Abstract
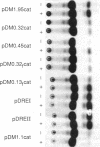
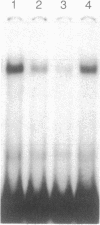
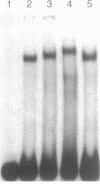
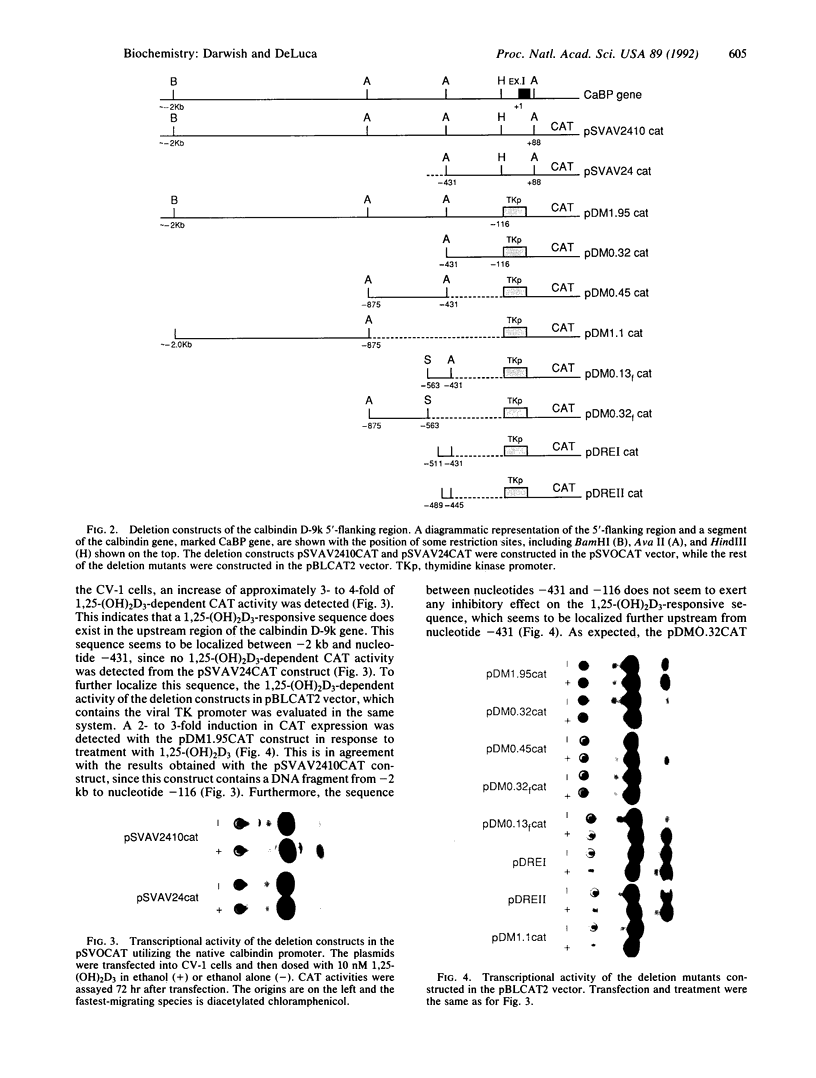
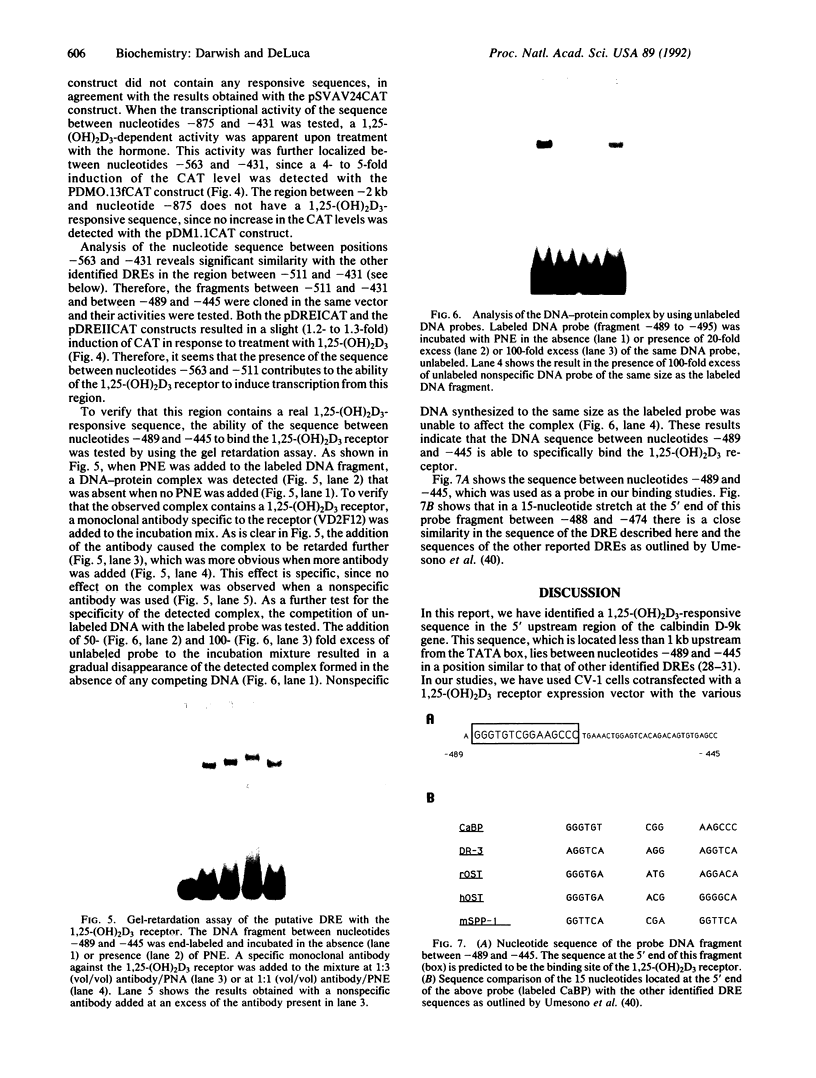
The rat calbindin D-9k gene is transcriptionally regulated by 1,25-dihydroxyvitamin D3 in the intestine. We have examined the 5'-flanking region of this gene and identified a 1,25-dihydroxyvitamin D3-responsive element (DRE) between nucleotides -489 and -445. This element confers 1,25-dihydroxyvitamin D3 responsiveness through its native promoter and the heterologous thymidine kinase promoter, and it contains the sequence GGGTGTCGGAAGCCC, which is homologous to the other previously identified DREs. Incubation of this element with the 1,25-dihydroxyvitamin D3 receptor produced a specific protein-DNA complex, which shifted to a higher molecular weight form upon the addition of a monoclonal antibody specific to the 1,25-dihydroxyvitamin D3 receptor. Therefore, the 5'-flanking region of the rat calbindin D-9k gene contains a DRE that mediates the enhanced expression of this gene by 1,25-dihydroxyvitamin D3 in the intestine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain N., Tisserand-Jochem E., Thomasset M., Cuisinier-Gleizes P., Mathieu H. Vitamin-D-dependent calcium-binding protein (CaBP-9K) in rat growth cartilage. Histochemistry. 1986;84(2):161–168. doi: 10.1007/BF00499828. [DOI] [PubMed] [Google Scholar]
- Bruns M. E., Kleeman E., Bruns D. E. Vitamin D-dependent calcium-binding protein of mouse yolk sac. Biochemical and immunochemical properties and responses to 1,25-dihydroxycholecalciferol. J Biol Chem. 1986 Jun 5;261(16):7485–7490. [PubMed] [Google Scholar]
- Bruns M. E., Overpeck J. G., Smith G. C., Hirsch G. N., Mills S. E., Bruns D. E. Vitamin D-dependent calcium binding protein in rat uterus: differential effects of estrogen, tamoxifen, progesterone, and pregnancy on accumulation and cellular localization. Endocrinology. 1988 Jun;122(6):2371–2378. doi: 10.1210/endo-122-6-2371. [DOI] [PubMed] [Google Scholar]
- Buckley M., Bronner F. Calcium-binding protein biosynthesis in the rat: regulation by calcium and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 1980 Jun;202(1):235–241. doi: 10.1016/0003-9861(80)90425-7. [DOI] [PubMed] [Google Scholar]
- Christakos S., Bruns M. E., Mehra A. S., Rhoten W. B., Van Eldik L. J. Calmodulin and rat vitamin D-dependent calcium-binding proteins: biochemical and immunochemical comparison. Arch Biochem Biophys. 1984 May 15;231(1):38–47. doi: 10.1016/0003-9861(84)90360-6. [DOI] [PubMed] [Google Scholar]
