Abstract
Background
To analyze the 10-year experience of single chamber permanent epicardial pacemaker placement for children with congenital heart diseases (CHD) after surgical repair.
Methods
Between 2002 and 2014, a total of 35 patients with CHD (age: 26.9 ± 23.2 months, weight: 9.7 ± 5.6 kg) received permanent epicardial pacemaker placement following corrective surgery. Echocardiography and programming information of the pacemaker, as well as major adverse cardiac events (MACE) as heart failure or sudden death, were recorded during follow-up (46.8 ± 33.8 months).
Results
Acute ventricular stimulation threshold was 1.34 ± 0.72 V and no significant increase was observed at the last follow-up as 1.37 ± 0.81 V (p = 0.93). Compared with initial pacemaker implantation, the last follow-up didn’t show significant increases in impedance (p = 0.327) or R wave (p = 0.635). Four patients received pacemaker replacement because of battery depletion. 7/35 (20 %) of patients experienced MACE. Although the age and body weight were similar between patients with and without MACE, the patients with MACE were with complex CHD (100 % vs.55.6 %, p = 0.04).
Conclusion
High-degree iatrogenic atrioventricular block was the primary reason for placement of epicardial pacemaker for patients with CHD after surgical repair. Pacemaker placement with the steroid-eluting leads results in acceptable outcomes, however, the pacemaker type should be optimized for the children with complex CHD.
Keywords: Congenital heart disease, Permanent epicardial pacemaker, Iatrogenic atrioventricular block
Background
Permanent epicardial pacemaker have been utilized in clinical practice for several decades and the numbers of patients who require them are increasing. Pediatric pacemaker implants comprise < 1 % of all pacemaker implantations [1]. The main indication for permanent pacemaker implantation in children is high-degree congenital atrioventricular block (AVB) with a frequency of about 1/2000 [2, 3]. Another important indication is high-degree AVB following open-heart surgery, occurring with an incidence of 1 %. It is the main reason for placement of permanent epicardial pacemaker after congenital heart surgery [4]. Although endocardial pacemaker have several advantages, such as longer battery life, better sensing and pacing thresholds, epicardial pacemakers are more suitable for infants and younger children who are still under somatic growth, especially for patients with complicated cardiac deformities [5, 6]. However, epicardial pacemakers are less durable and have a higher incidence of complications as compared to endocardial pacemakers [7]. With the development of steroid-eluting leads appears to offer a better alternative to epicardial pacemaker. Its performance and longevity have been demonstrated to be comparable with the conventional endocardial leads [8].
Because of low economic statuses and undeveloped insurance systems, the single chamber pacemakers are utilized in many heart center for sick children in China. The current study aimed to review and analyze our 10-year experience in placement of single chamber permanent epicardial pacemaker and its mid-term outcomes.
Methods
Patient characteristics
A total of 44 CHD patients aged < 8 years received placement of epicardial pacemakers between 2002 and 2014 in our hospital. Nine children were excluded because they were associated with congenital AVB and pacemaker was implanted during the surgical repair. Thirty-five patients received permanent pacemaker implantation for the iatrogenic AVB (age: 26.9 ± 23.2 months, weight: 9.7 ± 5.6 kg). The study was approved by the ethics committee of Fuwai Hospital. Patient data were reviewed retrospectively. The diagnoses of CHD were summarized in Table 1 and all the patients received the primary corrective operation.
Table 1.
Patient diagnoses
| ccTGA | DORV | TECD | TOF | VSD | ASD | Total | |
|---|---|---|---|---|---|---|---|
| Iatrogenic AVB | 8 | 6 | 4 | 4 | 11 | 2 | 35 |
ccTGA corrected transposition of great arteries, DORV double outlet right ventricle, TECD complete endocardial cushion defect, TOF tetralogy of Fallot, VSD ventricular septal defect, ASD atrial septal defect
Implantation procedure
All procedures were performed under general anaesthesia. Steroid-eluting bipolar epicardial pacing leads were inserted into the diaphragmatic surface of the right ventricular free wall through the previous median sternotomy, and then connected to the plus generator within the subrectus pocket. The patients received pacemaker placement at 26 ± 3.1 days post-operation.
