Abstract
Background
Depression presents a significant burden to both patients and society. One treatment that has emerged is vagus nerve stimulation (VNS), an FDA-approved physical treatment for depressive disorders. However, the application of this intervention has been limited by the involvement of surgery and potential side effects. The aim of this study is to explore the effectiveness of stimulating the superficial branches of the vagus nerve as a solo treatment for MDD.
Methods
This is a nonrandomized, controlled study. The first cohort of patients (n = 91) only received transcutaneous auricular VNS (taVNS) for 12 weeks. In the second cohort (n = 69), patients first received 4 weeks of sham taVNS followed by 8 weeks of taVNS. All treatments were self-administered by the patients at home after they received training from the hospitals. The primary outcome measurement was the 24-item Hamilton Depression Rating Scale measured at weeks 0, 4, 8, and 12. Data analysis included a timelag analysis comparing 1) real and sham taVNS groups at week 4; 2) the real taVNS group at week 4 vs the sham taVNS group at week 8 (fourth week of real taVNS following 4 weeks of sham); and 3) the real taVNS group at week 8 vs the sham taVNS group at week 12 (eighth week of real taVNS following sham).
Results
After four weeks of treatment, MDD patients in the taVNS group showed greater improvement than that of the sham taVNS group as indicated by both Hamilton score changes as well as response and remission rates at week four. In addition, we also found that the clinical improvements continued until week 12 during taVNS.
Limitations
Patients were not randomized in this study.
Conclusions
Our results suggest that taVNS is a promising, safe, and cost-effective therapeutic method for mild and moderate MDD.
Keywords: transcutaneous auricular vagus nerve stimulation, transcutaneous vagus nerve stimulation, Major Depressive Disorder, depression, Hamilton Depression Rating Scale
Introduction
Major depressive disorder (MDD) is the fourth leading cause of disability worldwide (Sackeim and Lisanby, 2001) and is projected to become the second leading cause of disability worldwide by the year 2020 (Michaud et al., 2001; Rush, 2003). Patients with MDD experience reduced quality of life in terms of psychological, physical, and social functioning, and this impairment increases with the severity of the disease (Daly et al., 2010). Antidepressant medication is considered as afirst-line treatment for depression, yet up to 68% of patients stop taking antidepressants within 3 months (Gartlehner et al., 2011). Approximately 50% of patients with MDD will experience a response to first-line antidepressant therapy and one-third of patients will achieve remission with any given antidepressant, but half of these patients will experience a relapse during continuous treatment before they achieve recovery (Rush et al., 2006). Thus, despite the critical need, current treatments for MDD are far from satisfactory (Rush, 2003; Sackeim and Lisanby, 2001).
Vagus nerve stimulation (VNS) is an FDA-approved somatic treatment for treatment-resistant depression (TRD) that can produce clinically significant antidepressant effects (Daban et al., 2008; George et al., 2003; Nemeroff et al., 2006; Sackeim and Lisanby, 2001). However, the surgical risks and potentially significant side effects have limited this treatment to MDD patients who have been treated for depression but failed to respond to at least 4 prescribed medications and/or established somatic treatment options such as electroconvulsive therapy (Fitzgerald, 2013; Ventureyra, 2000).
To overcome the potential barriers of applying VNS, a non-invasive transcutaneous vagus nerve stimulation (taVNS) method has been developed. Anatomical studies suggest that the ear is the only place on the surface of the human body where there is afferent vagus nerve distribution (Henry, 2002; Peuker and Filler, 2002). According to the “bottom-up” mechanism of the CNS, the propagation of electric stimuli might follow an inverse path from peripheral nerves toward the brain stem and central structures (Shiozawa et al., 2014). Consequently, direct stimulation of the afferent nerve fibers on the ear should produce an effect similar to classic VNS in reducing depressive symptoms, but without the burden of surgical intervention (Hein et al., 2013; Rong et al., 2012). In past years, taVNS has been applied to treat disorders such as epilepsy (Rong et al., 2014a; Stefan et al., 2012) and pre-diabetes (Huang et al., 2014) and has also been applied to boost associative memory in older individuals (Jacobs et al., 2015).
In a previous study (Hein et al., 2013), investigators explored the therapeutic effect of taVNS on 37 patients suffering from MDD using an add-on design (antidepressant therapy + real or sham taVNS). After two weeks of treatment, the taVNS group showed significant improvement on the Beck Depression Inventory (BDI) as compared with the sham condition. However, there was no significant difference on the Hamilton Depression Rating Scale (HAMD). Although the pilot study demonstrated that taVNS had potential as an MDD treatment, the small sample size, short length of treatment, and potential confounding of different antidepressant therapies have limited the significance of the study.
In this study, we applied a nonrandomized, controlled clinical trial to investigate the antidepressant effect of solo taVNS treatment in mild or moderate MDD patients. In the first cohort, patients received taVNS for 12 weeks to test the effectiveness of the treatment. In the second cohort, patients began with four weeks of sham taVNS followed by 8 weeks of taVNS. We hypothesize that taVNS will produce greater improvement in depression patients as compared with sham taVNS.
