Abstract
Background
Vascular dysfunction is a distinctive phenotype in diabetes mellitus. Current treatments mostly focus on the tight glycemic control and few of these treatments have been designed to directly recover the vascular dysfunction in diabetes. As a classical natural medicine, berberine has been explored as a possible therapy for DM. In addition, it is reported that berberine has an extra-protective effect in diabetic vascular dysfunction. However, little is known whether the berberine treatment could ameliorate the smooth muscle contractility independent of a functional endothelium under hyperglycemia. Furthermore, it remains unknown whether berberine affects the arterial contractility by regulating the intracellular Ca2+ handling in vascular smooth cells (VSMCs) under hyperglycemia.
Methods
Sprague–Dawley rats were used to establish the diabetic model with a high-fat diet plus injections of streptozotocin (STZ). Berberine (50, 100, and 200 mg/kg/day) were intragastrically administered to control and diabetic rats for 8 weeks since the injection of STZ. The intracellular Ca2+ handling of isolated cerebral VSMCs was investigated by recording the whole-cell L-type Ca2+ channel (CaL) currents, assessing the protein expressions of CaL channel, and measuring the intracellular Ca2+ in response to caffeine. Our results showed that chronic administration of 100 mg/kg/day berberine not only reduced glucose levels, but also inhibited the augmented contractile function of cerebral artery to KCl and 5-hydroxytryptamine (5-HT) in diabetic rats. Furthermore, chronic administration of 100 mg/kg/day berberine significantly inhibited the CaL channel current densities, reduced the α1C-subunit expressions of CaL channel, decreased the resting intracellular Ca2+ ([Ca2+]i) level, and suppressed the Ca2+ releases from RyRs in cerebral VSMCs isolated from diabetic rats. Correspondingly, acute application of 10 μM berberine could directly inhibit the hyperglycemia-induced CaL currents and suppress the hyperglycemia-induced Ca2+ releases from RyRs in cerebral VSMCs isolated from normal control rats.
Conclusions
Our study indicated that berberine alleviated the cerebral arterial contractility in the rat model of streptozotocin-induced diabetes via regulating the intracellular Ca2+ handling of smooth muscle cells.
Keywords: Berberine, Contractile function, Vascular smooth muscle cells (VSMCs), L-type Ca2+ channel (CaL), Ca2+ releases
Background
Diabetes mellitus (DM) is a kind of metabolic diseases characterized by chronic hyperglycemia due to either reduced insulin secretion (type 1 DM) or insulin resistance (type 2 DM). DM is also considered to be a kind of vascular disorders for diabetic macro- and microvascular complications, such as coronary arterial impairment, cerebrovascular injury, and diabetic retinopathy, nephropathy, and neuropathy, are the principal causes of morbidity and mortality in patients with type 1 or type 2 DM [1, 2]. It has been demonstrated that diabetic vascular complication is associated with impaired endothelial function, augmented vasoconstriction, increased oxidative stress, enhanced inflammation, and promoted thrombosis [2]. It is believed that hyperglycemia is the hallmark feature in the development of diabetic vascular dysfunction and so current treatments mostly focus on the tight glycemic control. However, few of these treatments have been designed to directly recover the vascular dysfunction in diabetes. Because the elevated concentration of intracellular Ca2+ ([Ca2+]i) is the primary stimulus for smooth muscle contraction, it is reported that diabetic vascular dysfunction is tightly coupled to the impairment of intracellular Ca2+ handling in vascular smooth muscle cells (VSMCs) [3, 4]. Ca2+ influx from the long-lasting voltage-dependent Ca2+ (L-type, CaL) channels in plasma membrane and Ca2+ releases from the ryanodine receptors (RyRs) in sarcoplasmic reticulum (SR) are the key factors to regulate the intracellular Ca2+ in VSMCs [3, 5]. An emerging body of evidence from human diabetes and different diabetic animal models indicated that hyperglycemia induced an increase of [Ca2+]i, promoted the Ca2+ influx by the activation of CaL channels, and altered the Ca2+ releases from the RyRs in VSMCs [3, 5, 6]. Therefore, the intracellular Ca2+ handling with their related proteins could be an important therapeutic target in diabetic vascular complications [3].
Berberine is a classical natural medicine, which has been widely used for bacteria-associated diarrhoea and other gastrointestinal infections in China [7]. In recent years, the herbal compound berberine has been explored as a possible therapy in DM for its metabolic activities of lowing blood glucose and regulating lipids, which has been studied and evidenced in the treatment of human diabetes and animal diabetic models [8, 9]. In addition, extensive research demonstrated that berberine treatment also had a cardiovascular protective effect in diabetic nephropathy, diabetic neuropathy, and diabetic cardiomyopathy [10–12]. For example, the berberine treatment was found to ameliorate diabetic endothelium-dependent relaxation by reducing oxidative stress and inflammatory response [10, 13–15]. It is believed that hyperglycemia may injury vascular function at both endothelium level and smooth muscle cell layers [2, 3]. However, previous researches on berberine mostly focused on the endothelial cells in the treatment of diabetic vascular dysfunction. Little is known whether the berberine treatment could ameliorate the smooth muscle contractility independent of a functional endothelium under hyperglycemia. Furthermore, it remains unknown whether berberine affects the smooth muscle contractility by regulating the intracellular Ca2+ handling in VSMCs under hyperglycemia.
The purpose of the present study was (1) to investigate the effects of berberine treatment on cerebrovascular contractile function independent of a functional endothelium in streptozotocin (STZ)-induced diabetic rats; (2) to investigate the effects of berberine on the intracellular Ca2+ handling of cerebral VSMCs in diabetic rats or when exposed to hyperglycemia condition, such as recording the whole-cell CaL currents, assessing the protein expressions of CaL channel, and measuring the intracellular Ca2+ in response to caffeine. Taken together, the present study provided initial evidences that berberine alleviates the cerebrovascular contractile function directly by the inhibition of CaL channels and the suppression of Ca2+ releases from the RyRs in cerebral VSMCs of streptozotocin-induced diabetic rats.
Methods
All animal procedures described in this study were in adherence with theGuide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996), with approval from Committee on the Ethics of Animal Experiments of the University of Xi’an Jiaotong University. In addition, the animal research performed in the present study was in compliance with the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines [16]. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering unless otherwise stated, all chemicals and reagents used in this study were obtained from Sigma Chemical Company (St. Louis, Missouri, USA).
Animal model
Male Sprague–Dawley rats (~190 g) used in this study were purchased from Medical Laboratory Animal Center of Xi’an Jiaotong University. After an adaptive feeding for 1 week, the SD rats were randomly divided into two groups: diabetic rats and control rats. The diabetic rat model was established with a high-fat diet plus injections of STZ, which was described previously [13, 17]. The high-fat diet consisted of 70 % standard laboratory chow, 5 % yolk powder, 10 % lard, 15 % carbohydrate. The control rats were given the regular diet. Following 4 weeks of dietary intervention, the diabetic rats were injected intraperitoneally with 45 mg/kg STZ for consecutively twice in 2 days, which was freshly dissolved in 0.1 M sodium citrate buffer (pH 4.5–5.0). In contrast, the control rats were injected intraperitoneally with vehicle citrate buffer in a dose volume of 1 ml/kg. Blood samples were collected by tail cutting for fasting blood glucose measurements by glucose oxidase/peroxidase method. Mean values of the fasting serum insulin level were measured by radioimmunoassay method. Successful induction of diabetes was considered as sustained fasting blood glucose levels >11.1 mM at 72 h after STZ-injection. Failed induction of diabetes was excluded during the whole study. The present study was divided into three experiments: Experiment I, Experiment II, and Experiment III. Experiment I was designed to investigate the effects of 100 mg/kg/day berberine on the contractile function of cerebral artery, the function of CaL channel, and the intracellular Ca2+ in response to caffeine in STZ-induced diabetic rats. Experiment II and Experiment III were designed to investigate the effects of 50 and 200 mg/kg/day berberine on the contractile function of cerebral artery, the function of CaL channel, and the intracellular Ca2+ in response to caffeine in STZ-induced diabetic rats. In Experiment I, rats were divided into four groups (n = 20/group): control rats (CON), control rats administered with berberine chloride (CON + 100 mg/kg/day berberine), diabetic rats, and diabetic rats administered with berberine chloride (Diabetic + 100 mg/kg/day berberine). In Experiment II and Experiment III, rats were divided into four groups (n = 10/group): control rats (CON), control rats administered with 50 or 200 mg/kg/day berberine chloride, diabetic rats, and diabetic rats administered with 50 or 200 mg/kg/day berberine chloride, respectively. After that, the diabetic and control rats were fed on the high-fat diet and the regular diet for another 8 weeks, respectively. Berberine chloride were dissolved and then intragastrically administered daily for 8 weeks. The other groups received equal volume of vehicles. The intragastric dose of berberine in this study was used according to our pre-experiments and the previous reports that the dose of 100 mg/kg/day is more close to the clinical practice than veno-injection or intraperitoneal administration [13, 18]. Eight weeks after the berberine treatment, animals were anesthetized with pentobarbital sodium (50 mg/kg ip) and killed by exsanguinations via the abdominal aorta. Body weight and fasting blood glucose were measured every week for monitoring diabetic condition. All groups were caged individually in a room maintained at 23 °C on a 12:12-h light–dark cycle and received water ad libitum.