Pacemaker parameters and pacing mode
Acute ventricular stimulation threshold, impedance, R wave, and pacing mode were recorded at implantation and at the last follow-up.
Echocardiographic assessment
Left ventricular ejection fraction (LVEF) and left ventricular end diastolic diameter (LVEDD) were recorded at pre-implantation (1 week before pacemaker implantation), post-implantation (1 week after pacemaker implantation), and the last follow-up.
Follow-up
The duration of follow-up was 46.8 ± 33.8 months. Pacemaker device-related complications were collected, including lead failure, elevated pacing threshold, poor sensing, and pocket infection. Age, body weight and type of cardiac deformities were compared between patients with and without major adverse cardiac events (MACE), which included heart failure or sudden death.
Statistical analysis
Statistical calculations were carried out using SPSS version 19.0. Continuous data were presented as mean ± standard deviation or median and range. Categorical data were expressed as frequency and percentage. The independent sample t-test and one way analysis of variance were performed to compare the normally distributed variables when variances were homogeneous. If variances are not homogeneous, Dunnett’s T3 test would be performed. For categorical data, a comparison was performed using the Chi-square test and Fisher’s exact test. The Kaplan-Meier method was used to study the battery longevity and patients survival curve. A P-value lower than 0.05 was considered statistically significant.
Results
Early postoperative results
The operation time for pacemaker implantation (skin to skin time) was 119.1 ± 33.5 min. After surgery, 11 patients were extubated in the operation room and the rest were extubated within 4.8 h (range, 1.2–66 h). No blood transfusions occurred in the perioperative period. Except for a patient that suffered early postoperative death, who died from the low cardiac output syndrome after surgical repair for complex congenital heart disease, all patients were discharged from the hospital.
Device characteristics
All the patients received a single chamber pacemaker with VVI pacing mode. The mode wasn’t changed during follow-up. Table 2 reported the data for ventricular stimulation threshold, impedance, and R wave.
Table 2.
Analysis of the data for ventricular stimulation threshold, impedance, and R wave
| Intraoperation | Last follow-up | P value | |
|---|---|---|---|
| Ventricular stimulation threshold(V) | 1.34 ± 0.72 | 1.37 ± 0.81 | p = 0.933 |
| Impedance (Ω) | 366.7 ± 88.0 | 331.9 ± 95.9 | p = 0.327 |
| R wave(mV) | 12.3 ± 3.5 | 11.4 ± 4.9 | p = 0.635 |
Echocardiographic findings
Compared with the post-operation, LVEF was significantly decreased in the last follow-up (65.6 ± 5.3 % vs. 59.6 ± 7.6 %, p = 0.03), while a significant increase was observed in LVEDD (25.5 ± 8.4 mm vs. 33.3 ± 9.6 mm, p = 0.005). Figure 1 depicts the tendency of LVEF and LVEDD at pre-operation, post-operation, and the last follow-up.
Fig. 1.
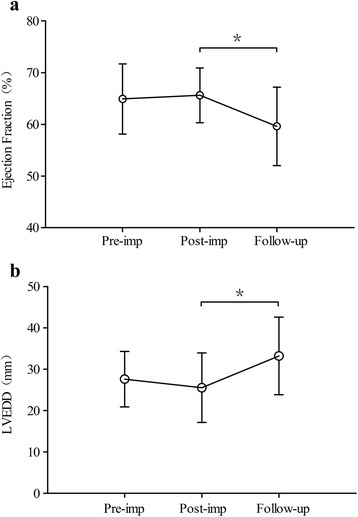
The tendency of LVEF and LVEDD at pre-implantation, post-implantation and the last follow-up: a Left ventricular ejection fraction:* p = 0.03. b Left ventricular end diastolic diameter:* p = 0.005
Follow-up
Four patients underwent pacemaker replacement at 50.8 months (range, 30–80 months) post-surgery due to battery depletion. No pocket infection or lead fracture occurred. Freedom from generator replacement was 94.1, 86.9, 77.2 and 61.8 % at 30, 36, 48, 57 months, respectively.
