximab, as her B cells were depleted.
As rituximab utilization continues to grow for MG treatment, we recommend that JC virus antibody titers be checked prior to treatment initiation. It also may be prudent to monitor CD19 and CD20 counts to screen patients with preexisting immune dysfunction, which may increase the PML risk.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
K.M. Kanth reports no disclosures. G.E. Solorzano serves on the editorial board of Frontiers in Hospitalist Neurology. His spouse receives research support from Kohl's Cares, NIH (NIAD, NICHD, NIDDK), University of Virginia Children's Hospital DuBose Family Fund, University of Virginia Children's Hospital, Jaeb Center for Health Research via Leona Helmsley Trust, as part of the T1D Exchange, Juvenile Diabetes Research Foundation, Helmsley Charitable Trust, American Diabetes Association, Juvenile Diabetes Research Foundation, National Center for Research Resources, and University of South Florida. M.D. Goldman serves on scientific advisory boards for Novartis, Biogen, Idec, Acorda, Questcor, and Genzyme; serves as an Associate Editor for Therapeutic Advances in Neurological Disorders; serves as a consultant for Concert Pharmaceuticals and Questcor; has received funding for travel and lodging for Acorda Therapeutics and Biogen; and receives research support from Biogen, Idec, Novartis, NIH/NINDS, and the National Science Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Steiner I, Berger JR. Update on progressive multifocal leukoencephalopathy. Curr Neurol Neurosci Rep 2012;12:680–686. [DOI] [PubMed] [Google Scholar]
- 2.Kabbaj DE. Mycophenolate mofetil associated with progressive multifocal leukoencephalopathy with successful outcome. Saudi J Kidney Dis Transpl 2012;23:790–793. [DOI] [PubMed] [Google Scholar]
- 3.Gedizlioglu M, Coban P, Ce P, Sivasli IE. An unusual complication of immunosuppression in myasthenia gravis: progressive multifocal leukoencephalopathy. Neuromuscul Disord 2009;19:155–157. [DOI] [PubMed] [Google Scholar]
- 4.Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol 2011;68:1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson DM. Progressive multifocal leukoencephalopathy in myasthenia gravis. Ann Neurol 1982;11:218. [DOI] [PubMed] [Google Scholar]
- 6.Sieb JP. Myasthenia gravis: an update for the clinician. Clin Exp Immunol 2013;175:408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan B, Kopyltsova Y, Khokhar A, Lam F, Bonagura V. Rituximab and immune deficiency: case series and review of the literature. J Allergy Clin Immunol Pract 2014;2:594–600. [DOI] [PubMed] [Google Scholar]