Examination of contractile function
As previously described [19], the segment of middle cerebral artery was transferred to the PSS containing (in mM): 119 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 5.5 glucose, and 0.026 EDTA, equilibrated with 21 % O2, 5 % CO2, and 74 % N2 at pH 7.4 adjusted with NaOH. The endothelial layer was mechanically removed by the injection of air bubbles and then cannulated by two pipettes with nylon suture in a vessel chamber. After cannulation, the chamber was transferred to the Pressure Myograph System P110 (DMT, Denmark) and the arterial segment was perfused under a pressure of 25 mmHg for 5–10 min to check the leaking and then remove the blood residue. The arterial segment was allowed to equilibrate at 37 °C and 50 mmHg for 1 h. After equilibration, the arterial viability was evaluated by its reactivity to 20 and 60 mM isotonic KCl. Then the pressure was cycled three times between 25 and 125 mmHg to reduce mechanical hysteresis. To determine the contractile function, concentration–response relationships were determined by the cumulative superfusion of isotonic KCl (0–100 mM) and 5-hydroxytryptamine (5-HT, 10−10–10−4 M) while the arteries were pressurized at 50 mmHg in Ca2+-contained PSS. Contractile response to cumulative superfusion of KCl or 5-HT was represented as the percentage of luminal diameter relative to the baseline internal diameter according to the formula: luminal diameter change (%) = (Di,a,s−Di,a,b)/Di,a,b × 100 %, where Di,a,b is the baseline internal diameter measured in active state at a pressure of 50 mmHg and Di,a,s is the steady-state internal diameter measured to each subsequent change in agonist concentration at the same pressure.
Isolation of VSMCs
Enzymatic isolation of single VSMC was carried out as previously described [20]. Briefly, brain tissues were removed rapidly and placed in 4 °C physiological salt solution (PSS) which contained (in mM) 137 NaCl, 5.6 KCl, 1 MgCl2, 0.42 Na2HPO4, 0.44 NaH2PO4, 4.2 NaHCO3, and 10 HEPES, equilibrated with 95 % O2 and 5 % CO2 at pH adjusted to 7.4 with NaOH. The cerebral arteries including superior, middle, and basilar arteries with the circle of Willis were dissected and digested for 18 min at 37 °C with the solution contained 4 mg/ml papain (Biochrom, Berlin, Germany), 2 mg/ml dithioerythritol (Amresco, St. Louis, Missouri, USA), 1 mg/ml bovien serum albumin (BSA), and 5 mM taurine in PSS. Arterial segments were then transferred to enzyme-free PSS containing 1 mg/ml BSA and 5 mM taurine at room temperature for 10 min and triturated with a flame-polished pipette to disperse VSMCs. Isolated VSMCs were suspended in Ca2+-free PSS containing 1 mg/ml BSA and 5 mM taurine and stored at 4 °C for use within 8 h.
Electrophysiological measurements
As previously described [21], patch-clamp recordings were performed with an amplifier (CEZ-2300, Nihon Kohden Co., Tokyo, Japan), a version interface (Axon Instruments, Foster City, California, USA), and the pCLAMP software (version 8.0, Axon Instruments). Patch pipettes (tip resistance 2–6 MΩ) were fabricated on an electrode puller (Narishige Instruments, Tokyo, Japan). Whole-cell CaL channel currents were measured with the conventional voltage clamp configuration. Cell capacitance (Cm) was estimated from the capacitive current transient evoked by applying a 20-mV pulse for 40 ms from a holding potential of −60 to −40 mV. The cell was held at −40 mV and then stepped in 10-mV increments from −30 to +60 mV. Voltage steps were 250 ms in duration with 2-s intervals between steps. Nonspecific membrane leakage and residual capacitive currents were subtracted using the P/4 protocol. Currents were sampled and averaged while the current amplitude was stabilized. Barium (Ba2+) was used as the charge carrier to increase unitary currents. Currents were normalized to Cm to obtain the current densities. To obtain the I–V curve of CaL, the current densities were plotted against the corresponding command potentials. Two kinds of external solutions were used, i.e., solution A and B. Solution A was used while making a gigaohm seal between the recording pipette and cell surface. It contained (in mM) 130 NaCl, 5.4 KCl, 1 MgCl2, 10 BaCl2, 10 HEPES, and 10 glucose, equilibrated with 95 % O2 and 5 % CO2 at pH 7.4 adjusted with NaOH. After a seal of 2 GΩ was obtained, the perfusion fluid was changed to solution B before current recording. It contained (in mM) 75 Tris–Cl, 50 BaCl2, 10 HEPES, and 10 glucose, equilibrated with 95 % O2 and 5 % CO2 at pH 7.4 titrated with Tris base. The pipette solution contained 150 CsCl, 1 MgCl2, 10 EGTA, 5 HEPES, 5 Na2ATP, and 5 Na2 creatine phosphate, equilibrated with 95 % O2 and 5 % CO2 at pH 7.2 titrated with CsOH. In the present study, extracellular application of 5 μM Bay K 8644 (the specific agonist of CaL) and 0.1 μM nifedipine (the specific blocker of CaL) were used to identify the properties of CaL as described before [21]. All measurements were performed at room temperature (22–24 °C).
Evaluation of CaL channel protein expression by Western blotting
According to our previous report [21], cerebral arteries were minced into small pieces and homogenized on ice containing tissue protein extraction reagent (T-PER, Pierce, Rockford, Illinois, USA) and protease inhibitor (Halt, Pierce, Rockford, Illinois, USA). Large tissue debris and nuclear fragments were removed by two centrifuge spins (1000 rpm for 5 min, 12,000 rpm for 15 min) at 4 °C and supernatants were obtained. The concentration of protein sample was determined by the bicinchoninic acid method (Pierce, Rockford, Illinois, USA) using BSA as a standard. Equivalent amounts of proteins from different groups were loaded to adjacent lanes for SDS-PAGE. Protein samples were run for 80 min at 30 mA for an electrophoretic size-separation using a 8 % Tris–Glycine gel (Invitrogen, Carlsbad, California, USA). After size separation, proteins were transferred onto a nitrocellulose membrane at 100 mA for 3 h and blocked with 5 % nonfat dry milk in PBS containing 0.1 % (w/v) Tween 20 (PBS-T) overnight at 4 °C. Subsequently, the membranes were incubated for 3 h with a 1:200 dilution of rabbit polyclonal antibody against amino acids 848–865, which corresponds to the C-terminus site of CaL channel α1C-subunit (Alomone Labs, Jerusalem, Israel). The membrane then incubated for 45 min with Infrared (IR)-labeled secondary antibodies (LI-COR) in PBS-T containing 0.01 % SDS. A monoclonal mouse antibody raised against the structural protein β-actin (Sigma) was used as a lane-loading control. The bound antibody was detected by the Odyssey infrared imaging system (LI-COR), and the densities of immunoreactive band were expressed as a percentage of the β-actin density for each lane. Densitometry analysis of bands was performed by Scion image (Scion, Frederick, MD).
Measurement of intracellular Ca2+ in response to caffeine
As previously described [21], we investigated the average changes of intracellular Ca2+ fluorescence intensity with Ca2+ indicator, Fluo-3-acetoxymethyl ester (Fluo-3/AM, Molecular Probes, Oregon, USA). Isolated cerebral VSMCs were incubated with Fluo-3/AM in a final concentration of 5 μM for 30 min at 37 °C. Then, the Fluo-3/AM-loaded cells were washed with Ca2+-free balanced salt solution (BSS) which contained (mM) 126 NaCl, 5 KCl, 0.3 NaH2PO4, 10 HEPES, 1 MgCl2, 10 glucose, 1 EGTA, equilibrated with 95 % O2 and 5 % CO2 at pH 7.4 adjusted with NaOH. The cells were scanned under a laser confocal microscope (Olympus FV1000, Tokyo, Japan) by illuminating with a krypton/argon laser at 488 nm emitted light and capturing the emitting fluorescence at 526 nm. To ensure efficient quantum capture, the cells were placed on the bottom of a recording chamber and images were recorded after 10–20 s when fluorescence intensity became stable. During continuously scanning, 10 mM caffeine in Ca2+-free BSS was administrated to the cell and a period of 3 min was recorded. To avoid any laser-induced change in Ca2+ signaling, each cell was scanned only once. The average fluorescence intensity was used to indicate the changes of intracellular Ca2+. The maximal increase of Ca2+ fluorescence intensity was used to indicate the function of ryanodine-sensitive Ca2+ releases from SR in VSMCs.
Statistical analysis
Except the data of body weight are given as mean ± SD, all other data are expressed as mean ± SEM. One-way ANOVA was used to determine the differences of CaL channel current densities in different groups, followed by a S–N–K-Post Hoc. Student’s t test was used to determine significant differences of body weights, blood glucose, the resting Ca2+ fluorescence intensity, and the maximal increase of Ca2+ fluorescence intensity in different groups. A value of P ≤ 0.05 was considered to be statistically significant.