Methods
This study was registered at the Chinese Clinical Trial Registry Center (ChiCTR-TRC-11001201). The Institutional Ethics Committee of the China Academy of Chinese Medical Sciences approved this study. All clinical investigative procedures were conducted according to the principles expressed in the Declaration of Helsinki. All patients signed a consent form prior to initiation of study procedures.
Due to ethical and safety concerns, we recruited two cohorts of patients. The patients in the first cohort received only real taVNS to test the effectiveness of the treatment; the patients in the second cohort received sham taVNS only for one month before shifting to real taVNS treatment for two months (Figure 1). The clinical outcomes (primary and secondary outcomes), inclusion and exclusion criteria, and the real and sham treatment procedures remain the same as originally registered. Some patients (n = 49) were also invited to participate in an fMRI study at baseline and after 4 weeks of treatment to investigate the brain resting state functional connectivity changes before and after one month of taVNS as compared to sham taVNS. Please see the original publication for more details (Fang et al., 2015). The present study will focus on the clinical outcomes of a 12-week treatment of the multiple-center clinical trial, which was not reported in the previous brain imaging study.
Figure 1.
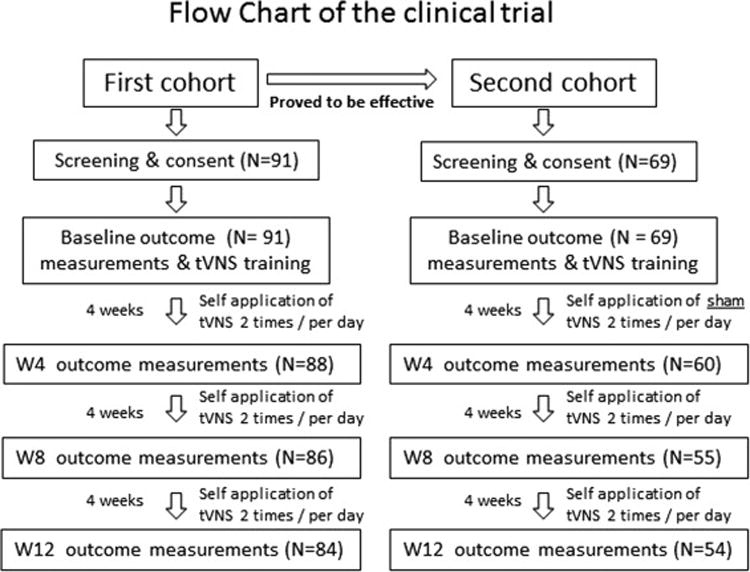
The flow diagram shows detailed information regarding recruited and excluded participants.
Inclusion criteria
1) Meets ICD-10 diagnosis standard of a depressive episode: mild (2 typical + 2 other core symptoms), moderate (2 typical + 3 other core symptoms); 2) HAM-D-24 score between 8 and 35; 3) 18–70 years of age; 4) Ceased taking anti-depressive or other psychiatric medications 2 weeks before beginning the intervention; 5) Junior high or higher level education (in order to understand the scales); 6) Exhibited symptoms for 2 weeks to 2 years.
Exclusion criteria
1) Ongoing addiction to drugs and alcohol; 2) Severe depression (HAM-D-24 score>35) or suicidal intent; 3) Bipolar disorder; 4) Organic mental disorder; 5) Drug-induced depression; 6) Seasonal affective disorder; 7) Severe medical disorders; 8) Pregnant women; 9) Postpartum depression; 10) Dementia or other cognitive disorders; 11) Patients who did not agree to sign the consent form.
Recruitment procedures
Investigators recruited patients with mild or moderate depressive symptoms from three participating hospitals through advertising and flyers. After passing a pre-screening performed by a qualified physician and in accordance with the inclusion and exclusion criteria, potentially eligible patients provided informed consent in the presence of a study physician.
Intervention and comparison
After receiving their group assignment, all patients were trained to apply taVNS or sham taVNS by themselves. Specifically, patients were trained on how to turn on/off the machine, how to apply the electrode to the ear and locate the stimulation position, how to increase the intensity, and how to fill out the diary booklet. The procedure was repeated until the subjects were capable of using the machine independently. The training process usually lasted about 40 minutes.
All subsequent treatments were self-administered by the patients at home, and assistance was available by phone or via site visits if patients had questions about treatment. Patients were also instructed to complete a patient diary booklet each day to describe any side effects corresponding with or temporally related to treatment. The investigators checked all booklets at assessments every four weeks to ensure compliance. All procedures performed in the sham taVNS treatment group were identical to the procedures for the taVNS group.
taVNS treatment
Location
The taVNS points are located in the auricular concha area, where there is rich vagus nerve branch distribution (Figure 2).
Figure 2.