7/35 (20 %) of patients experienced MACE as heart failure or sudden death. Among them, four patients suffered from heart failure, with cardiac deformities of double outlet right ventricle (n = 2) and complete endocardial cushion defect (n = 2). Their conditions were improved after standard drug treatment. The other three patients suffered from sudden death at 6, 8, and 9 months post-operation, with cardiac deformities of complete transposition of great arteries (n = 1), double outlet right ventricle (n = 1), and tetralogy of Fallot (n = 1). The average age and weight of patients with MACE were 21.6 ± 30.66 months and 9.5 ± 6.4 kg, respectively. For patients without MACE, average age and weight were 20.4 ± 22 months and 9.8 ± 5.3 kg. There was no significant differences in age and body weight between patients with and without MACE (p > 0.05). But the type of cardiac deformities in patients with MACE were complex CHD (100 % vs.55.6 %, p = 0.046). Survival analysis of the patients is presented in Fig. 2.
Fig. 2.
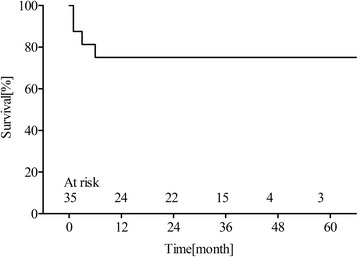
Estimated Kaplan-Meyer survival curve, which included the early mortality after pacemaker implantation
Discussion
CHD has become the leading type of birth defect in China. However, potential iatrogenic AVB is inevitable in a surgical operation and may result in postoperative pacemaker implantation. In our center, the primary reason for permanent pacemaker placement for children with CHD was iatrogenic AVB after congenital heart surgery. The most common type of cardiac deformities in patients with iatrogenic AVB is ventricle septal defect, followed by corrected transposition of great arteries (ccTGA), which was consistent with other developing counties such as Brazil and Egypt [9]. But in western countries, the most common type was complete transposition of the great arteries with ventricular septal defect, followed by complete endocardial cushion defect [10]. It is possibly because the surgeons in these countries prefer to perform corrective surgery on younger children with these two cardiac deformities, which could lead to a higher incidence of iatrogenic AVB.
For postoperative iatrogenic AVB, it is generally acknowledged that the proper duration of observation before permanent pacemaker placement is 7–14 days [11, 12]. However, in China, it is difficult for the patient’s family to accept postoperative pacemaker implantation, therefore, the observation time was extended, usually up to 3 weeks. Surgical placement of epicardial pacemaker is commonly known as the first choice for children with iatrogenic AVB who are younger than 4 years old, especially for the patient with complicated cardiac deformities, such as single ventricle and ccTGA.
All of our patients were implanted with a steroid-eluting lead. Compared with the traditional non-steroid-eluting lead, it could significantly reduce inflammation, with a low sensing threshold and is more durable [13, 14]. During follow-up, we didn’t observe a significant increase in the ventricular stimulation threshold, impedance, or R wave.
Generally speaking, children have a higher heart rate than adults, so their pulse generator is frequently exhausted earlier because of high pacing rates. In the patient less than 19 years old who had received permanent pacemaker implantation, it was reported that the mean longevity of the pacemaker generator was 5.5 years, and the lead was 10.8 years [6]. In the present study, no lead fracture was recorded, but four patients underwent reoperation for generator change because of battery depletion.
Although all patients’ cardiac functions were within the normal range (LVEF > 55 % is considered as normal [15]), the last follow-up examination showed significant decreases in LVEF compared with the post-operation examination. The major reason could be the pacing mode. Studies have shown that epicardial right ventricular free wall pacing could result in pathologic left ventricular dilatation and dysfunction, which could do harm to the patient’s cardiac functions [16, 17]. The significant risk factor for the development of LV dilatation and dysfunction was the presence of epicardial RV free wall pacing. One cross-sectional multicenter study showed that LV pacing especially apical and lateral wall pacing were associated with the best preservation of LV function, which appears to be related to preserved mechanical synchrony and contraction efficiency [15]. Surgical access to the LV is possible through the left lateral thoracotomy [18], therefore, the pacing site could be optimized in the future study.
