Abstract
The NADPH oxidase complex of a sclerotial mycoparasite Coniothyrium minitans, an important biocontrol agent against crop diseases caused by Sclerotinia sclerotiorum, was identified and its functions involved in conidiation and mycoparasitism were studied. Gene knock-out and complementary experiments indicated that CmNox1, but not CmNox2, is necessary for conidiation and parasitism, and its expression could be significantly induced by its host fungus. CmNox1 is regulated by CmRac1-CmNoxR and interacts with CmSlt2, a homolog of Saccharomyces cerevisiae Slt2 encoding cell wall integrity-related MAP kinase. In ΔCmNox1, CmSlt2-GFP fusion protein lost the ability to localize to the cell nucleus accurately. The defect of conidiation in ΔCmRac1 could be partially restored by over-expressing CmSlt2, indicating that CmSlt2 was a downstream regulatory factor of CmNox1 and was involved in conidiation and parasitism. The expressions of mycoparasitism-related genes CmPks1, Cmg1 and CH1 were suppressed in the knock-out mutants of the genes in CmNox1-CmSlt2 signal pathway when cultivated either on PDA. Therefore, our study infers that CmRac1-CmNoxR regulates CmNox1-CmSlt2 pathway in regulating conidiation and pathogenicity of C. minitans.
Sclerotinia sclerotiorum (Lib.) de Bary is a significant necrotrophic pathogen that could infect more than 400 plant species worldwide and cause huge economic losses every year1,2. Coniothyrium minitans is an important mycoparasite of Sclerotinia spp.; it could parasitize and destroy both sclerotium and hypha of its hosts. It is an effective agent for controlling crop diseases caused by S. sclerotiorum3,4,5. Like its hosts, C. minitans also occurs widely6. It shares the same growing season as its hosts, and its growth and proliferation are tightly related to the hosts. Without host fungi, C. minitans stays dormant for seasons in soil7. C. minitans is one of the commercialized fungal agents for biological control of crop diseases8.
Understanding the parasitism and conidiation of C. minitans at molecular level could help us to utilize this biological control agent more efficiently. Studies indicated that the primary cell wall degrading enzymes secreted by C. minitans are β-1,3 glucanase (encoded by Cmg1) and chitinase (encoded by CH1). The expression level of Cmg1 was enhanced when C. minitans parasitized S. sclerotiorum, indicating that β-1,3 glucanase played important roles in the parasitical process9. C. minitans also could secrete antifungal substances and degrade oxalic acid, an essential pathogenicity factor of S. sclerotiorum10,11. The parasitism of C. minitans is likely very complicated12, and shares some signal transduction pathways with conidiation. Fungal cell wall integrity-associated MAP kinase cascade, fatty acid beta-oxidation, reactive oxygen and nitrogen species, and possibly other unknown pathways in peroxisomes are required for both conidiation and mycoparasitism of C. minitans13,14. Recent research revealed that C. minitans can regulate ambient pH by degrading oxalic acid to facilitate mycoparasitism of S. sclerotiorum15,16. C. minitans requires a large amount of L-arginine during conidiation and L-arginine-derived nitric oxide was likely to be involved in conidiation with cyclic GMP functions as a second messenger17,18. Qin et al. found that phosphoribosylamidotransferase is essential for conidiation of C. minitans via adenosine related molecules, and C. minitans is able to obtain adenosine or related components from its host during parasitization19.
The NADPH oxidase complexes are conserved and play important roles in the life cycle of filamentous fungi20. In rice blast pathogen Magnaporthe oryzae, NADPH oxidase-derived reactive oxygen species (ROS) is essential for pathogenicity21, and further study found that NADPH oxidases are necessary for septin-mediated reorientation of the F-actin cytoskeleton to facilitate cuticle rupture and plant cell invasion22. NADPH oxidases are involved in sclerotial formation and pathogenicity of necrotrophic fungal pathogen Botrytis cinerea and S. sclerotiorum22,23,24,25, and fusion of conidial anastomosis tubes of B. cinerea26. ROS is critical in maintaining a mutualistic interaction between Epichloë festucae and perennial ryegrass27,28,29,30,31. In saprophytic fungi, such as Aspergillus spp., Neurospora crassa and Podospora anserina, NADPH oxidases are required for growth, cell differentiation, conidiation, and sexual reproduction32,33,34,35,36. Trichoderma harzianum NADPH oxidases are involved in the antagonism against Pythium ultimum37.
Besides NADPH oxidases mitogen-activated protein kinases (MAPKs) also play critical roles in pathogenicity and in fungal development38,39,40,41,42,43,44,45,46,47. In endophytic fungi, a stress-activated mitogen-activated protein kinase (sakA) of E. festucae is essential to maintain mutualistic symbiosis with perennial ryegrass. Deletion of sakA converted this endophytic fungus to a pathogen of its host48. MAP kinase cascade also is involved in the mycoparasitism and development of hyperparasitic fungi, such as C. minitans, Stachybotrys elegans and T. artoviride13,49,50. The phenotype of ΔsakA mutants are very similar to that of mutants whose genes in the Nox complex were disrupted, suggesting that there is a possible link between ROS signal and MAPK signal pathway on the maintenance of mutualistic symbiosis29. Similar phenomena were observed in tangerine pathogen Alternaria alternate51; Medina-Castellanos et al. found that extracellular ATP could promote the Nox1-derived ROS and activate a MAPK pathway in T. artoviride52. Recently, Jaimes-Arroyo et al. found that SrkA kinase could regulate stress responses and development in A. nidulans, and H2O2 could induce mitochondrial fragmentation and relocalize SrkA at the presence of SakA53.
Previously, we investigated a fungal cell wall integrity-associated MAP kinase cascade in C. minitans and found that this cascade was required for conidiation and mycoparasitism13. In this study, we analyzed the function of NADPH oxidases (Nox1/Nox2) complex of C. minitans, and found that CmNox1 played critical roles in conidiation and mycoparasitism, but not CmNox2. In C. minitans, CmNoxR interacts with CmRac1 to activate CmNox1, CmNox1 could also interact with CmSlt2 and thus adjust its location to the cell nucleus, and deliver the signal of conidiation and mycoparasitism.
Results
NADPH oxidases (Nox1/Nox2) in C. minitans
Two NADPH oxidase genes, CmNox1 and CmNox2, were isolated from C. minitans. The deduced amino acid sequence of CmNox1 (GenBank Accession No: KJ596434) shows high similarity to Nox1 homologs from other filamentous fungi, including Alternaria alternate (AaNox1, BAK52527.1, 89% identity), Curvularia lunata (ClNOX1, AHC53982.1, 89% identity), and Pyrenophora tritici-repentis (PrNOX1, XP_001935118.1, 88% identity). CmNox2 (GenBank Accession No: KJ596435) is highly similar to Nox2 homologs from other filamentous fungi. The multiple alignment analysis showed that both CmNox1 and CmNox2 contained NOX family signature regions. Phylogenetic analysis of NADPH oxidases in several fungi placed CmNox1 and CmNox2 homologs into two different clades based on the amino acid sequences (Supplementary Figure S1).
CmNox1 is essential for ROS production and conidiation
To study the function of CmNox1 in C. minitans, a replacement vector p3300neoCmNox1 (see Supplementary Figure S2a) was constructed and transformed into strain ZS-1 to disrupt CmNox1. Twenty transformants were obtained, and three of which, ΔCmNox1-1, ΔCmNox1-6, and ΔCmNox1-107 were selected randomly as candidates for further analyses. Furthermore, a complement vector pNox1 was transformed into mutant ΔCmNox1-6. The deletion and complementary events were confirmed by RT-PCR (see Supplementary Figure S2b) and Southern blot analysis (see Supplementary Figure S2c). Using the same methods, a replacement vector p3300neoCmNox2 was constructed (see Supplementary Figure S2a) and transformed into strain ZS-1 to disrupt CmNox2. Three deletion mutants ΔCmNox2-20, ΔCmNox2-323 and ΔCmNox2-347 were also confirmed by Southern blot analysis.
