Abstract
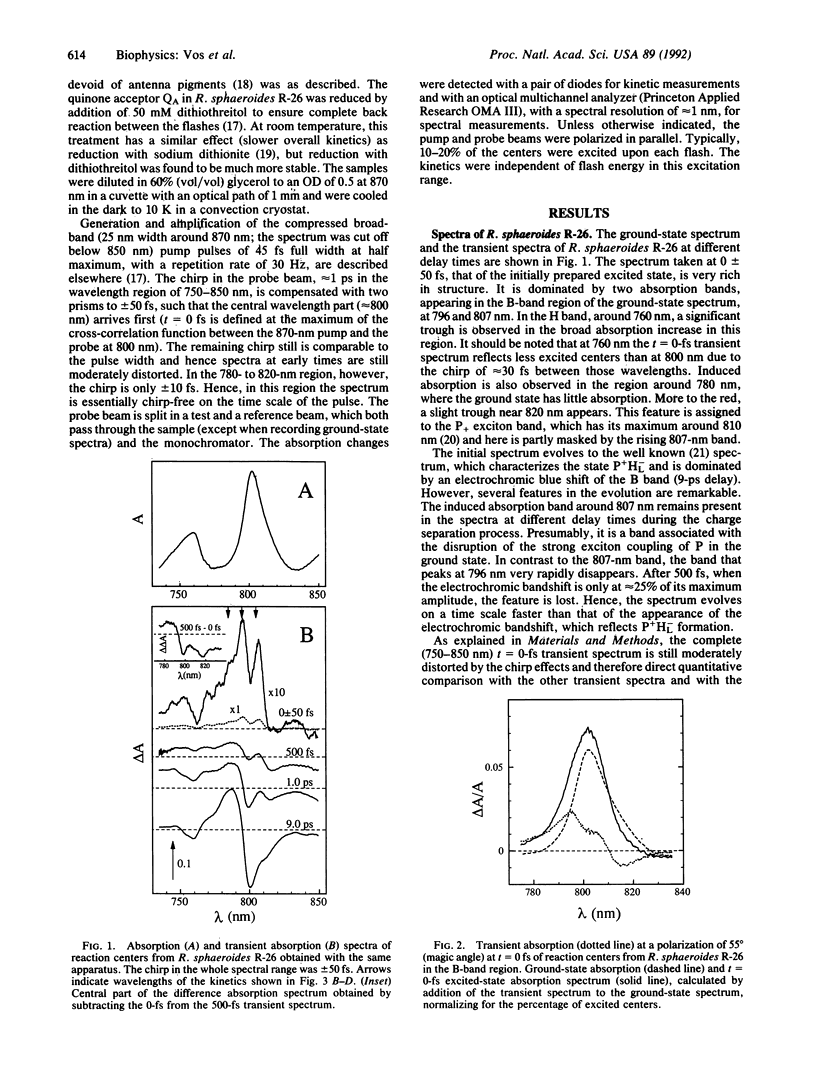
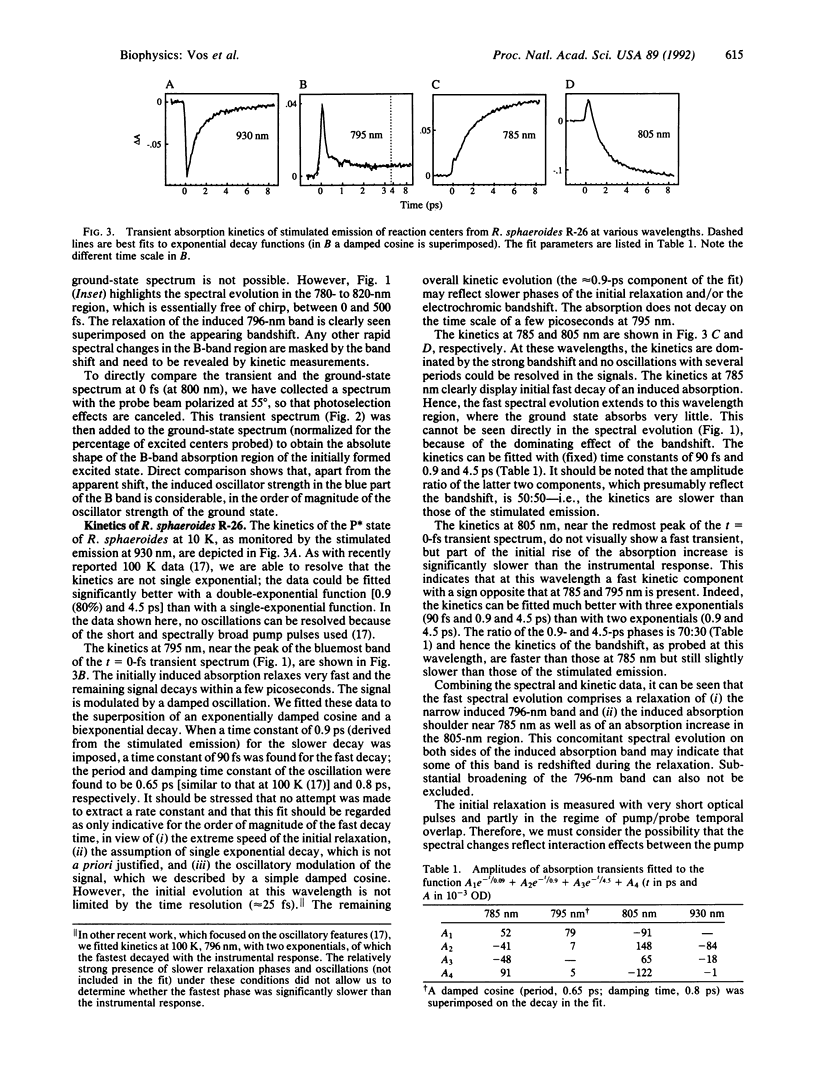
The femtosecond spectral evolution of reaction centers of Rhodobacter sphaeroides R-26 was studied at 10 K. Transient spectra in the near infrared region, obtained with 45-fs pulses (pump pulses centered at 870 nm and continuum probe pulses), were analyzed with associated kinetics at specific wavelengths. The t = 0-fs transient spectrum is very rich in structure; it contains separate induced bands at 807 and 796 nm and a bleaching near 760 nm, reflecting strong changes in interaction between all pigments upon formation of the excited state. A complex spectral evolution in the 800-nm region, most notably the bleaching of the 796-nm band, takes place within a few hundred femtosecond--i.e., on a time scale much faster than electron transfer from the primary donor P to the bacteriopheophytin acceptor HL. The remarkable initial spectral features and their evolution are presumably related to the presence of HL, as they were not observed in the DLL mutant of Rhodobacter capsulatus, which lacks this pigment. A simple linear reaction scheme with an intermediate state cannot account for our data; the initial spectral evolution must reflect relaxation processes within the excited state. The importance for primary photochemistry of long distance interactions in the reaction center is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G. Crystallization of reaction center from Rhodopseudomonas sphaeroides: preliminary characterization. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4795–4799. doi: 10.1073/pnas.81.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer S. G. Mechanisms of long-distance electron transfer in proteins: lessons from photosynthetic reaction centers. Annu Rev Biophys Biophys Chem. 1990;19:267–299. doi: 10.1146/annurev.bb.19.060190.001411. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Friesner R. A., Won Y. D. Spectroscopy and electron transfer dynamics of the bacterial photosynthetic reaction center. Biochim Biophys Acta. 1989 Nov 23;977(2):99–122. doi: 10.1016/s0005-2728(89)80062-3. [DOI] [PubMed] [Google Scholar]