Results
Physical characteristics of experimental animals
The rat model of STZ-induced diabetes is well-established using a toxin to destroy the pancreatic β-cells. As compared with control rats, the levels of blood glucose significantly increased and the body weights significantly decreased in diabetic rats from 4 to 8 week after STZ-injection, respectively, which indicated that diabetic rat model has been established successfully [13, 18]. In Experiment I, when the diabetic rats were treated with 100 mg/kg/day berberine for 4 or 8 weeks, there were a marked decrease in the blood glucose and an obvious increase in the body weight of diabetic rats, respectively, which is well in accordance with earlier studies [13, 18]. However, there were also the significant differences in the blood glucose and body weight between Diabetic + berberine and CON rats, which indicated that berberine treatment did not restore the blood glucose and body weight of diabetic rats to the normal control level (Table 1). When the control rats were treated with 100 mg/kg/day berberine, there was only a declined tendency and no significant difference both in the blood glucose and body weight between CON + berberine and CON rats. In addition, as shown in Table 2, diabetic rats showed a drastic increase in serum insulin level as compared with the normal control group, which is similar to previous report [13]. After 8 weeks of treatment of 100 mg/kg/day berberine, serum insulin levels also significantly decreased in diabetic rats. These results indicated that 100 mg/kg/day berberine treatment obviously has a hypoglycemic effect in diabetic rats.
Table 1.
Body weight and fasting blood glucose in CON, CON +100 mg/kg/day berberine, diabetic, and diabetic +100 mg/kg/day berberine rats at 4 and 8 weeks after STZ-injection in Experiment I
| Initial | 4 Week after STZ-injection | 8 Week after STZ-injection | ||||
|---|---|---|---|---|---|---|
| Body weight (g) | Blood glucose (mM) | Body weight (g) | Blood glucose (mM) | Body weight (g) | Blood glucose (mM) | |
| CON (n = 20) | 195.0 ± 25.7 | 4.2 ± 1.2 | 423.5 ± 55.8 | 5.2 ± 0.9 | 501.0 ± 75.6 | 5.6 ± 1.5 |
| CON + berberine (n = 20) | 186.5 ± 28.1 | 3.8 ± 2.2 | 417.9 ± 65.3 | 4.1 ± 1.2 | 483.5 ± 72.1 | 4.5 ± 1.7 |
| Diabetic (n = 20) | 190.0 ± 23.4 | 3.6 ± 1.3 | 270.3 ± 46.3* | 21.9 ± 5.4* | 251.0 ± 52.7* | 25.91 ± 6.6* |
| Diabetic + berberine (n = 20) | 187.0 ± 25.5 | 4.1 ± 1.6 | 327.0 ± 51.2#,* | 16.5 ± 4.5#,* | 353.0 ± 58.3#,* | 14.8 ± 4.3#,* |
CON control rats, CON + berberine control rats administrated with berberine (100 mg/kg/day), Diabetic diabetic rats, diabetic + berberine diabetic rats administrated with berberine (100 mg/kg/day). * P < 0.05 vs. CON and # P < 0.05 vs. diabetic rats
Table 2.
Mean values of the serum insulin level in CON, CON +100 mg/kg/day berberine, diabetic, and diabetic +100 mg/kg/day berberine rats at 8 weeks after STZ-injection in Experiment I
| Group | Insulin (μIU/ml) |
|---|---|
| CON (n = 20) | 9.0 ± 1.5 |
| CON + berberine (n = 20) | 8.7 ± 1.7 |
| Diabetic (n = 20) | 13.6 ± 1.8* |
| Diabetic + berberine (n = 20) | 11.5 ± 1.2#,* |
CON control rats, CON + berberine control rats administrated with berberine (100 mg/kg/day), Diabetic diabetic rats, diabetic + berberine diabetic rats administrated with berberine (100 mg/kg/day). * P < 0.05 vs. CON and # P < 0.05 vs. diabetic rats
In Experiment II and Experiment III, when the diabetic rats were treated with 50 mg/kg/day berberine for 8 weeks, there were no obvious effects on blood glucose and body weight in CON and diabetic rats, respectively. However, 200 mg/kg/day berberine for 8 weeks significantly decreased blood glucose in both CON and diabetic rats (Table 3).
Table 3.
Body weight and fasting blood glucose in CON, CON +50 or 200 mg/kg/day berberine, diabetic, and diabetic +50 or 200 mg/kg/day berberine rats at 8 weeks after STZ-injection in Experiment II and Experiment III
| Initial | 8 Week after STZ-injection | |||
|---|---|---|---|---|
| Body weight (g) | Blood glucose (mM) | Body weight (g) | Blood glucose (mM) | |
| Experiment II | ||||
| CON (n = 10) | 187.0 ± 22.8 | 4.9 ± 1.8 | 493.0 ± 55.2 | 5.2 ± 2.3 |
| CON +50 mg/kg/day berberine (n = 10) | 190.5 ± 18.3 | 4.3 ± 2.0 | 511.5 ± 62.1 | 5.7 ± 2.1 |
| Diabetic (n = 10) | 189.0 ± 17.6 | 4.6 ± 1.9 | 253.0 ± 72.7* | 19.9 ± 4.9* |
| Diabetic +50 mg/kg/day berberine (n = 10) | 191.0 ± 18.5 | 4.9 ± 2.5 | 261.0 ± 68.7#,* | 18.2 ± 5.3* |
| Experiment III | ||||
| CON (n = 10) | 195.0 ± 23.1 | 4.5 ± 1.2 | 503.0 ± 47.9 | 5.6 ± 1.2 |
| CON +200 mg/kg/day berberine (n = 10) | 196.5 ± 18.6 | 3.9 ± 2.5 | 406.5 ± 57.2* | 3.7 ± 1.1* |
| Diabetic (n = 10) | 200.0 ± 21.7 | 4.3 ± 1.8 | 296.0 ± 82.1* | 18.8 ± 4.5* |
| Diabetic +200 mg/kg/day berberine (n = 10) | 192.0 ± 17.8 | 4.4 ± 1.6 | 381.0 ± 55.8# | 10.2 ± 3.7#,* |
CON control rats, CON + berberine control rats administrated with berberine, Diabetic diabetic rats, diabetic + berberine diabetic rats administrated with berberine. * P < 0.05 vs. CON and # P < 0.05 vs. diabetic rats
Chronic treatment of 100 mg/kg/day berberine significantly inhibited the augmented contractile function of middle cerebral artery in diabetic rats
In the present study, removal of the endothelial layer was used to rule out the endothelium-dependent relaxation, which may affect the contractile responses of VSMCs. As compared with that in CON, contractile responsiveness of middle cerebral artery to KCl (Fig. 1a) and 5-HT (Fig. 1b) were both significantly increased in diabetic rats, which is in accordance with previous report [22]. In Experiment I, chronic administration of berberine (100 mg/kg/day) for 8 weeks significantly inhibited the augmented contractile responsiveness of middle cerebral artery to KCl (Fig. 1a) and 5-HT (Fig. 1b) in diabetic rats, respectively. For example, when the concentration of KCl was 60 mM, 100 mg/kg/day berberine treatment could markedly reduce the relative changes of luminal diameter from (−36.5 ± 3.2) % in diabetic rats to (−30.8 ± 3.0) % in diabetic + berberine rats (Fig. 2a). In addition, when the concentration of 5-HT was 10−6 M, 100 mg/kg/day berberine treatment could significantly decrease the relative changes of luminal diameter from (−42.5 ± 3.7) % in diabetic rats to (−35.9 ± 3.3) % in diabetic + berberine rats (Fig. 2b). However, there was also a significant difference in the contractile responsiveness of middle cerebral artery between Diabetic + berberine and CON rats, which indicated that berberine treatment did not restore the contractile responsiveness of middle cerebral artery in diabetic rats to the normal control level. When the control rats were treated with berberine (100 mg/kg/day), there was no significant difference in the contractile responsiveness of middle cerebral artery between CON + berberine and CON rats. These results clearly suggested that 100 mg/kg/day berberine treatment significantly inhibited the augmented contractile function of middle cerebral artery in diabetic rats.
Fig. 1.
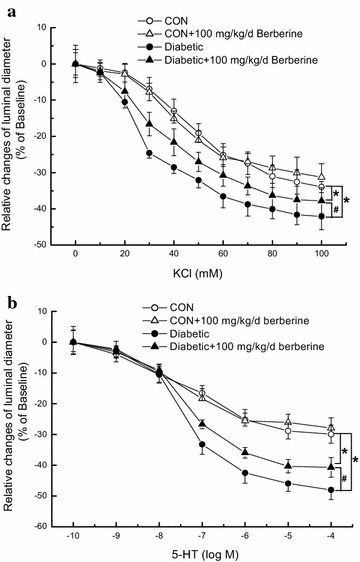
Comparison of contractile response in middle cerebral artery to cumulative superfusion of KCl (a) and 5-hydroxytryptamine (5-HT, b) in CON, CON +100 mg/kg/day berberine, diabetic, and diabetic +100 mg/kg/day berberine rats. Concentration–response relationships of middle cerebral artery were represented as the percentage of luminal diameter relative to the baseline internal diameter. Chronic administration of 100 mg/kg/day berberine significantly inhibited the augmented contractile responsiveness to KCl (a) and 5-HT (b) in diabetic rats, respectively. CON control rats, CON + berberine control rats administrated with berberine (100 mg/kg/day), Diabetic diabetic rats, diabetic + berberine: diabetic rats administrated with berberine (100 mg/kg/day). Values are expressed as mean ± SEM and n = 8 animals in each group. *P < 0.05 vs. CON rats and # P < 0.05 vs. diabetic rats
Fig. 2.