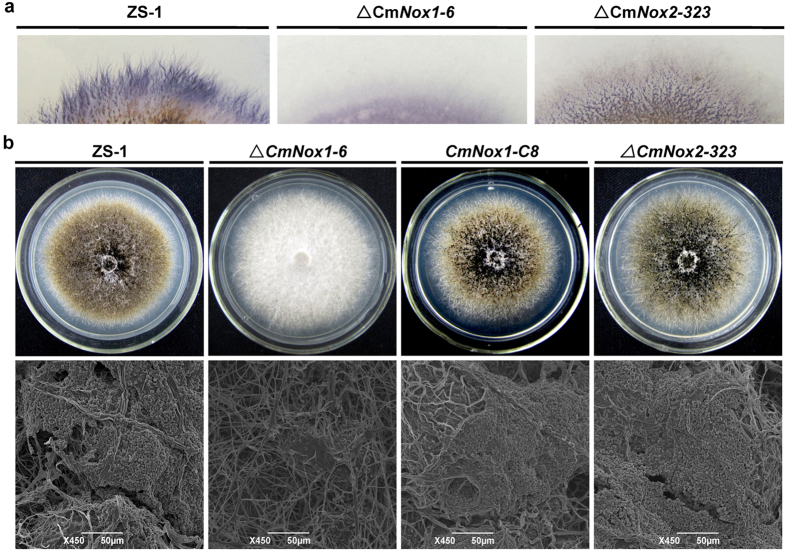
Colony staining with NBT solution showed that superoxide production was decreased significantly in ΔCmNox1-6, compared to ΔCmNox2-323 and the wild-type strain ZS-1 (Fig. 1a). The conidiation of the wild-type strain ZS-1, CmNox1 deletion mutants, CmNox1 complemented mutants, and CmNox2 deletion mutants were determined after incubating for 15 days on PDA (Fig. 1b). CmNox1 deletion mutants completely lost the ability to produce conidia (Table 1). In contrast, the wild-type strain ZS-1, CmNox1 complemented mutants and CmNox2 deletion mutants were normal in conidiation under the same condition (Table 1). Unlike strain ZS-1 and ΔCmNox2-323, which could form matured pycnidia and conidia, ΔCmNox1-6 could only form a few pycnidial primordia that could not further develop to mature pycnidia, and no conidium was produced (Fig. 1b). These data indicated that CmNox1, but not CmNox2, played significant roles in conidiation and production of superoxide. The experiments also suggested CmNox1 and CmNox2 are not essential for hyphal growth of C. minitans on PDA.
Figure 1. Production of superoxide and conidia of Coniothyrium minitans CmNox1 and CmNox2 mutants.
(a) Detection of superoxide using colony staining with NBT solution (0.05% w/v, 30 min) of cultures of C. minitans wild-type strain ZS-1, CmNox1 deletion mutant ΔCmNox1-6, CmNox2 deletion mutant ΔCmNox2-323 on PDA. (b) Pycnidial and conidial production of C. minitans wild-type strain ZS-1, CmNox1 deletion mutant ΔCmNox1-6, complementary transformant CmNox1-C8, and CmNox2 deletion mutant ΔCmNox2-323 12 days after incubation on PDA. Compared to ZS-1, CmNox1-C8 and ΔCmNox2-323, ΔCmNox1-6 lost the ability to produce pycnidia.
Table 1. Comparison of hyphal growth rate, conidial production, and parasitic ability among mutants and the wild-type strain ZS-1 of C. minitans.
| Strains | Growth ratea (mm/d) | Condiationb (x107condia/plate) | Rot indexc (%) |
|---|---|---|---|
| ZS-1 | 2.9 ± 0.1A | 128 ± 7.2A | 82 ± 3A |
| ΔCmNox1-1 | 2.8 ± 0.1A | 0B | 0B |
| ΔCmNox1-6 | 2.8 ± 0.1A | 0B | 0B |
| ΔCmNox1-107 | 2.9 ± 0.1A | 0B | 0B |
| CmNox1-C1 | 2.9 ± 0.1A | 115 ± 4.8A | 80 ± 2A |
| CmNox1-C7 | 2.8 ± 0.1A | 119 ± 4.8A | 81 ± 2A |
| CmNox1-C8 | 2.8 ± 0.1A | 121 ± 4.8A | 80 ± 2A |
| ΔCmNox2-20 | 2.9 ± 0.1A | 120 ± 5.4A | 81 ± 3A |
| ΔCmNox2-323 | 2.8 ± 0.1A | 122 ± 5.4A | 82 ± 3A |
| ΔCmNox2-347 | 2.9 ± 0.1A | 125 ± 5.4A | 82 ± 3A |
| ΔCmRac1-79 | 0.5 ± 0.1B | 0B | 0B |
| ΔCmRac1-95 | 0.5 ± 0.1B | 0B | 0B |
| ΔCmRac1-102 | 0.4 ± 0.1B | 0B | 0B |
| CmRac1-C1 | 2.9 ± 0.1A | 121 ± 9.8A | 81 ± 4A |
| CmRac1-C4 | 2.9 ± 0.1A | 119 ± 9.8A | 80 ± 4A |
| CmRac1-C7 | 2.8 ± 0.1A | 120 ± 9.8A | 81 ± 4A |
| OVS-Rac1-1 | 0.6 ± 0.1B | 0.6 ± 5A | 40 ± 3A |
| OVS-Rac1-3 | 0.5 ± 0.1B | 0.6 ± 5A | 45 ± 3A |
| OVS-Rac1-4 | 0.6 ± 0.1B | 0.5 ± 5A | 39 ± 3A |
| ΔCmNoxR-5 | 2.9 ± 0.1A | 0B | 0B |
| ΔCmNoxR-107 | 2.8 ± 0.1A | 0B | 0B |
| ΔCmNoxR-160 | 2.8 ± 0.1A | 0B | 0B |
| ΔCmBem1-344 | 2.9 ± 0.1A | 124 ± 14.2A | 84 ± 2A |
| ΔCmBem1-359 | 2.9 ± 0.1A | 125 ± 14.2A | 85 ± 2A |
| ΔCmBem1-370 | 2.9 ± 0.1A | 120 ± 14.2A | 80 ± 2A |
aGrowth rate was detected by measuring the colony diameter of cultures incubatedat 20 °C for 7 days.
bConidia produced by 14-day-old cultures and counted with a haematocytometer.
cRot index of sclerotia was calculated after infected by C. minitans for 30 days14.
dDifferent letters in the same column indicated statistically significant differences (P = 0.05). Means and standard errors were calculated from three replicates.
CmNox1 is essential to parasitize sclerotia of S. sclerotiorum
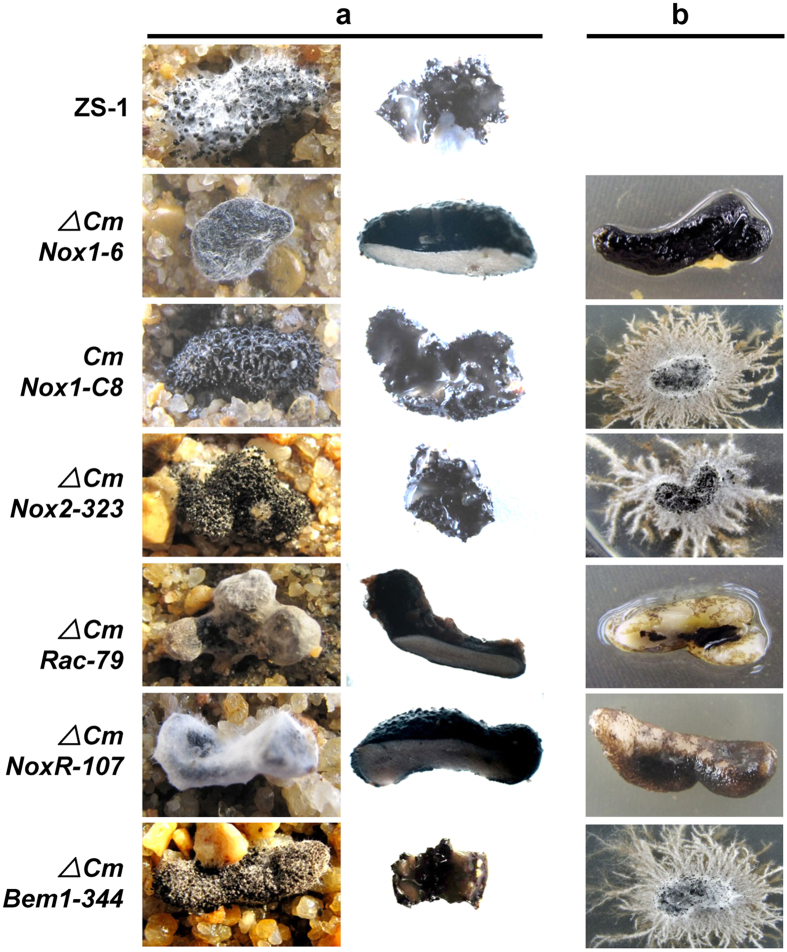
To determine whether CmNox1 is related to sclerotial parasitizing, the parasitic ability of CmNox1 mutants and the other strains to sclerotia of S. sclerotiorum were examined. The hyphae of ΔCmNox1-6 was inoculated on to sclerotia and incubated for 30 days at 20 °C, and no pycnidia and conidia were observed on either the surface or interior of the sclerotia (Fig. 2a, Table 1). The inoculated sclerotia were surface sterilized and then incubated on PDA containing 50 μg/ml hygromycin, and no C. minitans colony emerged from the sclerotia, suggesting that ΔCmNox1-6 did not invade the inner of sclerotia (Fig. 2b, Table 1). Meanwhile, CmNox1-C8, ΔCmNox2-323 and the wild-type strain ZS-1 could degrade sclerotia and produce mature pycnidia there. The results demonstrated CmNox1 played a significant role in sclerotial mycoparsitism.