- Go N., Noguti T., Nishikawa T. Dynamics of a small globular protein in terms of low-frequency vibrational modes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3696–3700. doi: 10.1073/pnas.80.12.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel W., Finkele U., Kaiser W., Oesterhelt D., Scheer H., Stilz H. U., Zinth W. Initial electron-transfer in the reaction center from Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5168–5172. doi: 10.1073/pnas.87.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmaier C., Holten D. Evidence that a distribution of bacterial reaction centers underlies the temperature and detection-wavelength dependence of the rates of the primary electron-transfer reactions. Proc Natl Acad Sci U S A. 1990 May;87(9):3552–3556. doi: 10.1073/pnas.87.9.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lous E. J., Hoff A. J. Exciton interactions in reaction centers of the photosynthetic bacterium Rhodopseudomonas viridis probed by optical triplet-minus-singlet polarization spectroscopy at 1.2 K monitored through absorbance-detected magnetic resonance. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6147–6151. doi: 10.1073/pnas.84.17.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lösche M., Feher G., Okamura M. Y. The Stark effect in reaction centers from Rhodobacter sphaeroides R-26 and Rhodopseudomonas viridis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7537–7541. doi: 10.1073/pnas.84.21.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech S. R., Hoff A. J., Wiersma D. A. Role of charge-transfer states in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9464–9468. doi: 10.1073/pnas.83.24.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles S. J., Breton J., Youvan D. C. Partial symmetrization of the photosynthetic reaction center. Science. 1990 Jun 15;248(4961):1402–1405. doi: 10.1126/science.2192455. [DOI] [PubMed] [Google Scholar]
- Vos M. H., Lambry J. C., Robles S. J., Youvan D. C., Breton J., Martin J. L. Direct observation of vibrational coherence in bacterial reaction centers using femtosecond absorption spectroscopy. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8885–8889. doi: 10.1073/pnas.88.20.8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A. Role of the chlorophyll dimer in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3105–3109. doi: 10.1073/pnas.77.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]