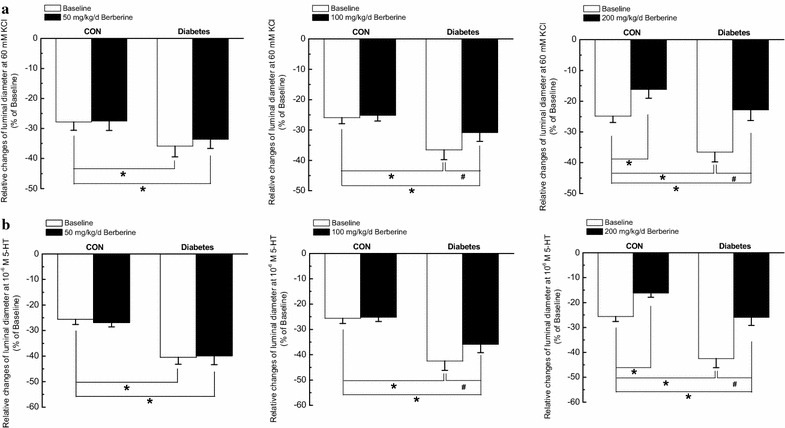
Comparison of contractile function in response to different dose of berberine (50, 100 and 200 mg/kg/day) at 60 mM KCl (a) and 10−6 M 5-hydroxytryptamine (5-HT, b) in middle cerebral artery isolated from CON, CON + berberine, Diabetic, and diabetic + berberine rats, respectively. Chronic administration of 50 mg/kg/day berberine had no obvious effects on contractile responsiveness to KCl (a) and 5-HT (b) in CON and diabetic rats, respectively. However, chronic administration of 100 mg/kg/day berberine did not change the contractile responsiveness in CON rats, whereas significantly inhibited the augmented contractile responsiveness to KCl (a) and 5-HT (b) in diabetic rats, respectively. In addition, chronic administration of 200 mg/kg/day berberine significantly inhibited the contractile responsiveness to KCl (a) and 5-HT (b) in both CON and diabetic rats, respectively. CON control rats, CON + berberine control rats administrated with different dose of berberine, Diabetic diabetic rats, diabetic + berberine: diabetic rats administrated with different dose of berberine. Values are expressed as mean ± SEM and n = 8 animals in each group. *P < 0.05 vs. CON rats and # P < 0.05 vs. diabetic rats
In Experiment II and Experiment III, chronic treatment with 50 mg/kg/day berberine for 8 weeks had no obvious effects on contractile responsiveness in CON and Diabetic rats, respectively. However, 200 mg/kg/day berberine for 8 weeks significantly inhibited contractile responsiveness in both CON and diabetic rats (Fig. 2).
Chronic administration of 100 mg/kg/day berberine markedly inhibited the increased CaL current densities of cerebral VSMCs in diabetic rats
To evaluate the function of CaL channels, whole-cell currents were recording with conventional patch clamp techniques. As shown in Fig. 3a, the typical time- and voltage-dependent inward currents were evoked by increasing depolarizations from a holding potential of −40 mV to +60 mV. The mean current–voltage relationship (I–V) curves which were further expressed in terms of current densities which were calculated by normalizing current to Cm (Fig. 3b) [20]. Whole-cell CaL currents of cerebral VSMCs in diabetic rats showed a larger inward components of trace as compared with that in CON (Fig. 3a), which is consistent with the previous reports [5, 22]. In Experiment I, chronic administration of berberine (100 mg/kg/day) for 8 weeks significantly inhibited the increased CaL channel current densities of cerebral VSMCs in diabetic rats. However, there was also a significant difference in CaL channel current densities of cerebral VSMCs between Diabetic + berberine and CON rats, which indicated that berberine treatment did not restore the CaL channel current densities of cerebral VSMCs to the normal control level. When the control rats were treated with berberine (100 mg/kg/day), there was no significant difference in the CaL channel current densities of cerebral VSMCs between CON + berberine and CON rats. These results clearly suggested that 100 mg/kg/day berberine treatment significantly inhibited the increased CaL channel current densities of cerebral VSMCs isolated from diabetic rats.
Fig. 3.
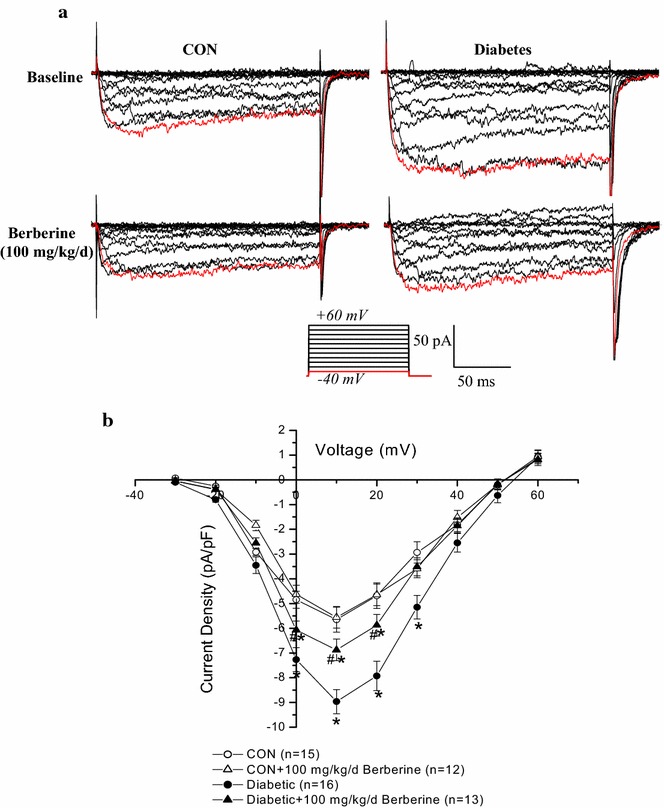
Comparison of whole-cell CaL current density in cerebral VSMCs isolated from CON, CON +100 mg/kg/day berberine, Diabetic, and diabetic +100 mg/kg/day berberine rats. Representative recording traces were used to show the whole-cell CaL currents (a) and the mean I–V curves were further expressed in terms of current densities (b) in different groups. Chronic administration of 100 mg/kg/day berberine markedly inhibited the increased CaL channel current density of cerebral VSMCs in diabetic rats. CON control rats, CON + berberine control rats administrated with berberine (100 mg/kg/day), Diabetic diabetic rats, diabetic + berberine: diabetic rats administrated with berberine (100 mg/kg/day). Values are mean ± SEM with the number of cells recorded in parentheses. *P < 0.05 vs. CON rats and # P < 0.05 vs. diabetic rats
In Experiment II and Experiment III, chronic treatment with 50 mg/kg/day berberine for 8 weeks had no obvious effects on CaL channel function in CON and diabetic rats, respectively. However, 200 mg/kg/day berberine for 8 weeks significantly inhibited CaL channel function in both CON and diabetic rats (Fig. 4).
Fig. 4.
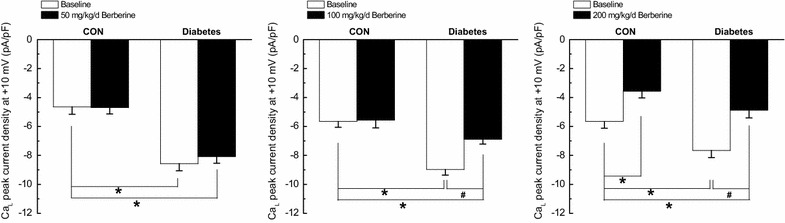
Comparison of CaL peak current density in response to different dose of berberine (50, 100 and 200 mg/kg/day) in cerebral VSMCs isolated from CON, CON + berberine, diabetic, and diabetic + berberine rats. Chronic administration of 50 mg/kg/day berberine had no obvious effects on CaL peak current density in CON and diabetic rats, respectively. However, chronic administration of 100 mg/kg/day berberine did not change the CaL peak current density in CON rats, whereas significantly inhibited the augmented CaL peak current density in diabetic rats, respectively. In addition, chronic administration of 200 mg/kg/day berberine significantly inhibited the CaL peak current density in both CON and diabetic rats, respectively. CON control rats, CON + berberine control rats administrated with berberine, diabetic diabetic rats, diabetic + berberine diabetic rats administrated with berberine. Values are expressed as mean ± SEM and at least n = 10 cells recorded in each group. *P < 0.05 vs. CON rats and # P < 0.05 vs. diabetic rats
Chronic administration of 100 mg/kg/day berberine significantly reduced the augmented α1C-subunit expressions of CaL channel in cerebral arteries isolated from diabetic rats
The apparent masses of 240 kD band were shown in the membrane, which correspond to the predicted sizes of α1C-subunit protein of CaL channel in cerebral arteries [21]. As the internal control, the expressions of β-actin (42 kDa) were similar in different lanes in the bottom membrane, showing the equal protein loading (Fig. 5a). The averaged data was expressed as the percentage of β-actin signal (Fig. 5b). As compared with that in CON rats, α1C-subunit expressions of CaL channel significantly increased in cerebral arteries of diabetic rats. In Experiment I, chronic administration of berberine (100 mg/kg/day) for 8 weeks significantly reduced the augmented α1C-subunit expressions of CaL channel in cerebral arteries of diabetic rats. However, there was also a significant difference in the α1C-subunit expressions of CaL channel in cerebral arteries between Diabetic + berberine and CON rats, which indicated that berberine treatment did not restore the α1C-subunit expressions of CaL channel in cerebral arteries of diabetic rat to the normal control level. When the control rats were treated with berberine (100 mg/kg/day), there was no significant difference in the α1C-subunit expressions of CaL channel in cerebral arteries between CON + berberine and CON rats. These results suggested that berberine treatment significantly reduced the augmented α1C-subunit expressions of CaL channel in cerebral arteries isolated from diabetic rats.
