Abstract
Diffuse pulmonary lymphangiomatosis (DPL) is a rare lymphatic disorder characterized by uncontrolled proliferation of lymphatic vessels. Histologically benign, however, it can lead to death because of its progression. In this paper, we would like to present an unusual case of diffuse pulmonary lymphangiomatosis involving a 28-year-old young female who was suffered chest pain and polypnea for one year, and also a lot of chylous effusion in left chest. Lymphoma and lymphangitic metastasis was the primary diagnosis in other hospitals. However, histologic sections of pleural biopsy revealed diffuse pleural lymphatic proliferation characteristic of DPL, wherein the histologic findings can be subtle and could be overlooked as nonspecific reactive changes or misdiagnosed as an idiopathic interstitial lung disease. Recognition of the characteristic lymphangitic distribution of abnormally dilated or reduplicated lymphatic spaces is the key to the correct diagnosis.
Keywords: Diffuse pulmonary lymphangiomatosis (DPL), diagnosis, pathology analysis
Introduction
Diffuse pulmonary lymphangiomatosis (DPL) is a rare lymphatic disorder characterized by uncontrolled proliferation of lymphatic vessels. It usually occurs in children and young adults, affects both sexes equally (1-3). Clinical symptoms such as chest pain, cough and dyspnea, pulmonary function with restrictively ventilatory dysfunction, and interstitial pneumonia, are all not specific (3). Histologically benign, however, it can lead to death because of its progression. No treatments were given and the patient was only subjected to routine follow up. This disease is different from lymphangiectasis and lymphangioleiomyomatosis but is often misdiagnosed. Pathology is helpful, mainly characterized by multifocal proliferation of lymphatic vessels and increased number of complex anastomosing channels. These channels tend to dilate with time. In this paper, We would like to present an unusual case of Diffuse pulmonary lymphangiomatosis involving a 28-year-old young female, who presented with progressive chest pain and polypnea of one year duration, and CT showed much left hydrothorax. In laboratory tests, hydrothorax in thoracic drainage tube was chylous, and pleural mesothelioma lymphoma and lymphangitic metastasis was the primary differential diagnosis. However, histologic sections of pleural biopsy revealed diffuse pleural and interlobular septal lymphatic proliferation characteristic of DPL.
Case presentation
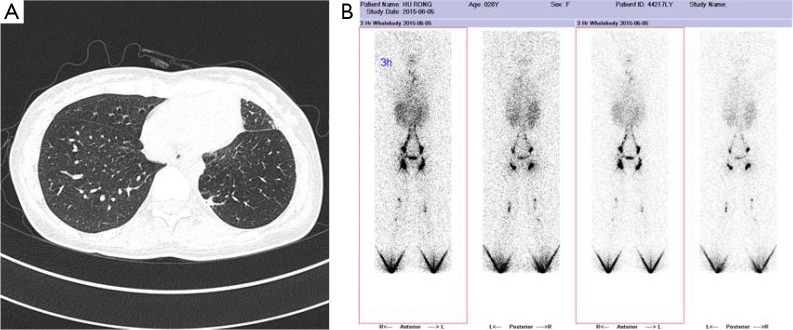
The patient was a 28-year-old female who had complained of chest pain and polypnea for one year. She had no symptoms of cough, expectoration and fever, and she had received antibiotic therapy at a local hospital for symptomatic treatment. This patient had no history of trauma, no history of surgery, and no other meaningful history. Upon physical examination, she only presented an anemic appearance, and other signs were all negative. Laboratory values upon admission showed microcytic hypochromic anemia, with hemoglobin value of 74 g/L and albumin value of 25.4 g/L. She was received lose concentration of red blood cells to correct anaemia. Pulmonary function examination reveals limitation ventilation dysfunction. The tumor markers (CEA, CA-125, CA-153 and CA-199) were within normal limits. A laboratory examination for tuberculosis (T-SPOT, TB test) was negative. And assay of serum, hydrothorax examination chylus experiment was positive, and the test of pleural effusion lipid probe [Total cholesterol was 1.47 mmol/L. Triglycerides was 5.34 mmol/L. High density lipoprotein (HDL) cholesterol and low density lipoprotein (LDL) was 0.26 and 0.71 mmol/L, each other] was abnormal. And what more, pleural effusion of biological and chemical target, etiological examination, tumor markers, and cytological examinations did not reveal specificity to diagnosis. In addition, lymphography showed that lymph fluid in left thorax was more density collective focus than the opposite. Chest computed tomography (CT) scan revealed a low-density lesion along the pulmonary vessels and a lot of red effusion in the left pleural cavity (Figure 1A). Abdominal CT revealed low density nearly round nodule without enhancement distributed in the spleen area, the biggest lesion reached 3.1×3.0 cm2. Lymphangiography show lymphatic chain structure of both lower limbs is complete, and also lymphatic drainage can be unblocked (Figure 1B). Bilateral inguinal and iliac blood lymph nodes is clear, but, contrast agent in the left chest is more concentrations than the other.