Twenty percent of patients in our study had MACE as heart failure or sudden death. All these patients were iatrogenic AVB following complex congenital heart surgery. These patients received a single chamber pacemaker. Currently, dual chamber pacing systems are the first choice for pediatric patients in western countries, but single chamber pacing systems are still widely used in China. It is no doubt that dual chamber pacing is better than single chamber pacing, because it more closely resembles cardiac physiology [19]. Therefore, with improvements in the public health insurance system, for the complex CHD patient who suffered from iatrogenic AVB after surgical repair, dual chamber pacing may reduce morbidity from heart failure and sudden death.
Conclusion
In summary, iatrogenic AVB following congenital heart surgery is the primary reason for the placement of permanent pacemaker in CHD patients. The epicardial approach for permanent pacemaker implantation provided acceptable outcomes for iatrogenic AVB. However, for the complex CHD patient with iatrogenic AVB, the pacemaker type should be optimized.
Acknowledgements
This study was supported by Program for New Century Excellent Talents in University, Foundation for Young Investigator at Peking Union Medical College (XHQN09 and XR01-YP) and Foundation for Professor in PUMC.
Abbreviations
- ASD
atrial septal defect
- AVB
atrioventricular block
- ccTGA
corrected transposition of great arteries
- CHD
congenital heart diseases
- DORV
double outlet right ventricle
- LVEDD
left ventricular end diastolic diameter
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiac events
- TECD
complete endocardial cushion defect
- TOF
tetralogy of Fallot
- VSD
ventricular septal defect
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TZ and YWL carried out the data collection, participated in the analysis and interpretation of clinical data, drafted the manuscript and performed the statistical analysis. CWZ participated in the design of the study and revised the intellectual content. HZ conceived of the study, performed all of the surgical procedures, and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Chengwei Zou, Email: cwzou@hotmail.com.
Hao Zhang, Email: drzhanghao@126.com.
References
- 1.McLeod KA. Cardiac pacing in infants and children. Heart. 2010;96:1502–8. doi: 10.1136/hrt.2009.173328. [DOI] [PubMed] [Google Scholar]
- 2.Kerstjens Frederikse MW, Bink Boelkens MT, de Jongste MJ, van der Heide JNH. Permanent cardiac pacing in children: morbidity and efficacy of follow-up. Int J Cardiol. 1991;33:207–14. doi: 10.1016/0167-5273(91)90348-S. [DOI] [PubMed] [Google Scholar]
- 3.Esperer HD, Singer H, Riede FT, Blum U, Mahmoud FO, Weniger J. Permanent epicardial and transvenous single- and dual-chamber cardiac pacing in children. Thorac Cardiovasc Surg. 1993;41:21–7. doi: 10.1055/s-2007-1013815. [DOI] [PubMed] [Google Scholar]
- 4.Bonatti V, Agnetti A, Squarcia U. Early and late postoperative complete heart block in pediatric patients submitted to open-heart surgery for congenital heart disease. Pediatr Med Chir. 1998;20:181–6. [PubMed] [Google Scholar]
- 5.Silvetti MS, Drago F, Grutter G, De Santis A, Di CV, Rava L. Twenty years of paediatric cardiac pacing: 515 pacemakers and 480 leads implanted in 292 patients. Europace. 2006;8:530–6. doi: 10.1093/europace/eul062. [DOI] [PubMed] [Google Scholar]