Fig. 5.
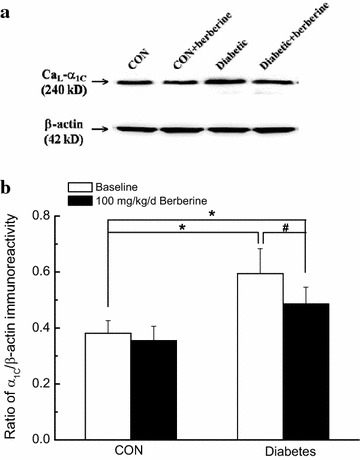
Comparison of α1C-subunit expressions of CaL channel in cerebral arteries isolated from CON, CON +100 mg/kg/day berberine, diabetic, and diabetic +100 mg/kg/day berberine rats. Representative band was used to show the protein expressions of CaL channel in different groups (a). Normalized band intensities are shown as a percentage of the β-actin density (b). Chronic administration of 100 mg/kg/day berberine markedly reduced the augmented α1C-subunit expressions of CaL channel in cerebral arteries isolated from diabetic rats. CON control rats, CON + berberine control rats administrated with berberine (100 mg/kg/day), Diabetic diabetic rats, Diabetic + berberine diabetic rats administrated with berberine (100 mg/kg/day). Values are expressed as mean ± SEM from four independent experiments, and each sample based on tissue pooled from 3–4 animals. *P < 0.05 vs. CON rats and # P < 0.05 vs. diabetic rats
Chronic administration of 100 mg/kg/day berberine not only markedly decreased the increased resting [Ca2+]i level, but also suppressed the augmented Ca2+ releases from RyRs in cerebral VSMCs isolated from diabetic rats
As previously described [21], a high concentration of caffeine (10 mM) was used to activate RyRs and then evoked a transient peak increase of [Ca2+]i which represents the Ca2+ release from the SR. Representative dot graph of Fluo-3/AM fluorescence recorded showed the typical transient increases of intracellular Ca2+ fluorescence intensity evoked by 10 mM caffeine (Fig. 6A). Summarized data indicated the average changes of the intracellular Ca2+ fluorescence intensity before and during the application of caffeine (Fig. 6B). We investigated the resting [Ca2+]i level and the maximal caffeine-induced increases of [Ca2+]i in cerebral VSMCs isolated from CON, CON + berberine, Diabetic, and Diabetic + berberine rats. As compared with that of CON rats, there is a significant higher resting [Ca2+]i level in cerebral VSMCs isolated from diabetic rats, which is consistent with previous report that high glucose contributes to elevate the global [Ca2+]i in VSMCs [4, 5]. The maximal increases of [Ca2+]i were also significantly increased in cerebral VSMCs isolated from diabetic rats as compared with that of CON rats. In Experiment I, chronic administration of berberine (100 mg/kg/day) for 8 weeks not only significantly decreased the resting [Ca2+]i level, but also markedly suppressed the maximal increases of [Ca2+]i in cerebral VSMCs isolated from diabetic rats. However, there were also significant differences in the resting [Ca2+]i level and the maximal increases of [Ca2+]i in cerebral VSMCs between diabetic + berberine and CON rats, which indicated that berberine treatment did not restore the resting [Ca2+]i level and the Ca2+ releases from RyRs in cerebral VSMCs of diabetic rats to the normal control level. When the control rats were treated with berberine (100 mg/kg/day), there was no significant difference in the resting [Ca2+]i level and the Ca2+ releases from RyRs in cerebral VSMCs between CON + berberine and CON rats. These results indicated that berberine treatment not only markedly decreased the increased resting [Ca2+]i level, but also suppressed the augmented Ca2+ releases from RyRs in cerebral VSMCs isolated from diabetic rats.
Fig. 6.
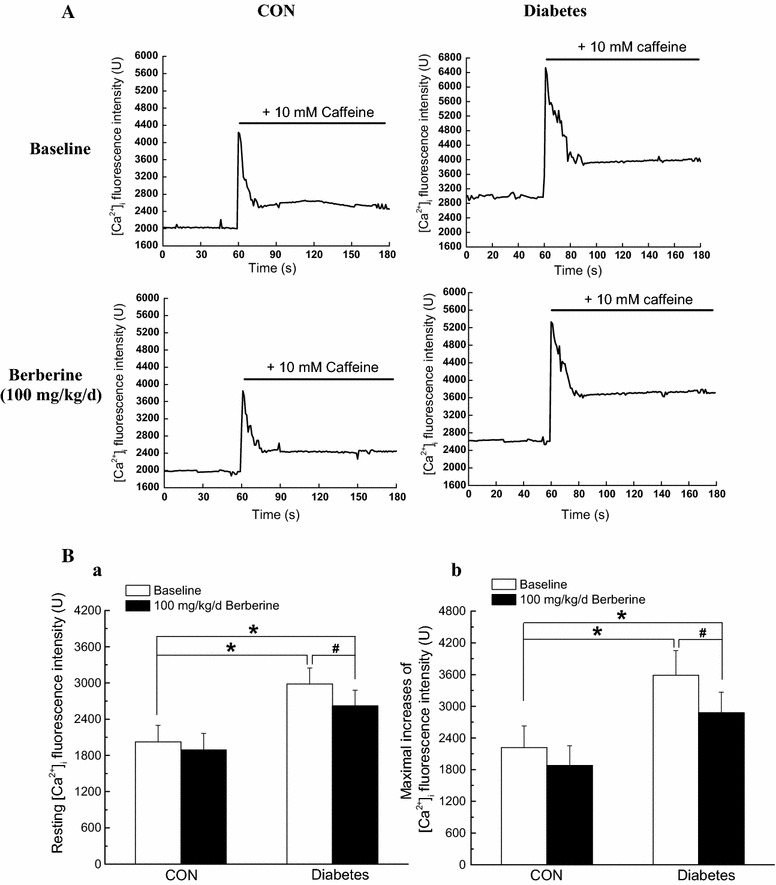
Comparison of resting level of Ca2+ fluorescence intensity and maximal increases of Ca2+ fluorescence intensity in cerebral VSMCs isolated from CON, CON +100 mg/kg/day berberine, Diabetic, and diabetic +100 mg/kg/day berberine rats. Representative dot graph of Fluo-3/AM fluorescence recorded showed the typical transient increases of intracellular Ca2+ fluorescence intensity evoked by 10 mM caffeine (A). Summarized data indicated the average changes of the intracellular Ca2+ fluorescence intensity before and during the application of caffeine (B). Chronic administration of 100 mg/kg/day berberine significantly not only decreased the resting [Ca2+]i level, but also suppressed the Ca2+ releases from RyRs in cerebral VSMCs isolated from diabetic rats. CON control rats, CON + berberine control rats administrated with berberine (100 mg/kg/day), Diabetic diabetic rats, diabetic + berberine diabetic rats administrated with berberine (100 mg/kg/day). Values are expressed as mean ± SEM and at least n = 10 cells recorded in each group. *P < 0.05 vs. CON rats and # P < 0.05 vs. diabetic rats
In Experiment II and Experiment III, chronic treatment with 50 mg/kg/day berberine for 8 weeks had no obvious effects on maximal increases of Ca2+ fluorescence intensity in CON and diabetic rats, respectively. However, 200 mg/kg/day berberine for 8 weeks significantly inhibited maximal increases of Ca2+ fluorescence intensity in both CON and diabetic rats (Fig. 7).
Fig. 7.