Figure 2. Parasitism of C. minitans mutants to sclerotia of S. sclerotiorum.
(a) Microscopic observation of the surface and interior of sclerotia infected by C. minitans. (b) Colony formed by all inoculated sclerotia. All inoculated sclerotia were surface sterilized and then placed on hygromycin (50 μg/ml) amended PDA for 7 days.
Expression of CmNox1 is highly induced by S. sclerotiorum
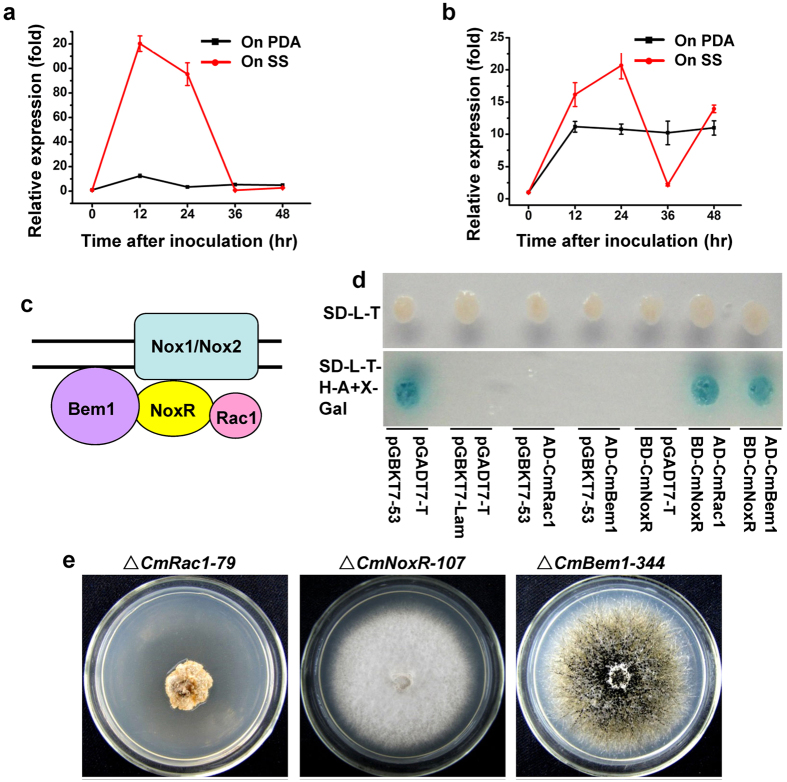
When dual cultured with S. sclerotiorum, the expression of CmNox1 was highly induced. Compared to growing on PDA, the expression of CmNox1 peaked at 12 hpi, and the high expression was maintained till 24 hpi, and then sharply declined to undetectable level at 36 hpi (Fig. 3a). This phenomenon suggested that CmNox1 played an important role in parasitism. Expression of CmNox2 also was up-regulated slightly by S. sclerotiorum at 12 hpi and 24 hpi, but compared to culture on PDA, the induction was much lower (Fig. 3b). The results showed that S. sclerotiorum could induce the expression of CmNox1 and CmNox2, with stronger induction to CmNox1.
Figure 3. NADPH oxidase complexes in C. minitans.
Expression analysis of CmNox1 (a) and CmNox2 (b). Total RNA was isolated from mycelia of ZS-1 cultured alone or dual-incubated with S. sclerotiorum on PDA, and used for cDNA synthesis. The transcript level of CmActin was used to normalize different samples. Bars represent means and standard deviations (three replications). (c) Schematic drawing showing the NADPH oxidase complexes in C. minitans. (d) Yeast two-hybrid assay of interaction between CmNoxR, CmBem1, and CmRac1. (e) Colony morphology of CmRac1 deletion mutant ΔCmRac1-79, CmNoxR deletion mutant ΔCmNoxR-107 and CmBem1 deletion mutant ΔCmBem1-344 grown on PDA at 20 °C for 12 days.
CmRac1 interacts with CmNoxR together to regulate CmNox1 and to control conidiation in C. minitans
It is reported that Bem1 and Cdc24 are components of the NADPH oxidase complex in filamentous fungi, and NoxR interacting with RacA in vitro together regulates ROS production and control hyphal branching and patterning in E. festucae27,29. Since CmNox1 is involved in the production ROS in C. minitans, we identified C. minitans homologs of NoxR, GTPase Rac1 and Bem1, named CmNoxR (GenBank Accession No: KJ596436), CmRac1 (GenBank Accession No: KJ596436) and CmBem1, respectively. The interaction between CmRac1 and CmNoxR, CmBem1 and CmNoxR were confirmed by Yeast two-hybrid (Fig. 3c,d).
To investigate the role of CmNoxR, CmBem1 and CmRac1 in C. minitans, replacement vector for CmNoxR (p3300neoCmNoxR), CmRac1 (p3300neoCmRac1), and CmBem1 (p3300neoCmBem1) were constructed and transformed into ZS-1. The CmNoxR deletion mutants (ΔCmNoxR-5 and ΔCmNoxR-107), CmBem1 deletion mutant (ΔCmBem1-344) and CmRac1 deletion mutant (ΔCmRac1-79) were obtained and confirmed by RT-PCR. In contrast to the wild-type strain ZS-1, the CmNoxR deletion mutants and the CmRac1 deletion mutant lost the ability to produce conidia and to parasitize S. sclerotiorum. In addition to the conidiation and parasitizing phenotype, growth rate also was reduced significantly in the CmRac1 deletion mutant. The growth rate of ΔCmRac1-79 was less than 0.6 mm/day on average on PDA, while it was about 3mm/day for strain ZS-1 under the same conditions. However, there was no significant difference in the growth rate between CmNoxR deletion mutants and ZS-1, and the deletion mutant displayed a phenotype similar to CmNox1 deletion mutant (Fig. 3e, Table 1). ΔCmBem1-344 shared the same phenotype as strain ZS-1. It suggests that CmRac1and CmNoxR are required to activate CmNox1 to adjust conidiation and parasitism, while CmBem1 is not involved.