- Christakos S., Gabrielides C., Rhoten W. B. Vitamin D-dependent calcium binding proteins: chemistry, distribution, functional considerations, and molecular biology. Endocr Rev. 1989 Feb;10(1):3–26. doi: 10.1210/edrv-10-1-3. [DOI] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., DeLuca H. F. Identification of the porcine intestinal 1,25-dihydroxyvitamin D3 receptor on sodium dodecyl sulfate/polyacrylamide gels by renaturation and immunoblotting. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7825–7829. doi: 10.1073/pnas.82.23.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., Prahl J. M., Hayes C. E., DeLuca H. F. Monoclonal antibodies to the porcine intestinal receptor for 1,25-dihydroxyvitamin D3: interaction with distinct receptor domains. Biochemistry. 1986 Aug 12;25(16):4523–4534. doi: 10.1021/bi00364a011. [DOI] [PubMed] [Google Scholar]
- Darwish H. M., Krisinger J., Strom M., DeLuca H. F. Molecular cloning of the cDNA and chromosomal gene for vitamin D-dependent calcium-binding protein of rat intestine. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6108–6111. doi: 10.1073/pnas.84.17.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish H., Krisinger J., Furlow J. D., Smith C., Murdoch F. E., DeLuca H. F. An estrogen-responsive element mediates the transcriptional regulation of calbindin D-9K gene in rat uterus. J Biol Chem. 1991 Jan 5;266(1):551–558. [PubMed] [Google Scholar]
- Davie M. Calcium-ion-binding activity in human small-intestinal mucosal cytosol. Purification of two proteins and interrelationship of calcium-binding fractions. Biochem J. 1981 Jul 1;197(1):55–65. doi: 10.1042/bj1970055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A. C., Danan J. L., Acker M. G., Ripoche M. A., Mathieu H. In rat uterus 17 beta-estradiol stimulates a calcium-binding protein similar to the duodenal vitamin D-dependent calcium-binding protein. Endocrinology. 1983 Oct;113(4):1340–1347. doi: 10.1210/endo-113-4-1340. [DOI] [PubMed] [Google Scholar]
- Delorme A. C., Danan J. L., Mathieu H. Biochemical evidence for the presence of two vitamin D-dependent calcium-binding proteins in mouse kidney. J Biol Chem. 1983 Feb 10;258(3):1878–1884. [PubMed] [Google Scholar]
- Delorme A. V., Cassier P., Geny B., Mathieu H. Immunocytochemical localization of vitamin D-dependent calcium-binding protein in the yolk sac of the rat. Placenta. 1983 Jul-Sep;4(3):263–270. doi: 10.1016/s0143-4004(83)80005-8. [DOI] [PubMed] [Google Scholar]