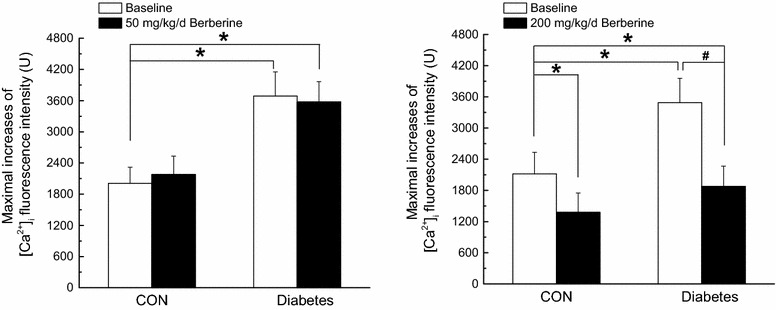
Comparison of maximal increases of Ca2+ fluorescence intensity in response to different dose of berberine (50 and 200 mg/kg/day) in cerebral VSMCs isolated from CON, CON + berberine, Diabetic, and diabetic + berberine rats. Chronic administration of 50 mg/kg/day berberine had no obvious effects on maximal increases of Ca2+ fluorescence intensity in CON and diabetic rats, respectively. In addition, chronic administration of 200 mg/kg/day berberine significantly inhibited the Ca2+ releases from RyRs in both CON and diabetic rats, respectively. CON control rats, CON + berberine control rats administrated with berberine, Diabetic diabetic rats, diabetic + berberine diabetic rats administrated with berberine. Values are expressed as mean ± SEM and at least n = 8 cells recorded in each group. *P < 0.05 vs. CON rats and # P < 0.05 vs. diabetic rats
Acute application of berberine directly inhibited the hyperglycemia-induced CaL currents in cerebral VSMCs isolated from normal rats
To determinate whether the inhibition of CaL channel in diabetic rats is mainly due to a direct effect of berberine or only a secondary consequence of the lowing blood glucose in response to berberine treatment, we investigated the acute effects of berberine on the function of CaL channel in cerebral VSMCs isolated from normal control rats under high glucose condition. As shown in Fig. 5, extracellular application of the high glucose (d-glucose, 20 mM for 2–7 min, as described previously [5]) significantly evoked an increase of CaL channel currents at +10 mV test potential. Subsequently, acute extracellular application of 10 μM berberine (as described previously [23]) markedly reduced the amplitude of the inward currents in the presence of 20 mM d-glucose. These findings suggested that hyperglycemia increased CaL channel current and then the acute extracellular application of berberine could directly reduced the hyperglycemia-induced CaL currents in cerebral VSMCs of normal control rats.
Acute application of berberine directly suppressed the hyperglycemia-induced Ca2+ releases from RyRs in cerebral VSMCs isolated from normal rats
To determinate whether the suppression of Ca2+ releases from RyRs diabetic rats is mainly due to a direct effect of berberine or only a secondary consequence of the lowing blood glucose in response to berberine treatment, we investigated the acute effects of berberine on the Ca2+ releases from RyRs in cerebral VSMCs isolated from normal control rats under high glucose condition. After the load of Fluo-3/AM, the isolated VSMCs were incubated with 20 mM d-glucose and the combination of 10 μM berberine and 20 mM d-glucose for 10 min at 37 °C, respectively. As shown in Fig. 6, incubation with 20 mM d-glucose significantly increased the resting [Ca2+]i level (Fig. 6Ba) and maximal increases of [Ca2+]i fluorescence intensity (Fig. 6Bb). However, incubation with combination of 10 μM berberine and 20 mM d-glucose markedly suppressed the resting [Ca2+]i level (Fig. 6Ba) and maximal increases of [Ca2+]i fluorescence intensity (Fig. 6Bb). These findings suggested that hyperglycemia increased the resting [Ca2+]i and Ca2+ releases from RyRs and then the acute application of berberine could directly reduced the hyperglycemia-induced Ca2+ releases from RyRs in cerebral VSMCs of normal control rats.
Discussion
There are two principal and novel findings in the present work. First, the berberine treatment could inhibit the increased cerebrovascular contractility independent of a functional endothelium in the rat model of streptozotocin-induced diabetes. Secondly, the berberine treatment not only inhibited the function of CaL channel, but also suppressed the Ca2+ releases from the RyRs of cerebral VSMCs in diabetic rats or when exposed to hyperglycemia condition. Our study indicated that berberine alleviated the cerebral arterial contractility in the rat model of streptozotocin-induced diabetes via regulating the intracellular Ca2+ handling of smooth muscle cells.
Hyperglycemia impaired the intracellular Ca2+ handling in diabetic vascular dysfunction
Diabetic vascular complication has been demonstrated to be not only responsible for the increased risk of stroke and heart attack, but also contribute to the diabetic organ damage in diabetic patients [3]. Under diabetic conditions, multiple factors may intend to impair the function and structure of artery, including hyperglycemia, insulin resistance, hyperinsulinemia, excess free fatty acid (FFAs) release, advanced glycation end products (AGEs), lipemia, and obesity [1, 2]. However, hyperglycemia is still considered to be the most important feature in the development of diabetic vascular complication.
CaL channels in plasma membrane and RyRs in SR are important mediators to control arterial excitation–contraction coupling by handling intracellular Ca2+ in VSMCs. It is currently believed that when the membrane is depolarized, extracellular Ca2+ entries through CaL channels and then activates RyRs to produce the transient local Ca2+ release events, which is termed Ca2+-induced Ca2+ release (CICR). This Ca2+ event not only contribute to the overall rise in the concentration of intracellular Ca2+, but also in turn activate nearby Ca2+-activated K+ (KCa) channels in plasma membrane, leading to membrane hyperpolarization, inhibition of CaL channels, and thereby favoring vasodilation by reducing the Ca2+ influx. In the present work, the rat model of STZ-induced diabetic rats showed a higher blood glucose level (Table 1) and a significant increase in the contractile function of cerebral artery (Fig. 1) as compared with that in CON. In addition, the function of CaL channel (Figs. 3, 5), the resting [Ca2+]i level (Fig. 6), and the Ca2+ releases from RyRs (Fig. 6) significantly increased in cerebral VSMCs isolated from STZ-induced diabetic rats. Furthermore, we also observed that hyperglycemia directly increased the function of CaL channel (Fig. 8) and the Ca2+ releases from RyRs (Fig. 9) in cerebral VSMCs isolated from normal control rats. All these results are in agreement with previous reports that hyperglycemia induced diabetic vascular dysfunction by elevating global intracellular calcium ([Ca2+]i) [4, 5], up-regulating the function of CaL channel [5, 22], and altering the function of Ca2+ releases from the RyRs [6, 24]. It is also reported that cardiac dysfunction in diabetic cardiomyopathy is associated with a time-dependent decline in Ca2+ sparks of diabetic SD rat [25]. However, overexpression of silent information regulator 1 (SIRT1) reduces diabetes-exacerbated injury of myocardial ischemia and reperfusion and oxidative stress [26]. In addition, corin, a cardiac serine protease, also exerts cardioprotective action via activating pro-atrial natriuretic peptide pathway in diabetic cardiomyopathy [27].
Fig. 8.
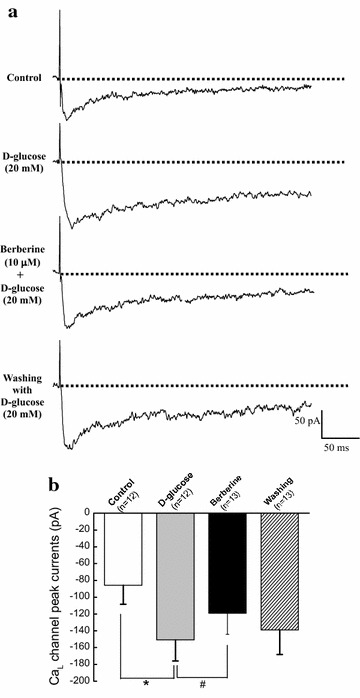
Acute application of berberine directly inhibited the hyperglycemia-induced CaL currents in cerebral VSMCs isolated from normal control rats. Representative traces of CaL channel current at +10 mV in cerebral VSMCs when exposed to 20 mM d-glucose and then subsequent acute application of 10 μM berberine in the presence of 20 mM d-glucose. At last, the cerebral VSMCs were washed with 20 mM d-glucose (a). Summarized data indicated the amplitudes of CaL channel current at +10 mV before and after application of 20 mM d-glucose, 10 μM berberine in the presence of 20 mM d-glucose, and then washing with 20 mM d-glucose (b). Values are mean ± SEM with the number of cells recorded in parentheses. *P < 0.05 vs. control condition and # P < 0.05 vs. high glucose condition
Fig. 9.