CmSlt2 is CmNox1 effector required for conidiation and parasitism
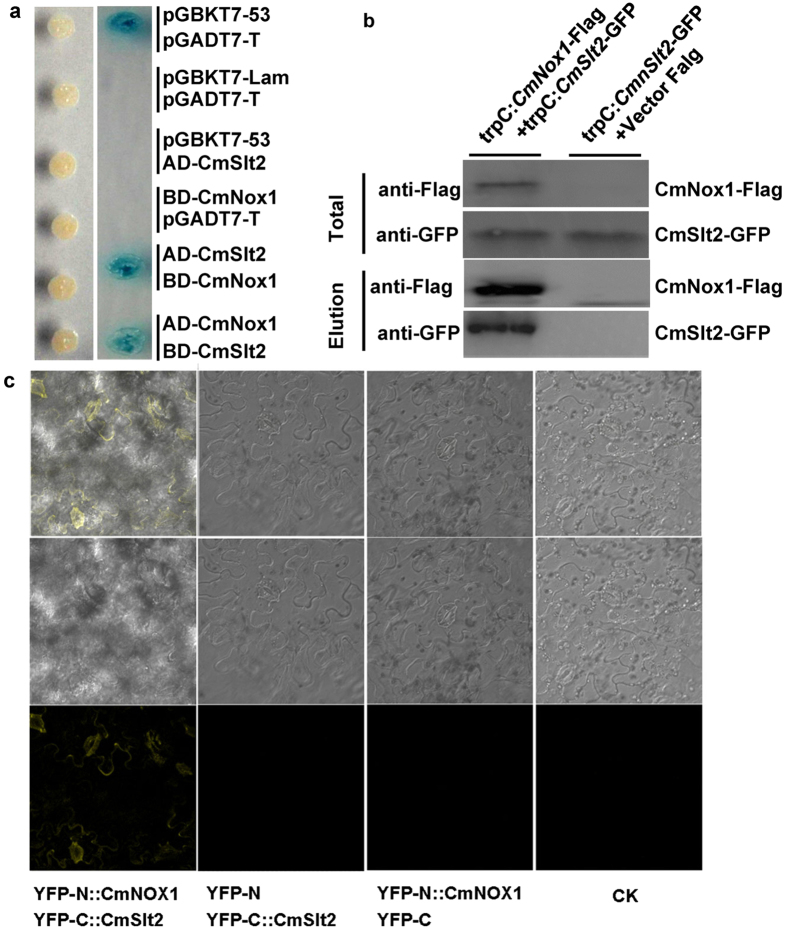
To understand the mechanism of CmNox1-mediated conidiation and parasitism in C. minitans, we further investigated the functional relationship between CmNox1 and CmSlt2. We reported previously that CmSlt2 (cell wall integrity-related MAP kinase) is essential for conidiation of C. minitans. ΔCmSlt2 lost the ability to produce pycnidia and conidia, and had dramatically reduced sclerotial mycoparasitism13. The phenotype of ΔCmSlt2 was similar to that of ΔCmNox1, thus CmSlt2 and CmNox1 might have somewhat interaction directly or indirectly. To identify whether CmSlt2 interact with CmNox1, full length gene of CmSlt2 and CmNox1 were cloned into yeast two-hybrid vector pGADT7 and pGBDT7 to test the possible interaction, and the results showed that CmSlt2 could interact with CmNox1 (Fig. 4a). Furthermore, we generated fusions of CmSlt2 and CmNox1 with N- and C-terminal domains of YFP and assessed their interaction in tobacco (Nicotiana benthamiana) using bimolecular fluorescence complementation (BiFC) (Fig. 4c). To confirm this interaction in C. minitans, a transformant with TrpC-CmNox1-Flag and TrpC-CmSlt2-GFP and a transformant with TrpC-CmSlt2-GFP and Vector-Flag in ZS-1 were constructed. In Western blot analysis with total protein, the anti-Flag and anti-GFP antibodies detected a 65-kDa and an 80-kDa band, respectively. In proteins eluted from anti-Flag agarose, the 80-kDa CmSlt2-GFP band was detected with an anti-GFP antibody in transformant with CmSlt2 and CmNox1, but not in transformant with CmSlt2 only (Fig. 4b). The results showed that CmSlt2 and CmNox1 interacted directly in C. minitans.
Figure 4. Interaction of CmNox1 with CmSlt2.
(a) Yeast two-hybrid assay of interaction between CmNox1 and CmSlt2. (b) Co-IP assays. Western blots of total proteins and proteins eluted from anti-Flag agarose from transformant (CmSlt2-GFP and vector Flag) and transformant (CmSlt2-GFP and CmNox1-Flag) were detected with anti-Flag or anti-GFP antibodies. (c) Bimolecular fluorescence complementation (BiFC) analysis of the interaction between CmNox1 and CmSlt2 tagged with YFP in Nicotiana benthamiana.
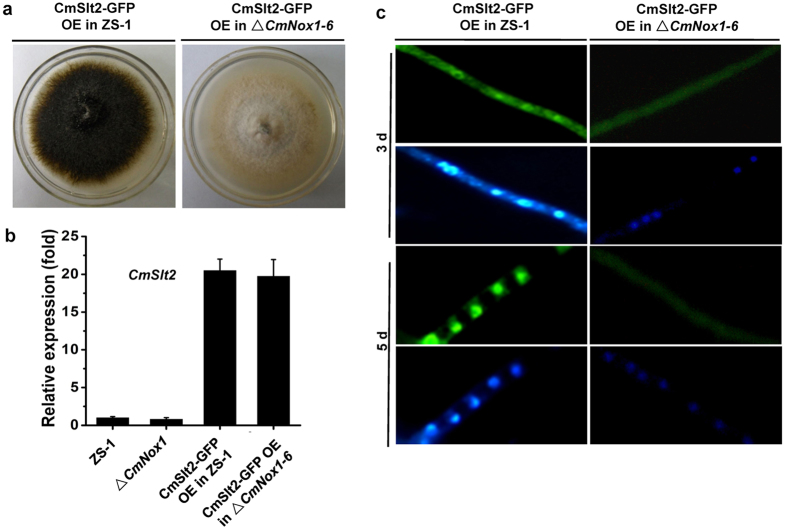
We expressed the CmSlt2-GFP fusion in the wild-type strain ZS-1 and ΔCmNox1, and found that over-expressing CmSlt2-GFP could not rescue the deficiency of conidiation and parasitism in ΔCmNox1 (Fig. 5a,b). In the wild-type strain ZS-1, CmSlt2 was located in the cytoplasm during the hyphal growth period (3-day-old hyphae), and was located in the nucleus in 40% of the hyphae at the later stage of conidiation (5-day-old hyphae) observed under a fluorescence microscope; While in ΔCmNox1, no fluorescence signal of CmSlt2- GFP was observed in the nucleus at any stages (Fig. 5c). The results suggested that CmSlt2 was a downstream regulatory factor of CmNox1 and was involved in conidiation and parasitism.
Figure 5. CmNox1 controls the nucleus location of CmSlt2.
(a) Colony morphology of CmSlt2-GFP-overexpressed ZS-1 and ΔCmNox1-6. (b) Relative expression of CmSlt2 in CmSlt2-GFP-overexpressed ZS-1 and ΔCmNox-1-6. The expression of ZS-1 was set as level one. (c) CmSlt2-GFP nuclear localization in CmSlt2-GFP-overexpressed ZS-1 and ΔCmNox1-6.
Over expressing CmSlt2 in ΔCmRac1 could partially restore conidiation and parasitism
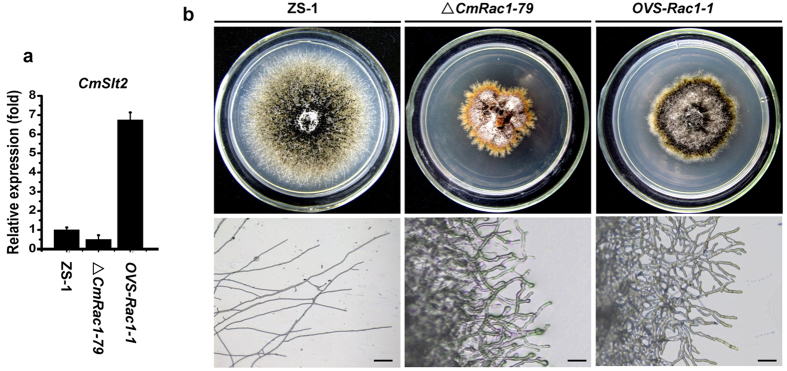
Since CmRac1 interacts with CmNoxR together in regulating CmNox1, and CmNox1 mediates the localization of CmSlt2 in nucleus, we suspected that CmRac1 is likely to regulate the expression of CmSlt2. qRT-PCR analysis showed that the expression of CmSlt2 was obviously decreased in ΔCmRac1 by 0.5 fold, compared to the wild-type strain (Fig. 6a). We fused CmSlt2 with a trpC promoter from Aspergillus and transformed into ΔCmRac1, and found that over-expressing of CmSlt2 could partially restore conidiation and parasitic ability of ΔCmRac1 (Fig. 6b, and Table 1), in spite of no significant improvement of the hyphal growth.
Figure 6. Over-expression of the MAP kinase gene CmSlt2 partially restored the conidiation of ΔCmRac1-79.
(a) The relative expression of CmSlt2 in the wild type strain ZS-1, ΔCmRac1-79 and OVS-Rac1-1 (CmSlt2 over-expressing in ΔCmRac1-79). (b) Comparison of colony morphology and tip hyphae branching pattern among the wild-type strain ZS-1, ΔCmRac1-79, and OVS-Rac1-1 grown on PDA medium at 20 °C for 20 days.