- Demay M. B., Gerardi J. M., DeLuca H. F., Kronenberg H. M. DNA sequences in the rat osteocalcin gene that bind the 1,25-dihydroxyvitamin D3 receptor and confer responsiveness to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Jan;87(1):369–373. doi: 10.1073/pnas.87.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. The amino acid sequence of bovine intestinal calcium-binding protein. J Biol Chem. 1981 Jun 10;256(11):5669–5674. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T., Kawakami M., Hitchman A. J., Harrison J. E., Dorrington K. J. The amino acid sequence of porcine intestinal calcium-binding protein. Can J Biochem. 1979 Jun;57(6):737–748. doi: 10.1139/o79-092. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Yates J. R., 3rd, Shabanowitz J., Bruns M. E., Bruns D. E. Amino acid sequence analysis of two mouse calbindin-D9k isoforms by tandem mass spectrometry. Protein modification by internal insertion of a single amino acid. J Biol Chem. 1989 Apr 15;264(11):6580–6586. [PubMed] [Google Scholar]
- Hunziker W. The 28-kDa vitamin D-dependent calcium-binding protein has a six-domain structure. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7578–7582. doi: 10.1073/pnas.83.20.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. A., Lamm L., Jarnagin K., DeLuca H. F. 1,25-Dihydroxyvitamin D3-stimulated mRNAs in rat small intestine. Arch Biochem Biophys. 1986 Dec;251(2):403–412. doi: 10.1016/0003-9861(86)90346-2. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Kohwi Y. Torsional stress stabilizes extended base unpairing in suppressor sites flanking immunoglobulin heavy chain enhancer. Biochemistry. 1990 Oct 16;29(41):9551–9560. doi: 10.1021/bi00493a009. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Krisinger J., Darwish H., Maeda N., DeLuca H. F. Structure and nucleotide sequence of the rat intestinal vitamin D-dependent calcium binding protein gene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8988–8992. doi: 10.1073/pnas.85.23.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisinger J., Strom M., Darwish H. D., Perlman K., Smith C., DeLuca H. F. Induction of calbindin-D 9k mRNA but not calcium transport in rat intestine by 1,25-dihydroxyvitamin D3 24-homologs. J Biol Chem. 1991 Jan 25;266(3):1910–1913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus J. P., Watson D. C., Yaguchi M. The purification and complete amino acid sequence of the 9000-Mr Ca2+-binding protein from rat placenta. Identity with the vitamin D-dependent intestinal Ca2+-binding protein. Biochem J. 1986 Apr 15;235(2):585–595. doi: 10.1042/bj2350585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C. L., Mills S. E., Burnett S. H., Cloney D. L., Bruns D. E., Bruns M. E. The presence and estrogen control of immunoreactive calbindin-D9k in the fallopian tube of the rat. Endocrinology. 