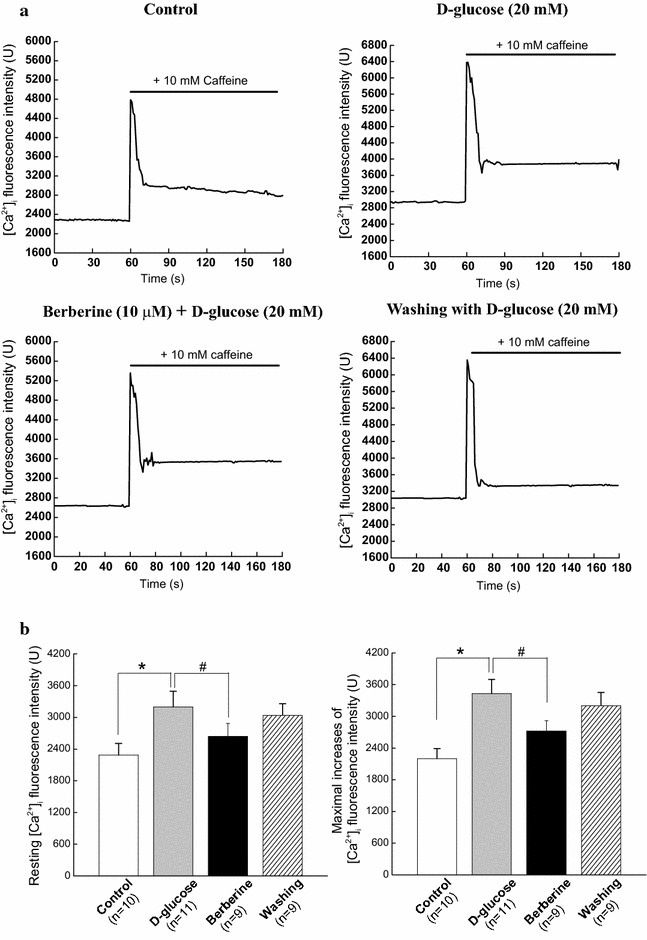
Acute application of berberine directly suppressed the hyperglycemia-induced Ca2+ releases from RyRs in cerebral VSMCs isolated from normal control rats. Typical transient increases of intracellular Ca2+ evoked by 10 mM caffeine were recorded when cerebral VSMCs exposed to the 20 mM d-glucose or the combination of 10 μM berberine and 20 mM d-glucose, respectively. At last, the cerebral VSMCs were washed with 20 mM d-glucose (a). Summarized data indicated the average changes of the resting intracellular Ca2+ and Ca2+ releases from RyRs before and after application of the 20 mM d-glucose and the combination of 10 μM berberine and 20 mM d-glucose, respectively (b). Values are mean ± SEM with the number of cells recorded in parentheses. *P < 0.05 vs. control condition and # P < 0.05 vs. high glucose condition
The berberine treatment alleviates the cerebrovascular contractility in diabetic rats by regulating the intracellular Ca2+ handling in VSMCs
Berberine [18, 5, 6-dihydro-9, 10-dimethoxybenzo (g)-1, 3-benzodioxolo (5, 6-a) quinolizinium] is an isoquinoline alkaloid, which is extracted from berberine-containing herbs, such as Goldenseal (Hydrastis canadensis), Oregon Grape (Berberis aquifolium), and Coptis chinensis (Chinese goldthread) [7]. It has been demonstrated that berberine exhibits a variety of pharmacological properties and has been extensively used for the immune enhancement, antibacterial and antiparasitic effects, and gastrointestinal infections [12]. Recently, berberine was found to have a long-term effect on lowering blood glucose, which could delay the diabetic complications in patients and various animal models [9]. The anti-hyperglycemic mechanisms of berberine are very complex, including the improving insulin secretion and sensitivity, activating the adenosine monophosphate-activated protein kinase (AMPK) pathway, modulating gut microbiota, reducing intestinal absorption of glucose, inhibiting gluconeogenesis and glucose transporter in liver, stimulating glycolysis in peripheral tissue cells [8, 12]. In addition, extensive studies indicated that berberine treatment also exerts an extra-benefit on the diabetic cardiovascular complications. Besides the anti-hyperglycemic and cholesterol-lowering activity, berberine also has the anti-inflammatory and anti-oxidant properties to protect hyperglycemia-induced endothelial injury [10, 12]. For example, berberine could improve the endothelium-dependent vasodilatation by activation of the AMPK pathway [14], increasing the phosphorylation of endothelial nitric oxide synthase (eNOS) [15, 28], down-regulating expression of NADPH oxidase [13], and inhibiting the formation of advanced glycation end products (AGEs) [29]. It has been demonstrated that berberine treatment attenuates palmitate-induced reduction in glucose uptake and consumption, in part, through reduction in cellular 1,2-diacyl-sn-glycerol (DAG) levels and accumulation of 1,2,3-triacyl-sn-glycerol (TAG) in H9c2 cells [30].
In the present work, chronic administration of 100 mg/kg/day berberine not only significantly reduced glucose levels in diabetic rats (Table 1), but also markedly inhibited the augmented contractile function of cerebral artery in diabetic rats (Fig. 1). In addition, chronic administration of 100 mg/kg/day berberine not only markedly inhibited the increased CaL channel current densities (Fig. 3), but also reduced the augmented α1C-subunit expressions of CaL channel (Fig. 5) in cerebral VSMCs isolated from diabetic rats. Furthermore, 100 mg/kg/day chronic administration of berberine not only markedly decreased the increased resting [Ca2+]i level, but also suppressed the augmented Ca2+ releases from RyRs (Fig. 6) in cerebral VSMCs isolated from diabetic rats. Correspondingly, acute application of berberine could directly inhibit the hyperglycemia-induced CaL currents (Fig. 8) and suppress the hyperglycemia-induced Ca2+ releases from RyRs (Fig. 9) in cerebral VSMCs isolated from normal control rats. Our results suggested 100 mg/kg/day berberine treatment could alleviate the cerebral arterial contractility by regulating the intracellular Ca2+ handling of VSMCs in diabetic rats or when exposed to hyperglycemia condition.
From our results, chronic administration of 50 mg/kg/day berberine had no obvious effects on contractile responsiveness (Fig. 2), CaL peak current density (Fig. 4), and maximal increases of Ca2+ fluorescence intensity (Fig. 7) in CON and Diabetic rats, respectively. However, chronic administration of 200 mg/kg/day berberine significantly inhibited the contractile responsiveness (Fig. 2), CaL peak current density (Fig. 4), and maximal increases of Ca2+ fluorescence intensity (Fig. 7) in both CON and diabetic rats, respectively. We concluded that the dose of berberine is very important for treatment and 100 mg/kg/day berberine could produce significant effects on DM rats rather than CON rats in terms of contractile function, CaL channel and [Ca2+]i.
Practical implications of the present study
It has been reported that several therapeutic strategies are administered to ameliorate the deteriorated vascular function in diabetes, such as increasing the endothelium-dependent NO production, reducing the oxidative stress damage, inhibiting the inflammatory responses [7, 8]. Our study may provide a novel therapeutic approach to treat vascular dysfunction in diabetic patients by regulating the intracellular Ca2+ handling with their related proteins in VSMCs. In addition, the safety and efficacy of berberine have been generally accepted as a kind of traditional Chinese medicine. It is believed that berberine performs a good clinical practice and our study provided new evidence that application of berberine is a novel potential application in the treatment of diabetic vascular dysfunction.
Study limitations
First, there are some disparities between our work and other previous reports. The present work showed that the function of CaL channel significantly increased in the rat model of STZ-induced diabetic rats (Figs. 3, 5) or when exposed to hyperglycemia condition (Fig. 8), which are in consistence with previous reports [5, 22]. However, there is also a report that CaL channel currents significantly reduced in STZ-induced diabetic rats [3]. In addition, we observed that Ca2+ releases from RyRs significantly increased in the diabetic rats (Fig. 6) or when exposed to hyperglycemia condition (Fig. 9), which are in agreement with previous report that there was a dramatic increase in ryanodine receptor (RyR) levels of VSMCs from diabetic animals [6]. However, reduced expression of RyR has also been reported in several diabetic models [3]. These discrepancies may be related to different species or rat strain, the method of diabetic induction, the vascular bed studied or the severity of diabetes [3, 31]. Second, RyRs and inositol 1,4,5-trisphosphate receptors (IP3Rs) are the two mainly important families of Ca2+ release channels in the SR. Activation of IP3Rs could also evoke the localized Ca2+ transients and contribute to the global elevation of intracellular Ca2+. Thus, it is necessary to study the role of IP3Rs in the berberine treatment for the diabetic vascular dysfunction. Third, we observed that berberine inhibited the function of CaL channel in the present work. However, the present work did not study the underlying mechanism. It is believed that there are three subclasses of calcium channel blockers which are wildly used to block CaL channel by binding to the poreforming α1C subunit, such as dihydropyridine, phenylalkylamine, and benzothiazepine. Whether berberine inhibited the function of CaL channel by directly binding to poreforming α1C subunit or other indirect pathway needs the further investigation.
Conclusion
The present work showed that berberine treatment could alleviate the cerebral arterial contractility independent of a functional endothelium in the rat model of streptozotocin-induced diabetes. In addition, the berberine treatment not only directly inhibited the function of CaL channels, but also suppressed the function of Ca2+ releases from the RyRs of cerebral VSMCs in diabetic rats or when exposed to hyperglycemia condition. Taken together, our study indicated that berberine alleviated the cerebral arterial contractility in the rat model of streptozotocin-induced diabetes via regulating the intracellular Ca2+ handling of smooth muscle cells.