The expression of CmPks1 was suppressed in CmNox1 signal pathway mutants
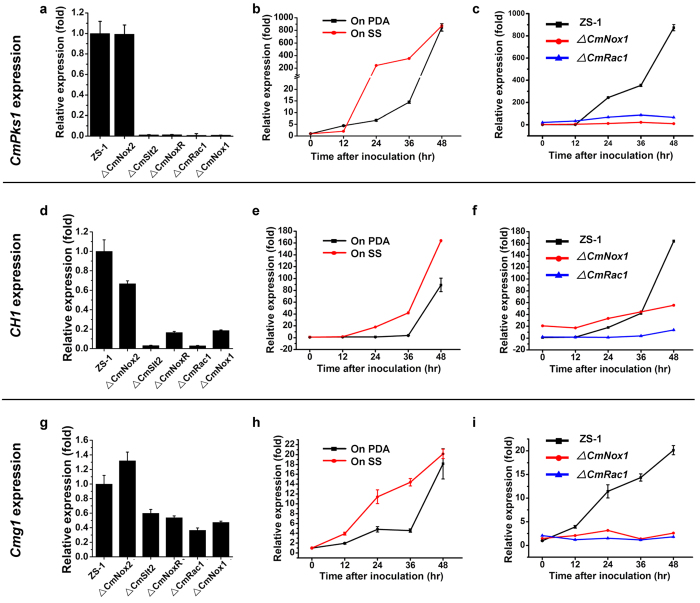
C. minitans produces dark pigment and black pycnidia during the late stage of growth. However, all ΔCmRac1, ΔCmNoxR, ΔCmNox1, and ΔCmSlt2 produce little dark pigment, and colonies were whitish. It is likely that the expression of CmPks1, a melanin biosynthesis associated polyketide synthase-encoding gene, was suppressed in those mutants. The transcript profile of CmPks1 was monitored by qRT-PCR, and the result showed that the expression of CmPks1 peaked at 96 hpi in the wild type ZS-1 and was significantly suppressed in ΔCmNoxR, ΔCmRac1, ΔCmNox1 and ΔCmSlt2, whereas there was no obvious difference in the transcript level between ΔCmNox2 and ZS-1 (Fig. 7a). Further experiments proved that the expression of CmPks1 in ZS-1 was enhanced by 300–400 fold when dual cultured with S. sclerotiorum than cultured on PDA alone at 24 hpi and 36 hpi (Fig. 7b), while it could not be induced in ΔCmRac1 and ΔCmNox1 (Fig. 7c). These results indicated that the expression of CmPks1 could be induced at the early stages of interaction with S. sclerotiorum, while could not in all of the CmNox1 signal pathway mutants either cultured on PDA or interacted with S. sclerotiorum. It suggested that the CmNox1 signal pathway is involved in regulating the expression of CmPks1.
Figure 7. Gene expression analysis in the wild-type strain ZS-1 and deletion mutants of C. minitans.
Relative transcript accumulations of CmPks1 (a), CH1 (d) and Cmg1 (g) detected with qRT-PCR amplification in ZS-1, ΔCmNox2, ΔCmSlt2, ΔCmNoxR, ΔCmRac1 and ΔCmNox1 after growing on PDA for 96 hr. The expression of ZS-1 was set as level one. Relative transcript patterns of CmPks1 (b), CH1 (e) and Cmg1 (h) in ZS-1 after contacting with S. sclerotiorum (red) or growing on PDA (black) for 0–48 hr. The gene expression of ZS-1 inoculated in plate at 0 hr was set as level one. Relative transcript patterns of CmPks1 (c), CH1 (f) and Cmg1 (i) genes in ZS-1 (black), ΔCmNox1 (red) and ΔCmRac1 (blue) after growing on PDA for 0–48 hr. The gene expression of ZS-1 at 0 hr was set as level one. The relative level of transcript was calculated by the comparative Ct method. The level of CmActin transcript was used to normalize different samples. Bars represent means and standard deviations (three replications).
The CmNox1 signal pathway is involved in regulating cell wall degrading enzyme genes
The parasitic ability of all mutants in CmNox1 signal pathways decreased obviously and it is also reported that the cell wall degrading enzyme Cmg1 (β-1,3 glucanase-encoding gene) and CH1 (chitinase gene) play important roles in the process of parasitizing sclerotia of S. sclerotiorum9. Our results of qRT-PCR demonstrated that the transcript levels of both Cmg1 and CH1 in ZS-1 were enhanced significantly at 24 hpi and 36 hpi when dual cultured with S. sclerotiorum (Fig. 7e,h), while were suppressed in ΔCmNoxR, ΔCmRac1, ΔCmNox1 or ΔCmSlt2 when incubated on PDA compared to that in ZS-1 (Fig. 7d,f,g,i). The results indicated that Cmg1 and CH1 were involved in parasitism and could be regulated by the CmNox1 signal pathway.
Discussion
The mycoparasitism system of C. minitans/S. sclerotiorum is unique and important to probe fungi and fungi interaction. In this study, the role of NADPH oxidases complex of C. minitans on growth, conidiation and mycoparasitism were studied. We found that CmNox1, but not CmNox2, played essential roles on conidiation and mycoparasitism. Furthermore, we found that CmRac1 could interact with CmNoxR, and CmNox1 could interact with CmSlt2. Expression of CmSlt2 in ΔCmRac1 could partially restore the conidiation and parasitism. These findings broadened and strengthened our knowledge of mycoparasitism, and supplied a possible link between Nox complex signal and MAPK cascade signal in fungi.
Fungal Nox isoforms have different roles in fungi lifecycle. In M. oryzae, both Nox1 and Nox2 are essential for pathogenicity21. Nox1 is required for penetration, hyphal elongation, and Nox2 is required for assembly of a toroidal F-actin network during the penetration peg formation22; In S. sclerotiorum, Nox1 is required for virulence, oxalic acid production and sclerotial development, while, Nox2 might be involved in sclerotial development24. In P. anserine, PaNox1 mutants are impaired in the differentiation of fruiting bodies from their progenitor cells, and deletion of the PaNox2 specifically blocked ascospore germination33,54. New Nox proteins were also identified from P. anserine, a Nox isoform Nox3 was found to play a minor role36. In N. crassa, Nox1 is required for female sterility, and involved in asexual development and hyphal growth, while Nox2 might be involved in ascospore germination34. In E. festucae, both ΔNoxA and ΔNoxB mutants could produce conidia, while NoxA, but not NoxB, is essential for hyphal polarized growth and hyphal funsion, NoxB did not affect conidiation29. In Sordaria macrospora, Nox1 is required for fruiting body formation, normal hyphal growth, and hyphal fusion, while Nox2 is involved in strict melanin-dependent ascospore germination55. In C. purpurea, CpNox1 is essential for infection, but CpNox2 is not essential for infection. Interestingly, Δcpnox2 mutants converted endophytic lifestyle to pathogenic lifestyle, and CpNox2 functioned in the infection process and moderates damage to the host56. In this study, the Nox1 of C. minitans is essential for mycoparasitism, conidiation and pigmentation, while deletion of Nox2 did not affect these bioprocesses. However, how Nox1 regulates these bioprocesses is necessary to be unraveled.
Rac1, a member of the Rho-family GTPases, widely exists in eukaryotes and in fungi as well. Sequence alignment of RAC1 homologs from fungi revealed significant conservation in amino acid composition and RAC1 from M. grisea could rescue the conidiation, parasitization and growth in ΔRac1 mutantof C. minitans (data not shown). Fungal Rac1 is crucial for the growth, virulence and development in many fungi, such as M. grisae, U. maydis, A. fumigatus, Claviceps purpurea and F. graminearum57,58,59,60,61. In M. grisea, MgRac1 is essential for conidiogenesis, and contributes to the formation of appressorium and pathogenicity through activating its downstream factors: the PAK kinase Chm1 and NADPH oxidases58. In Candida albicans, Rac1 is an upstream regulatory factor of the MAP kinase Cek1 and Mkc1, but the control mechanism is still unclear62. In this study, the qRT-PCR assay demonstrated that the expression level of CmSlt2 was obviously decreased in ΔCmRac1. When CmSlt2 was over expressed in ΔCmRac1, the conidiation could be partially restored, further confirming that Rac1was an upstream regulatory factor of MAPK cascade signal pathway.
The fact that the Nox complex mutants and the MAPK cascade pathway mutants of fungi sharing highly similar phenotypes suggests that these two signal pathways are cross-linked. This study provides further evidence supporting this cross-link since CmNox1 could interact with CmSlt2, and CmRac1 could interact with CmNoxR. Lalucque et al. reported that PaNox1 acted on the PaASK1/PaMKK1/PaMpk1 MAPK module by promoting nuclear translocation of the PaMpk1 MAP kinase in P. anserina63. More recently, H2O2 was found to induce the relocalization of a putative MAPK-activated protein kinase SrkA to nuclei and mitochondria under the presence of SakA in A. nidulans53. We further found that CmNox1 could regulate the nucleus location of a cell wall integrity-associated MAP kinase (CmSlt2). In ∆CmNox1, CmSlt2 could not move into fungal nuclei, suggesting that Nox complex-derived ROS may function on the localization of CmSlt2.