1989 Nov;125(5):2745–2750. doi: 10.1210/endo-125-5-2745. [DOI] [PubMed] [Google Scholar]
- Morrison N. A., Shine J., Fragonas J. C., Verkest V., McMenemy M. L., Eisman J. A. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science. 1989 Dec 1;246(4934):1158–1161. doi: 10.1126/science.2588000. [DOI] [PubMed] [Google Scholar]
- Noda M., Vogel R. L., Craig A. M., Prahl J., DeLuca H. F., Denhardt D. T. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret C., Desplan C., Thomasset M. Cholecalcin (a 9-kDa cholecalciferol-induced calcium-binding protein) messenger RNA. Distribution and induction by calcitriol in the rat digestive tract. Eur J Biochem. 1985 Jul 1;150(1):211–217. doi: 10.1111/j.1432-1033.1985.tb09009.x. [DOI] [PubMed] [Google Scholar]
- Perret C., Lomri N., Gouhier N., Auffray C., Thomasset M. The rat vitamin-D-dependent calcium-binding protein (9-kDa CaBP) gene. Complete nucleotide sequence and structural organization. Eur J Biochem. 1988 Feb 15;172(1):43–51. doi: 10.1111/j.1432-1033.1988.tb13853.x. [DOI] [PubMed] [Google Scholar]
- Sandgren M. E., Deluca H. F. An immunoradiometric assay for 1,25-dihydroxyvitamin D3 receptor. Anal Biochem. 1989 Nov 15;183(1):57–63. doi: 10.1016/0003-2697(89)90171-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Umesono K., Mangelsdorf D. J., Bolado J., Pike J. W., Evans R. M. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990 May 4;61(3):497–504. doi: 10.1016/0092-8674(90)90531-i. [DOI] [PubMed] [Google Scholar]
- Takagi T., Nojiri M., Konishi K., Maruyama K., Nonomura Y. Amino acid sequence of vitamin D-dependent calcium-binding protein from bovine cerebellum. FEBS Lett. 1986 May 26;201(1):41–45. doi: 10.1016/0014-5793(86)80567-1. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warembourg M., Perret C., Thomasset M. Analysis and in situ detection of cholecalcin messenger RNA (9000 Mr CaBP) in the uterus of the pregnant rat. Cell Tissue Res. 1987 Jan;247(1):51–57. doi: 10.1007/BF00216546. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Fullmer C. S. Calcium transport proteins, calcium absorption, and vitamin D. Annu Rev Physiol. 1983;45:375–390. doi: 10.1146/annurev.ph.45.030183.002111. [DOI] [PubMed] [Google Scholar]