Figure 1.
A 28-year-old female with diffuse pulmonary lymphangiomatosis. (A) High resolution CT with diffuse pulmonary lymphangiomatosis. There is diffuse, smooth subpleural and interlobular septal thickening, peribronchovascular thickening is also present; (B) lymphangiography show lymphatic chain structure of both lower limbs is complete, and also lymphatic drainage can be unblocked.
Pathology
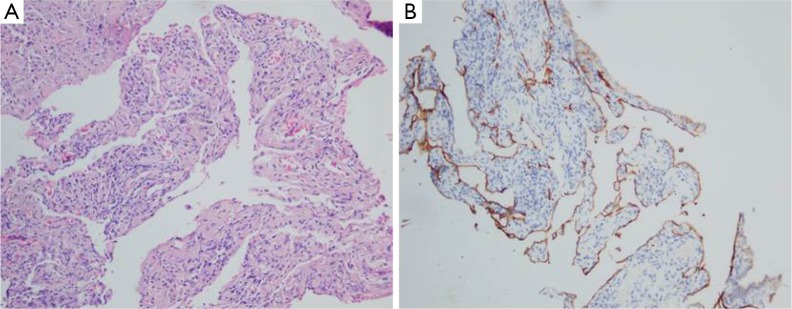
To confirm diagnosis, thoracoscopy were performed, therefore, pleura biopsy was implemented in follow. Pathologically, the pleura biopsy showed diffuse and complex dilation of irregular lymphatic vessels, lined by a single layer of flat endothelial cells, with variable amounts of fibrosis, and anastomosing spaces filled with eosinophilic material or chyle. Moreover, focal haemorrhage and edema, and aggregates of inflammatory cells mainly containing small lymphocytes were often observed (Figure 2A). The dilated lymphatic spaces usually had suffered muscularization, it could easily result in misclassification as veins. Immunohistochemical staining showed that the abnormal lymphatic channels were positive for the lymphatic endothelial antigen recognized by D2-40 (4) (Figure 2B) and CD31. Spindle cells can be found arranged in poorly delineated fascicles. The spindle cells vary in their positivity to antibodies, which react with antigens commonly found in smooth muscle cells (e.g., vimentin, desmin, alpha smooth muscle). In this case, a diagnosis of diffuse pulmonary lymphangiomatosis was established on account of the above histological findings.
Figure 2.
A 28-year-old female with diffuse pulmonary lymphangiomatosis. (A) HE-section of lesion showing proliferation of thin-walled, anastomosing lymphatic vessels lined by single layer of endothelial cells lacking cytological atypia (arrows, 200×); (B) immunohistochemical staining with D2-40 revealing proliferative lymphatic channels (200×).
Discussion
Diffuse pulmonary lymphangiomatosis are benign, cystic and focal regions of lymphatic proliferation, which can be localized to the lung or chest or more widespread with multiple organ (2,5). This disease can frequently present in childhood, and lead to infiltrative disease, restrictive and/or obstructive ventilation dysfunction, chylous effusions and moreover, respiratory failure (6), wherein the histologic findings can be subtle and could be often misdiagnosed or never diagnosed for nonspecific reactive changes. Therefore, recognition of the characteristic lymphangitic distribution of abnormally dilated or reduplicated lymphatic channel is key to the correct diagnosis.