The Nox complex signal and MAPK cascade signal affect fungal gene expression globally to regulate complicated processes in physiology, pathogenicity and development. In S. macrospora, Nox1 affects the expression of genes involved in cytoskeleton remodeling, hyphal fusion, metabolism, and mitochondrial respiration55. In P. anserine, the expressions of 15% genes were modified in ΔPaMpk1, ΔPaMpk2 and ΔPaNox1, and about 1000 genes were regulated similarly in these three mutants64. We attempted to explain how the Nox complex signal and MAPK cascade signal regulate mycoparasitism by monitoring the expression levels of CmPks1, Cmg1 and CH1 in C. minitans. We found that expression of these genes was obviously enhanced when interacted with its host fungus S. sclerotiorum., while could not be induced in ΔCmNox1, ΔCmRac1 and ΔCmSlt2, indicating that these three genes were regulated by the Nox complex signal and MAPK cascade signal pathways, thus to affect parasitism. Mycoparasitism of C. minitans is a very complicated process. There are many other genes that are also involved in the mycoparasitism and remain to be investigated.
Briefly, we analyzed the function of Nox complex on mycoparasitism and conidiation of C. minitans, and we found that it is CmNox1, but not CmNox2, that is required for mycoparasitism and conidiation. We further found that CmNox1 interacts with MAP kinase CmSlt2 and effects the location of CmSlt2. Our finding suggests that Nox complex signal pathway and MAPK cascade signal pathway are cross-linked via CmNox1.
Methods
Strains and cultural conditions
The C. minitans wild-types train ZS-1 (CCAM 041057) was isolated from garden soil at Zhushan County, Hubei Province, P R China67. Strain Ep-1PNA367 was a virulent and virus-free strain of S. sclerotiorum, derived from a single ascospore of the hypovirulent strain Ep-1PN67. The strains used in this research were maintained and cultured on PDA at 20–22 °C and stored in PDA slants at 4 °C17,67. Cultures for genomic DNA and RNA isolation were conducted on PDA at 20–22 °C for 4 d. Conidia were prepared from 15-day-old cultures grown on PDA. The selective PDA was supplemented with 50 μg/ml of hygromycin B (Sigma) or 80 μg/ml of G418 (Sigma), depending on the selection marker in the plasmid vector.
Analysis of colony morphology, growth rate, conidiation and parasitic ability
Colony morphology was observed on PDA after incubating at 20 °C for 12 days. Observation of the pycnidial formation was performed by following the method described14. To characterize the biological properties of the mutants, growth rate, conidial production and parasitic ability were examined as described13,14. For all the transformants obtained, three individuals were examined (Table1).
ROS detection assay
Production of superoxide was evaluated with NBT using the method modified from Chen et al.58. The wild-type ZS-1, mutants ΔCmNox1 and ΔCmNox2 were grown on PDA for 7 days. Mycelia were incubated in 0.05 M sodium phosphate buffer, pH 7.5, containing 0.05% (w/v) NBT (Sigma-Aldrich). After 1 h of incubation, the culture was fixed in ethanol to stop the reaction. The stained sample was examined with a compound microscope at ×400 magnification.
Isolation of CmNox1/CmNox2
A PCR primer pair 1F1/1R1 (Table S1) was designed based on the C. minitans genome database. CmNox1 was amplified from the ZS-1 genomic DNA by a 32-cycle PCR reaction (94 °C, 1 min; 58 °C, 1 min; 72 °C, 2 min), followed by a 10 min extension at 72 °C. The PCR product was cloned into the pMD18-T vector (TaKaRa) and confirmed by DNA sequencing. The cDNA of CmNox1 was isolated by RT-PCR from total RNA of C. minitans with the primer pair 1F1 and 1R1, followed by cloning into the pMD18-T vector and direct DNA sequencing. The same method was used to clone CmNox2 in C. minitans with the primer pair 2F1/2R1 (Table S1).
Vector construction and Agrobacterium-mediated transformation
The CmNoxR, CmRac1, CmNox1, CmNox2 and CmBem1 replacement constructs were generated according to Qin et al.19. The CmNox1 (primer pairs 9F1/9R1 and 10F1/10R1), CmNox2 (primer pairs 11F1/11R1 and 12F1/12R1), CmNoxR (primer pairs 13F1/13R1 and 14F1/14R1), CmRac1 (primer pairs 15F1/15R1 and 16F1/16R1), and CmBem1 (primer pairs 17F1/17R1 and 18F1/18R1) deletion constructs were made by PCR amplification of the 5′- and 3′- flanks of the respective ORFs (0.8 –1.2 kb) with genomic DNA of the wild-type strain ZS-1as template. The fragments were cloned upstream and downstream of hph cassette in pMD18 respectively, and then the structures were cloned into the corresponding sites of vector pneo-P3300III, which carries a neomycin resistance gene cassette (neo) as the second selection marker.
In order to construct the complementary vector of CmNox1, a 2.7 kb fragment containing the native promoter and ORF of CmNox1 was amplified by PCR with primer pair 19F1/19R1 (Table S1) and cloned into vector pneoP3300. The complementary mutant CmNox1-C was generated by introducing the vector into ΔCmNox1-6, followed by screening for neomycin resistance and RT-PCR confirmation.
cDNA of CmSlt2 was amplified by RT-PCR with primer pair 20F/20R (Table S1) and cloned into the HindIII/SmaI sites of pCIT vector, which contains the trpC promoter and terminator, and then the gene cassette was cloned into vector pneoP3300 to get the CmSlt2 over-expression vector pOVSlt2. The over-expression mutant OVS-Rac1-1 was generated by introducing pOVSlt2 into ΔCmRac1-79, followed by screening for neomycin resistance and qRT-PCR confirmation.
To tag CmSlt2 with the GFP, cDNA of CmSlt2 without stop codon was amplified by RT-PCR with primer pair 20F/20R (Table S1) and cloned into the HindIII/SmaI sites of pCIT vector. The GFP coding sequence was excised from plasmid pEGFP-1 with SmaI/BamHI, and the corresponding fragment was purified. Both the CmSlt2 and GFP coding sequences were cloned into vector pneoP3300 digested with XhoI to yield plasmid pSGFP1. Mutants were generated by introducing of pSGFP1 into ΔCmNox1-6 and the wide-type strain ZS-1, followed by screening for neomycin resistance. GFP signal was observed under a Nikon Eclipse 80i fluorescent microscope (Nikon, Japan).
DNA extraction and Southern blot analysis
The genomic DNA of the wild-type strain ZS-1 and generated mutants in this study was extracted according to the standard protocols67. Southern blot analysis was performed according to Gong et al.16. Genomic DNA (ZS-1 and CmNox1 mutants) aliquots of 15 μg were digested with HindIII, separated by electrophoresis on 0.8% agarose gel and transferred onto a Hybond N+ membrane (Amersham Pharmacia Biotech). Interior probe was amplified with the primer pair 30F and 30R (Fig. 2c, Table S1), while the exterior probe was amplified with the primer pair 31F and 31R (Fig. 2c, Table S1). CmNox2 mutants were also confirmed by Southern blot, with the probe amplified using primer pair 32F and 32R.
RNA manipulation and qRT-PCR analysis
The total RNA sample of fungal strain was isolated with TriZOL reagent (Invitrogen, USA) according to the manufacturer’s protocols and potential DNA contamination was removed by DNase I treatment (RNase Free) (TaKaRa, Dalian, China). The first-strand cDNA was synthesized with RevertAid™ First Strand cDNA Synthesis Kit (MBI Fermentas, Lithuania) by following the manufacturer’s instructions. In quantitative Real-time PCR, CmPks1 (primer pair 21F/21R), CmRac1 (primer pair 22F/22R), CmNox1 (primer pair 23F/23R), CmNox2 (primer pair 24F/24R), CmNoxR (primer pair 25F/25R), Cmg1 (primer pair 26F/26R), CH1 (primer pair 27F/27R) and CmSlt2 (primer pair 34F/34R) were amplified by using the respective primer pairs (Table S1). As an endogenous control, a 154-bp amplicon of actin was amplified with primer pair 28F and 28R (Table S1).