Authors’ contributions
Conceived and designed the experiments: Y-GM, Y-BZ, and M-JX. Performed the experiments: Y-GM, Y-BZ, Y-GB, ML, H-TG. Analyzed the data: LL, ML, H-TG. Wrote the paper: M-JX. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 31270904 and 81471032). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- DM
diabetes mellitus
- [Ca2+]i
concentration of intracellular Ca2+
- VSMCs
vascular smooth muscle cells
- CaL
long-lasting voltage-dependent Ca2+ (L-type Ca2+) channel
- RyRs
ryanodine receptors
- STZ
streptozotocin
- 5-HT
5-hydroxytryptamine
- CICR
Ca2+-induced Ca2+ release
- KCa
Ca2+-activated K+ channel
- AMPK
adenosine monophosphate-activated protein kinase
- eNOS
endothelial nitric oxide synthase
- AGEs
advanced glycation end products
Footnotes
Yu-Guang Ma and Yin-Bin Zhang contributed equally to this work
Contributor Information
Yu-Guang Ma, Email: mygxmj@hotmail.com.
Yin-Bin Zhang, Email: 23227119@qq.com.
Yun-Gang Bai, Email: baiyungang@163.com.
Zhi-Jun Dai, Email: dzj0911@126.com.
Liang Liang, Email: liangliang@163.com.
Mei Liu, Email: liumei@163.com.
Man-Jiang Xie, Phone: 86-29-84774809, Email: manjiangxie@hotmail.com.
Hai-Tao Guan, Email: guanhaitao@cscoorg.cn.
References
- 1.Tousoulis D, Papageorgiou N, Androulakis E, Siasos G, Latsios G, Tentolouris K, Stefanadis C. Diabetes mellitus-associated vascular impairment. J Am Coll Cardiol. 2013;62(8):667–676. doi: 10.1016/j.jacc.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 2.Sena CM, Pereira AM, Seiça R. Endothelial dysfunction—a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832(12):2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Velasco M, Ruiz-Hurtado G, Gómez AM, Rueda A. Ca2 + handling alterations and vascular dysfunction in diabetes. Cell Calcium. 2014;56(5):397–407. doi: 10.1016/j.ceca.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Dunn KM, Nelson MT. Calcium and diabetic vascular dysfunction. Focus on “Elevated Ca2 + sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells”. Am J Physiol Cell Physiol. 2010;298(2):C203–C205. doi: 10.1152/ajpcell.00499.2009. [DOI] [PubMed] [Google Scholar]
- 5.Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2 + sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol. 2010;298(2):C211–C220. doi: 10.1152/ajpcell.00267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Searls YM, Loganathan R, Smirnova IV, Stehno-Bittel L. Intracellular Ca2 + regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia. Cardiovasc Diabetol. 2010;9:8. doi: 10.1186/1475-2840-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang W, Chen L, Hatch GM. Berberine as a therapy for type 2 diabetes and its complications: from mechanism of action to clinical studies. Biochem Cell Biol. 2014:1–8. [DOI] [PubMed]
- 8.Pang B, Zhao L, Zhou Q, Zhao T, Wang H, Gu C, Tong X. Application of berberine on treating type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:1–12. doi: 10.1155/2015/905749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Chen L. Berberine in type 2 diabetes therapy: a new perspective for an old antidiarrheal drug? Acta Pharm Sinica B. 2012;2(4):379–386. doi: 10.1016/j.apsb.2012.06.004. [DOI] [Google Scholar]
- 10.Li Z, Geng Y, Jiang J, Kong W. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid-Based Compl Alt Med. 2014;2014:1–12. doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Li X, Li Q, Fu Y, Yu H, Sun Y, Zhang L, Shan H. Berberine alleviates ischemic arrhythmias via recovering depressed Ito and ICa currents in diabetic rats. Phytomedicine. 2012;19(3–4):206–210. doi: 10.1016/j.phymed.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Ni W, Ding H, Tang L. Berberine as a promising anti-diabetic nephropathy drug: an analysis of its effects and mechanisms. Eur J Pharmacol. 2015;760:103–112. doi: 10.1016/j.ejphar.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Wu D, Wen W, Qi C, Zhao R, Lü J, Zhong C, Chen Y. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine. 2012;19(8–9):712–718. doi: 10.1016/j.phymed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Huang Y, Lam KSL, Li Y, Wong WT, Ye H, Lau CW, Vanhoutte PM, Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009;82(3):484–492. doi: 10.1093/cvr/cvp078. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Li J, Lv X, Zhang M, Song Y, Chen L, Liu Y. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur J Pharmacol. 2009;620(1–3):131–137. doi: 10.1016/j.ejphar.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansor LS, Gonzalez ER, Cole MA, Tyler DJ, Beeson JH, Clarke K, Carr CA, Heather LC. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovasc Diabetol. 2013;12:136. doi: 10.1186/1475-2840-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghaddam HK, Baluchnejadmojarad T, Roghani M, Goshadrou F, Ronaghi A. Berberine chloride improved synaptic plasticity in STZ induced diabetic rats. Metab Brain Dis. 2013;28(3):421–428. doi: 10.1007/s11011-013-9411-5. [DOI] [PubMed] [Google Scholar]
- 19.Lin LJ, Gao F, Bai YG, Bao JX, Huang XF, Ma J, Zhang LF. Contrasting effects of simulated microgravity with and without daily—Gx gravitation on structure and function of cerebral and mesenteric small arteries in rats. J Appl Physiol. 2009;107(6):1710–1721. doi: 10.1152/japplphysiol.00493.2009. [DOI] [PubMed] [Google Scholar]
- 20.Xie MJ, Ma YG, Gao F, Bai YG, Cheng JH, Chang YM, Yu ZB, Ma J. Activation of BKCa channel is associated with increased apoptosis of cerebrovascular smooth muscle cells in simulated microgravity rats. Am J Physiol Cell Physiol. 2010;298(6):C1489–C1500. doi: 10.1152/ajpcell.00474.2009. [DOI] [PubMed] [Google Scholar]
- 21.Xue JH, Chen LH, Zhao HZ, Pu YD, Feng HZ, Ma YG, Ma J, Chang YM, Zhang ZM, Xie MJ. Differential regulation and recovery of intracellular Ca2 + in cerebral and small mesenteric arterial smooth muscle cells of simulated microgravity rat. PLoS ONE. 2011;6(5):e19775. doi: 10.1371/journal.pone.0019775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinho JF, Medeiros M, Capettini L, Rezende BA, Campos PP, Andrade SP, Cortes SF, Cruz JS, Lemos VS. Phosphatidylinositol 3-kinase-δ up-regulates L-type Ca2 + currents and increases vascular contractility in a mouse model of type 1 diabetes. Brit J Pharmacol. 2010;161(7):1458–1471. doi: 10.1111/j.1476-5381.2010.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, Ho K, Huang Y. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol. 2000;399(2–3):187–196. doi: 10.1016/S0014-2999(00)00339-3. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Zhu B, Chen X, Liu J, Guan Y, Ren J. Abnormalities of sarcoplasmic reticulum ca2 + mobilization in aortic smooth muscle cells from streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol. 2008;35(5–6):568–573. doi: 10.1111/j.1440-1681.2007.04832.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhao SM, Wang YL, Guo CY, Chen JL, Wu YQ. Progressive decay of Ca2 + homeostasis in the development of diabetic cardiomyopathy. Cardiovasc Diabetol. 2014;13:75. doi: 10.1186/1475-2840-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding M, Lei J, Han H, Li W, Qu Y, Fu E, Fu F, Wang X. SIRT1 protects against myocardial ischemia-reperfusion injury via activating eNOS in diabetic rats. Cardiovasc Diabetol. 2015;14:143. doi: 10.1186/s12933-015-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang A, Hu Y, Zhou P, Long G, Tian X, Men L, Shen Y, Liu Y, Cui Y. Corin is down-regulated and exerts cardioprotective action via activating pro-atrial natriuretic peptide pathway in diabetic cardiomyopathy. Cardiovasc Diabetol. 2015;14:134. doi: 10.1186/s12933-015-0298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallejo S, Palacios E, Romacho T, Villalobos L, Peiro C, Sanchez-Ferrer CF. The interleukin-1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2014;13:158. doi: 10.1186/s12933-014-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao M, Li SY, Sun CK, Jingyu-Xu V, Lin Y, Liu KX, Wang L, Li CX, Zhou Q, Du JL, et al. Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro. Eur J Pharmacol. 2011;654(3):320–325. doi: 10.1016/j.ejphar.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Chang W, Chen L, Hatch GM. Berberine treatment attenuates the palmitate-mediated inhibition of glucose uptake and consumption through increased 1,2,3-triacyl-sn-glycerol synthesis and accumulation in H9c2 cardiomyocytes. Biochim Biophys Acta. 2016;1864(4):352–362. doi: 10.1016/j.bbalip.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Velmurugan GV, White C. Calcium homeostasis in vascular smooth muscle cells is altered in type 2 diabetes by Bcl-2 protein modulation of InsP3R calcium release channels. Am J Physiol Heart Circ Physiol. 2011;302(1):H124–H134. doi: 10.1152/ajpheart.00218.2011. [DOI] [PubMed] [Google Scholar]