In this report, lymphangiomatosis involved in pleura and spleen, and the patient suffered mainly complained of chest pain and polypnea for one year, and amount of chylothorax was chief symptoms. Findings on chest CT are nonspecific and can reveal peribronchovascular and interlobular septal thickening, furthermore, diffuse interstitial infiltrates and pleural thickening with effusions. In spleen, these findings are suggestive—but are not pathognomonic—for DPL. Histological findings of pleura biopsy are characterized by abnormal proliferation of anastomosing lymphatic channels. We confirmed this by immunohistochemical staining for the lymphatic endothelial antigen with D2-40 and CD31 antibodies. Our case is showed characteristic histological changes and special immunophenotype of diffuse pulmonary lymphangiomatosis. This disease is often misdiagnosed or never diagnosed, therefore, recognition of the characteristic lymphangitic distribution of abnormally dilated or reduplicated lymphatic channel is key to the correct diagnosis (the same with pathology).
Histologically, diffuse pulmonary lymphangiomatosis is difficult to differentiate DPL from other lymphatic diseases such as lymphangioma, lymphangiomyomatosis, lymphangiectasia etc. Pulmonary lymphangioleiomyomatosis (LAM) is a benign, uncommon disorder of unknown etiology affecting women of child-bearing age (7). CT image showed that multiple thin-walled and air-filled cysts distributed in size-ranging throughout both lungs (8). In addition, pathologically, because there is no infiltration into the lung parenchyma, or the typical HMB45 and MalanA-positive in LAM cells and also positive for myogenic markers and estrogen receptors (ER) and progestin receptors (PR) are specific and Sensitive. Instead, complex anastomosing lymphatic channels are contained within the normal lymphatic pathways of the lung. The small portion of mature smooth muscle cells further distinguishes DPL from LAM. Finally, the presence of endothelial markers such as CD31, factor VIII-related antigen and D2-40 on immunohistochemical staining is characteristic of DPL. Lymphangiectasis can rarely present in adults and should include thorough clinical, radiologic, and pathologic correlation. Chest CT usually showed thickening of the bronchovascular bundles, interlobular septa, and pleura (9). The key to recognizing these conditions on biopsy is the lymphangitic distribution of abnormal lymphatics, with dilation in lymphangiectasis and anastomosing proliferation in lymphangiomatosis. D2-40 immunostain should aid in differentiating them from veins when muscularization of the lymphatics is present.
Kaposi’s sarcoma is a cancer caused by infection with human herpesvirus 8 (HHV8), also resulted in patches of abnormal tissue to grow under the skin, in the lining of the mouth, nose, and throat or in other organs. Under the microscope, it is different from DPL that anastomosing of spindle cell and small lacunae vasorum formed in tumor (10-12). Capillary hemangiomas are the most common vascular anomaly and the most common tumor of childhood and infancy, and pathologically, unencapsulated aggregates of closely packed, thin-walled capillaries, usually with endothelial lining (13).
Conclusions
DPL can often have a progressive course giving rise to respiratory failure, even death in child and adolescents. Clinical presentations varies from mild wheezing and moderate cough to marked respiratory insufficiency due to progressive in infiltrative disease and recurrent pleural effusions (14). Pleural effusions are often chylous, the same with our case, and patients may have associated asthma syndrome, chyloptysis, hemoptysis or chylopericardium. Chest X-ray or CT can help us the location and degree of the disease, which present diffuse mediastinal soft tissue infiltration, pulmonary interstitial parenchymal infiltration, and pleural effusion (15). Bronchoscopy can display airway mucosal erythema and edema, bronchial narrowing and, in advanced cases, thin-walled vesicles containing chylous fluid, even it is nonspecific. Although DPL could be made a definite diagnosis through bronchoscopy with transbronchial biopsy, most cases in the literature were confirmed through open lung biopsy. Prognosis is generally poor and treatment is largely supportive, on account of no specific targeting treatments available for this disorder, only with routine symptomatic follow-up (16).As discussed above, combining clinical symptoms, chest X-ray/CT, bronchoscopy and particularly immunohistological indentification of appropriate tissue biopsies are mainly approach in exactly diagnosis of DPL.