Yeast two-hybrid system
Yeast two-hybrid analysis was carried out using a GAL4-based yeast two-hybrid system-Matchmaker™ Gold Systems (Clontech, Palo Alto, CA). cDNA of CmNox1 (1F1/1R1), CmSlt2 (2F2/2R2), CmRac1 (3F/3R) and CmNoxR (4F/4R), were amplified with the respective primer pairs (Table S1) and inserted into the yeast vector pGADT7. To test the specificity of the interaction, the bait plasmid and the prey plasmids were co-transformed into yeast strain Y2HGold. The transformants were assayed on SD (synthetic dropout)/-Trp-Leu-His-Ade plates and SD/-Trp-Leu-His-Ade plates with X-α-gal for β-galactosidase test.
Bimolecular fluorescence complementation experiments
For bimolecular fluorescence complementation experiments (BiFC), CmNox1 and CmSlt2 were tagged with separated halves of YFP, based on Walter et al.68, with the following modifications. To fuse YFP-N to CmSlt2, CmSlt2 cDNA without stop codon was amplified with primer pair 7F and 7R and cloned into the SpeI/SmaI sites of pUC-SPYNE1. To fuse YFP-C to CmNox1, CmNox1 cDNA without stop codon was amplified with primer pair 8F and 8R and cloned into the SpeI/SmaI sites of pUC-SPYCE1. The two vectors were co-transformed into tobacoo (Nicotiana benthamiana). Leaf samples were collected 2 days after agro-infiltration. YFP Fluorescence signal was observed under a Nikon Eclipse 80i fluorescent microscope (Nikon, Japan).
In vivo Co-IP
Agrobacterium strain EHA105 carrying the TrpC-CmSlt2-GFP and TrpC-CmNox1-Flag expression vectors was coinfiltrated into C. minitans. The over-expression mutant was generated by screening for neomycin/hygromycin resistance. Mutant transformed with TrpC-CmSlt2-GFP and empty Vector-Flag was generated and used as a control. For in vivo Co-IP, 8-day-old mutant total protein was extracted in IP buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton, 1 mM EDTA, 1 mM MgCl2, and protease inhibitor cocktail). A 15 ul aliquot of anti-Flag agarose (Beyotime) was added to the samples, and the mixtures were incubated for 4 h at 4 °C with shaking. The immunoprecipitated proteins were washed three times with IP buffer and eluted with SDS sample buffer. Eluted sample were loaded on the protein gels for immunoblot analysis using anti-GFP antibody or anti-Flag antibody (Sigma).
Additional Information
How to cite this article: Wei, W. et al. Nox Complex signal and MAPK cascade pathway are cross-linked and essential for pathogenicity and conidiation of mycoparasite Coniothyrium minitans. Sci. Rep. 6, 24325; doi: 10.1038/srep24325 (2016).
Supplementary Material
Acknowledgments
The research was financially supported by the National `973’ Basic Research Program of China (Grant No. 2012cb114000), the National Natural Science Foundation of China (grant 31371895), the Programme of Introducing Talents of Discipline to Universities in China (the 111 Project no. B14032), and the Program for Changjiang Scholars and Innovative Research Team in University of China (IRT1247).
Footnotes
Author Contributions W.W., D.J., W.C. and Y.F. designed the research and wrote the paper; W.W., W.Z. and J.C. executed the experiments. J.C., J.X., G.L. and Y.F. performed the data and bioinformatics analyses. All authors read and approved the final manuscript.
References
- Bolton M., Thomma B. P. H. J. & Nelson B. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol 7, 1–16 (2006). [DOI] [PubMed] [Google Scholar]
- Amselem J. et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet 7, e1002230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps J. M. & Gerlagh M. Biology of Coniothyrium minitans and its potential for use in disease biocontrol. Mycol Res 96, 897–907 (1992). [Google Scholar]
- Li G. et al. Biological control of Sclerotinia diseases of rapeseed by aerial applications of the mycoparasite Coniothyrium minitans. Eur J Plant Pathol 114, 345–355 (2006). [Google Scholar]
- Whipps J. M., Sreenivasaprasad S., Muthumeenakshi S., Rogers C. W. & Challen M. P. Use of Coniothyrium minitans as a biocontrol agent and some molecular aspects of sclerotial mycoparasitism. Eur J Plant Pathol 121, 323–330 (2008). [Google Scholar]
- Sandys-Winsch C., Whipps J. M., Gerlagh M. & Kruse M. World distribution of the sclerotial mycoparasite Coniothyrium minitans. Mycol Res 97, 1175–1178 (1993). [Google Scholar]
- Yang L. et al. Effects of soil temperature and moisture on survival of Coniothyrium minitans conidia in central China. Biol Control 55, 27–33 (2010). [Google Scholar]
- Paulitz T. C. & Belanger R. R. Biological control in greenhouse systems. Annu Rev of phytopathol 39, 103–33 (2001). [DOI] [PubMed] [Google Scholar]
- Giczey G., Kernyi Z., Fulop L. & Hornok L. Expression of cmg1, an exo-β- glucanase gene from Coniothyrium minitans, increase during sclerotial parasitism. Appl Environ Microbiol 67, 865–87 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Han Y., Li G., Jiang D. & Huang H. Suppression of Sclerotinia sclerotiorum by antifungal substances produced by the mycoparasite Coniothyrium minitans. Eur J Plant Patho 119, 411–420 (2008). [Google Scholar]
- Ren L., Li G., Han Y., Jiang D. & Huang H. Degradation of oxalic acid by Coniothyrium minitans and its effects on production and activity of beta-1,3-glucanase of this mycoparasite. Biol Control 43, 1–11 (2007). [Google Scholar]
- Muthumeenakshi S., Sreenivasaprasad S., Rogers C. W., Challen M. P. & Whipps J. M. Analysis of cDNA transcripts from Coniothyriumminitans reveals a diverse array of genes involved in key processes during sclerotial mycoparasitism. Fungal Genet Biol 44, 1262–1284 (2008). [DOI] [PubMed] [Google Scholar]
- Zeng F. Y. et al. A fungal cell wall integrity-associated MAP kinase cascade in Coniothyrium minitans is required for conidiation and mycoparasitism. Fungal Genet Biol 49, 347–357 (2012). [DOI] [PubMed] [Google Scholar]
- Wei W. et al. CmPEX6, a gene involved in peroxisome biogenesis, is essential for parasitism and conidiation of sclerotial parasite Coniothyrium minitans. Appl Environ Microbiol 79, 3658–3666 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L. M. et al. Degradation of oxalic acid by the mycoparasite Coniothyrium minitans plays an important role in interacting with Sclerotinia sclerotiorum. Environ Microb 16, 2591–2610 (2014). [DOI] [PubMed] [Google Scholar]
- Lou Y. et al. CmpacC regulates mycoparasitism, oxalate degradation and antifungal activity in the mycoparasitic fungus Coniothyrium minitans. Environ Microb 17(11), 4711–4729 (2015). [DOI] [PubMed] [Google Scholar]
- Gong X. Y. et al. L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet Biol 44, 1368–1379 (2007). [DOI] [PubMed] [Google Scholar]
- Li B. et al. Cyclic GMP as a second messenger in the nitric oxide-mediated conidiation of the mycoparasite Coniothyrium minitans. Appl Environ Microbiol 76, 2830–2836 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L. et al. Phosphoribosylamidotransferase, the first enzyme for purine de novo synthesis, is required for conidiation in the sclerotial mycoparasite Coniothyrium minitans. Fungal Genet Biol 48, 956–965 (2011). [DOI] [PubMed] [Google Scholar]
- Scott B. Conservation of fungal and animal nicotinamide adenine dinucleotide phosphate oxidase complexes. Mol Microb 95, 910–913 (2015). [DOI] [PubMed] [Google Scholar]
- Egan M. J., Wang Z. Y., Jones M. A., Smirnoff N. & Talbot N. J. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci USA 104, 11772–11777 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder L. S. et al. NADPH oxidases regulate septin-mediated cytoskeletal remodeling during plant infection by the rice blast fungus. Proc Natl Acad Sci USA 110, 3179–3184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segmuller N. et al. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol Plant Microbe Interact 21, 808–819 (2008). [DOI] [PubMed] [Google Scholar]
- Kim H. J., Chen C. B., Kabbage M. & Dickman M. B. Identification and characterization of Sclerotinia sclerotiorum NADPH oxidases. Appl Environ Microbiol 77, 7721–7729 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund U., Marschall R. & Tudzynski P. BcNoxD, a putative ER protein, is a new component of the NADPH oxidase complex in Botrytis cinerea. Mol Microbiol 95, 988–1005 (2015). [DOI] [PubMed] [Google Scholar]
- Roca M. G. et al. Germling fusion via conidial anastomosis tubes in the grey mould Botrytis cinerea requires NADPH oxidase activity. Fungal Biol 116, 379–387 (2012). [DOI] [PubMed] [Google Scholar]