- 6.Kwak JG, Kim SJ, Song JY, Choi EY, Lee SY, Shim WS, et al. Permanent epicardial pacing in pediatric patients: 12-year experience at a single center. Ann Thorac Surg. 2012;93:634–9. doi: 10.1016/j.athoracsur.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 7.McLeod CJ, Attenhofer JCH, Warnes CA, Hodge D, Hyberger L, Connolly HM, et al. Epicardial versus endocardial permanent pacing in adults with congenital heart disease. J Interv Card Electrophysiol. 2010;28:235–43. doi: 10.1007/s10840-010-9494-4. [DOI] [PubMed] [Google Scholar]
- 8.Beaufort-Krol GC, Mulder H, Nagelkerke D, Waterbolk TW, Bink-Boelkens MT. Comparison of longevity, pacing, and sensing characteristics of steroid-eluting epicardial versus conventional endocardial pacing leads in children. J Thorac Cardiovasc Surg. 1999;117:523–8. doi: 10.1016/S0022-5223(99)70332-6. [DOI] [PubMed] [Google Scholar]
- 9.Lotfy W, Hegazy R, AbdElAziz O, Sobhy R, Hasanein H, Shaltout F. Permanent cardiac pacing in pediatric patients. Pediatr Cardiol. 2013;34:273–80. doi: 10.1007/s00246-012-0433-2. [DOI] [PubMed] [Google Scholar]
- 10.Sachweh JS, Vazquez-Jimenez JF, Schondube FA, Daebritz SH, Dorge H, Muhler EG, et al. Twenty years experience with pediatric pacing: epicardial and transvenous stimulation. Eur J Cardiothorac Surg. 2000;17:455–61. doi: 10.1016/S1010-7940(00)00364-X. [DOI] [PubMed] [Google Scholar]
- 11.Weindling SN, Saul JP, Gamble WJ, Mayer JE, Wessel D, Walsh EP. Duration of complete atrioventricular block after congenital heart disease surgery. Am J Cardiol. 1998;82:525–7. doi: 10.1016/S0002-9149(98)00375-0. [DOI] [PubMed] [Google Scholar]
- 12.Epstein AE, DiMarco JP, Ellenbogen KA, Mrak Estes KA, Freedman RA, Gettes LS. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Horenstein MS, Hakimi M, Walters H, 3rd, Karpawich PP. Chronic performance of steroid-eluting epicardial leads in a growing pediatric population: a 10-year comparison. Pacing Clin Electrophysiol. 2003;26:1467–71. doi: 10.1046/j.1460-9592.2003.t01-1-00212.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MI, Bush DM, Vetter VL, Tanel RE, Wieand TS, Gaynor JW, et al. Permanent epicardial pacing in pediatric patients: seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103:2585–90. doi: 10.1161/01.CIR.103.21.2585. [DOI] [PubMed] [Google Scholar]
- 15.Janousek J, van Geldorp IE, Krupickova S, Rosenthal E, Peacock K, Tomaske M. Permanent cardiac pacing in children: choosing the optimal pacing site: a multicenter study. Circulation. 2013;127:613–23. doi: 10.1161/CIRCULATIONAHA.112.115428. [DOI] [PubMed] [Google Scholar]
- 16.Gebauer RA, Tomek V, Salameh A, Marek J, Chaloupecky V, Gebauer R, et al. Predictors of left ventricular remodelling and failure in right ventricular pacing in the young. Eur Heart J. 2009;30:1097–104. doi: 10.1093/eurheartj/ehp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Geldorp IE, Delhaas T, Gebauer RA, Frias P, Tomaske M, Friedberg MK, et al. Impact of the permanent ventricular pacing site on left ventricular function in children: a retrospective multicentre survey. Heart. 2011;97:2051–5. doi: 10.1136/heartjnl-2011-300197. [DOI] [PubMed] [Google Scholar]
- 18.Dodge-Khatami A, Dave H, Rahn M, Pretre R, Bauersfeld U. Left heart atrial and ventricular epicardial pacing through a left lateral thorcacotomy in children: a safe approach with excellent functional and cosmetic results. Eur J Cardiothorac Surg. 2005;28:541–5. doi: 10.1016/j.ejcts.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 19.Dretzke J, Toff WD, Lip GY, Raftery J, Fry-Smith A, Taylor RS. Dual chamber versus single chamber ventricular pacemakers for sick sinus syndrome and atrioventricular block. Cochrane Database Syst Rev. 2004;2 doi: 10.1002/14651858.CD003710.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