Acknowledgements
The authors thank Longguang Li in the Division of Respiratory Pathology Center at the State Key Laboratory of Respiratory Disease (Guangzhou Medical University) for taking Pathological figures.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Swensen SJ, Hartman TE, Mayo JR, et al. Diffuse pulmonary lymphangiomatosis: CT findings. J Comput Assist Tomogr 1995;19:348-52. 10.1097/00004728-199505000-00002 [DOI] [PubMed] [Google Scholar]
- 2.Faul JL, Berry GJ, Colby TV, et al. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 2000;161:1037-46. 10.1164/ajrccm.161.3.9904056 [DOI] [PubMed] [Google Scholar]
- 3.Noonan JA, Walters LR, Reeves JT. Congenital pulmonary lymphangiectasis. Am J Dis Child 1970;120:314-9. [DOI] [PubMed] [Google Scholar]
- 4.Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest 2002;82:1255-7. 10.1097/01.LAB.0000028824.03032.AB [DOI] [PubMed] [Google Scholar]
- 5.Taylor JR, Ryu J, Colby TV, et al. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med 1990;323:1254-60. 10.1056/NEJM199011013231807 [DOI] [PubMed] [Google Scholar]
- 6.Satria MN, Pacheco-Rodriguez G, Moss J. Pulmonary lymphangiomatosis. Lymphat Res Biol 2011;9:191-3. 10.1089/lrb.2011.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly J, Moss J. Lymphangioleiomyomatosis. Am J Med Sci 2001;321:17-25. 10.1097/00000441-200101000-00004 [DOI] [PubMed] [Google Scholar]
- 8.Adachi K, Miki Y, Saito R, et al. Intracrine steroid production and mammalian target of rapamycin pathways in pulmonary lymphangioleiomyomatosis. Hum Pathol 2015;46:1685-93. 10.1016/j.humpath.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 9.Brown M, Pysher T, Coffin CM. Lymphangioma and congenital pulmonary lymphangiectasis: a histologic, immunohistochemical, and clinicopathologic comparison. Mod Pathol 1999;12:569-75. [PubMed] [Google Scholar]
- 10.Whitby D, Howard MR, Tenant-Flowers M, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 1995;346:799-802. 10.1016/S0140-6736(95)91619-9 [DOI] [PubMed] [Google Scholar]
- 11.Pammer J, Plettenberg A, Weninger W, et al. CD40 antigen is expressed by endothelial cells and tumor cells in Kaposi's sarcoma. Am J Pathol 1996;148:1387-96. [PMC free article] [PubMed] [Google Scholar]
- 12.Arul AS, Kumar AR, Verma S, et al. Oral Kaposi's sarcoma: Sole presentation in HIV seropositive patient. J Nat Sci Biol Med 2015;6:459-61. 10.4103/0976-9668.160041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tajima S, Koda K. A case of cord capillary hemangioma of the spleen: a recently proven true neoplasm. Pathol Int 2015;65:254-8. 10.1111/pin.12277 [DOI] [PubMed] [Google Scholar]
- 14.Banieghbal B, Davies MR. Guidelines for the successful treatment of lymphangioma with OK-432. Eur J Pediatr Surg 2003;13:103-7. 10.1055/s-2003-39581 [DOI] [PubMed] [Google Scholar]
- 15.Kotecha R, Mascarenhas L, Jackson HA, et al. Radiological features of Gorham's disease. Clin Radiol 2012;67:782-8. 10.1016/j.crad.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 16.Ozeki M, Fukao T, Kondo N. Propranolol for intractable diffuse lymphangiomatosis. N Engl J Med 2011;364:1380-2. 10.1056/NEJMc1013217 [DOI] [PubMed] [Google Scholar]