- Tanaka A., Christensen M. J., Takemoto D., Park P. & Scott B. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell 18, 1052–1066 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D., Tanaka A. & Scott B. A. p67 Phox-like regulator is recruited to control hyphal branching in a fungal-grass mutualistic symbiosis. Plant Cell 18, 2807–2821 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton C. J., Cox M. P. & Scott B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci 180, 190–195 (2011). [DOI] [PubMed] [Google Scholar]
- Takemoto D. et al. Polarity proteins Bem1 and Cdc24 are components of the filamentous fungal NADPH oxidase complex. Proc Natl Acad Sci USA 108, 2861–2866 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano Y., Tanaka A., Akano F. & Scott B. Differential roles of NADPH oxidases and associated regulators in polarized growth, conidiation and hyphal fusion in the symbiotic fungus Epichloe festucae. Fungal Genet Biol 56, 87–97 (2013). [DOI] [PubMed] [Google Scholar]
- Lara-Ortiz T., Riveros-Rosas H. & Aguirre J. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol 50, 1241–1255 (2003). [DOI] [PubMed] [Google Scholar]
- Malagnac F., Lalucque H., Lepère G. & Silar P. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet Biol 41, 982–997 (2004). [DOI] [PubMed] [Google Scholar]
- Cano-Domínguez N., Álvarez-Delfí K., Hansberg W. & Aguirre J. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot Cell 7, 1352–1361 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semighini C. P. & Harris S. D. Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics 179, 1919–1932 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun S., Malagnac F., Bidard F., Lalucque H. & Silar P. Functions and regulation of the Nox family in the filamentous fungus Podospora anserina: a new role in cellulose degradation. Mol. Microbiol 74, 480–496 (2009). [DOI] [PubMed] [Google Scholar]
- Montero-Barrientos M., Hermosa R., Cardoza R. E., Gutierrez S. & Monte E. Functional analysis of the Trichoderma harzianum nox1 gene, encoding an NADPH oxidase, relates production of reactive oxygen species to specific biocontrol activity against Pythium ultimum. Appl Environ Microbiol 77, 3009–3016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. R. MAP kinases in fungal pathogens. Fungal Genet Biol 31, 137–152 (2000). [DOI] [PubMed] [Google Scholar]
- Zhao X. H., Mehrabi R. & Xu J. R. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot Cel l6, 1701–1714 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. H., Kim Y., Park G. & Xu J. R. A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea. Plant Cell 17, 1317–1329 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispail N. et al. Comparative genomics of MAP kinase and calcium-calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet Biol 46, 287–298 (2009). [DOI] [PubMed] [Google Scholar]
- Wang C. F. et al. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathog 7, e1002460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel L. P., Nicole M. C., Duplessis S. & Ellis B. E. Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct message from conserved messenger. Plant Cell 24, 1327–1351 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert I. et al. PRO40 is a scaffold protein of the cell wall integrity pathway, linking the MAP kinase module to the upstream activator protein kinase C. PLoS Genet 10, e1004582 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrà D., Segorbe D. & Di Pietro A. Protein kinases in plant-pathogenic fungi: conserved regulators of infection. Annu Rev of Phytopathol 52, 267–288 (2014). [DOI] [PubMed] [Google Scholar]
- Yun Y. et al. The MAPKK FgMkk1 of Fusarium graminearum regulates vegetative differentiation, multiple stress response, and virulence via the cell wall integrity and high-osmolarity glycerol signaling pathways. Environ Microbiol 16, 2023–2037 (2014). [DOI] [PubMed] [Google Scholar]
- Gu Q., Chen Y., Liu Y., Zhang C. & Ma Z. The transmembrane protein FgSho1 regulates fungal development and pathogenicity via the MAPK module Ste50-Ste11-Ste7 in Fusarium graminearum. New Phytologist 206, 315–328 (2015). [DOI] [PubMed] [Google Scholar]
- Eaton C. J. et al. Disruption of signaling in a fungal-grass symbiosis leads to pathogenesis. Plant Physiol 153, 1780–1794 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun R., Aliferis K. A. & Jabaji S. H. Characterization and transcriptional regulation of Stachybotrys elegans mitogen-activated-protein kinase gene smkA following mycoparasitism and starvation conditions. Current Genet 59, 43–54 (2013). [DOI] [PubMed] [Google Scholar]
- Gruber S. & Zeilinger S. The transcription factor Ste12 mediates the regulatory role of the Tmk1 MAP kinase in mycoparasitism and vegetative hyphal fusion in the filamentous fungus Trichoderma atroviride. PLoS One 9, e111636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. L. & Chung K. R. Similar and distinct roles of NADPH oxidase components in the tangerine pathotype of Alternaria alternate. Mol Plant Pathol 14, 543–556 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Castellanos E., Esquivel-Naranjo E. U., Heil M. & Herrera-Estrella A. Extracellular ATP activates MARK and ROS signaling during injury response in the fungus Trichoderma atroviride. Front in Plant Sci 5, 659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes-Arroyo R. et al. The SrkA kinase is part of the SakA mitogen-activated protein kinase interactome and regulates stress responses and development in Aspergillus nidulans. Eukaryot Cel l 14, 495–510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambou K. et al. The crucial role of the Pls1 tetraspanin during ascospore germination in Podospora anserine provides an example of the convergent evolution of morphogenetic processes in fungal plant pathogens and saprobes. Eukaryot Cell 7, 1809–1818 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirschnabel D. E. et al. New insights into the roles of NADPH oxidases in sexual development and ascospore germination in Sordaria macrospora. Genetics 196, 729–744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann J., Buttermann D. & Herrmann A. Molecular characterization of the NADPH oxidase complex in the ergot fungus Claviceps purpurea: CpNox2 and CpPls1 are important for a balanced host-pathogen interaction. Mol Plant Microbe Interact 26, 1151–1164 (2013). [DOI] [PubMed] [Google Scholar]
- Mahlert M., Levelek L., Hlubek A., Sandrock B. & Bolker M. Rac1 and Cdc42 regulate hyphal growth and cytokinesis in the dimorphic fungus Ustilago maydis. Mol Microbiol 59, 567–578 (2006). [DOI] [PubMed] [Google Scholar]
- Chen J. et al. Rac1 is required for pathogenicity and Chm1-dependent conidiogenesis in rice fungal pathogen Magnaporthe grisea. PLoS Pathog 4, e1000202 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke Y. & Tudzynski P. The small GTPase Rac and the p21- activated kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development and pathogenicity. Mol Microbiol 68, 405–423 (2008). [DOI] [PubMed] [Google Scholar]
- Li H. Y. et al. The small GTPase RacA mediates intracellular reactive oxygen species production, polarized growth, and virulence in the human fungal pathogen Aspergillus fumigatus. Eukaryot Cell 10, 174–186 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. et al. Functional characterization of Rho family small GTPases in Fusarium graminearum. Fungal Genet Biol 61, 90–99 (2013). [DOI] [PubMed] [Google Scholar]
- Hope H., Schmauch C., Arkowitz R. A. & Bassilana M. The Candida albicans ELMO homologue functions together with Rac1 and Dck1, upstream of the MAP Kinase Cek1, in invasive filamentous growth. Mol Microbiol 76, 1572–1590 (2010). [DOI] [PubMed] [Google Scholar]
- Lalucque H., Malagnac F., Brun S., Kicka S. & Silar P. A Non-mendelian MAPK-generated hereditary unit controlled by a second MAPK pathway in Podospora anserine. Genetics 191, 419–433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard F., Coppin E. & Silar P. The transcriptional response to the inactivation of the PaMpk1 and PaMpk2 MAP kinase pathways in Podospora anserine. Fungal Genet Biol 49, 643–652 (2012). [DOI] [PubMed] [Google Scholar]
- Cheng J. S. et al. Production, survival and efficacy of Coniothyrium minitans conidia produced in shaken liquid culture. FEMS Microbiol Lett 227, 127–131 (2003). [DOI] [PubMed] [Google Scholar]
- Xie J. et al. Characterization of debilitation-associated mycovirus infecting the plant-pathogenic fungus Sclerotinia sclerotiorum. J Gen Virol 87: 241–249 (2006). [DOI] [PubMed] [Google Scholar]
- Sambrook J. & Russell D. W. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. (2001). [Google Scholar]
- Walter M. et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40, 428–438 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.