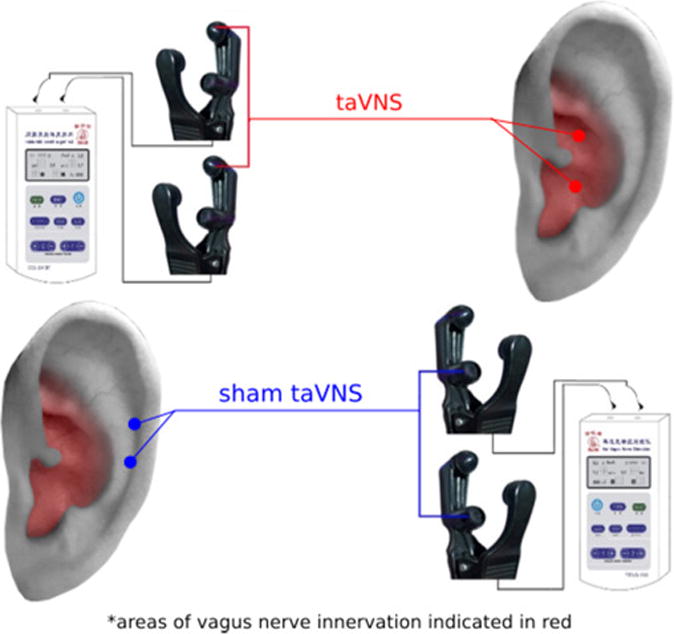
Location of taVNS and sham taVNS. Red coloring indicates area with vagus nerve distribution. Red dots indicate location of taVNS; blue dots indicate the location of sham taVNS.
Intervention procedure
All treatments were applied with an ear vagus nerve stimulator developed through the cooperation of the Institute of Acupuncture and Moxibustion, China Academy to Chinese Medicine Science (Beijing, China) and Suzhou Medical Appliance Factory (Jiangsu Province, China) with special ear clips (electrodes) (Huang et al., 2014; Rong et al., 2014b; Rong et al., 2012). Patients took a seated position or lay on their sides. After stimulation points were disinfected according to standard practice, ear clips were attached to the auricular concha. Similar to previous studies (Rong et al., 2014b; Rong et al., 2012), stimulation parameters included: 1) a 20 Hz continuous sinusoidal wave (wave width of 0.2 ms) (Aihua et al., 2014; He et al., 2013; La Marca et al., 2010; Wang et al., 2012), and 2) stimulation intensity increased gradually (starting from 0) to the highest point that the patients could tolerate (typically between 4–6 mA). Each treatment lasted for 30 minutes and was carried out twice a day (once in the morning, once after dinner) (Huang et al., 2014).
Sham taVNS treatment
Location
The stimulation points for sham taVNS are located at the superior scapha (outer ear margin midpoint), where there is no vagus nerve distribution (Figure 2). A specially designed ear clip (electrode) that looks identical to a real taVNS clip was applied for sham treatment.
Intervention procedure
All procedures and stimulation parameters in the sham taVNS treatment group were identical to those of the real taVNS group. After 4 weeks, patients shifted to taVNS treatment for 8 weeks by changing a pair of ear clips (electrodes) with a line designed specifically for real taVNS. To blind the patients, all patients were told that the line and clips would be regularly changed to ensure the reliability of stimulation.
Clinical outcomes
All endpoints were measured at weeks 0, 4, 8, and 12. The primary endpoint was the 24-item Hamilton Depression Rating Scale (HAM-D-24) (Tang, 1984) and the secondary endpoints included the Self-rating Depression Scale (SDS), 17-item Hamilton Anxiety Rating Scale (HAM-A-17), and Self-rating Anxiety Scale (SAS). At the end of weeks 4, 8, and 12, we also assessed the differences in treatment response and remission rates between the two groups using HAM-D-24, where a response is defined as a 50% or greater reduction in HAM-D-24 scores and remission is defined as HAM-D-24 scores of 7 or less. Trained interviewers blind to treatment condition conducted the evaluations.
Statistical analysis
The effect of taVNS was estimated by comparing HAM-D-24 score differences between Week 4 and Week 0 using mixed-model regression with hospitals, group (real and sham taVNS), and week (Week 0 and Week 4) as fixed effects and patients as a random effect on patients who completed the trial at week 4. The analysis was performed using R Version 3.1.0, with the lme4 (http://CRAN.R-project.org/package=lme4) and lmerTest packages (http://CRAN.R-project.org/package=lmerTest). For this model, the treatment effect is the group and time interaction; the between hospital treatment difference is measured by the group, time, and hospital interaction. We omitted data from hospital H3 for this analysis because all H3 patients received the real treatment. Similar analyses were also performed on other clinical outcomes.
In addition, we compared the categorical classification of treatment response between the real and sham taVNS groups including the data from all three hospitals. We define the treatment response as a 50% or greater reduction in HAM-D-24 scores following treatment. Response rates between the two groups were compared using the chi square (x2) test.
Power calculation
Since no prior study had used taVNS as the solo treatment for MDD, as a novel treatment, we present here our power analysis for the primary outcome (HAM-D-24) at week 4. For comparison of the pre- and post-treatment differences between real and sham taVNS, with 91 patients in the taVNS group and 69 patients in the sham taVNS group, we will have 80% power to test the effect size of 0.45 between the two groups based on the two sample t-test at a significance level of 0.05.
Results
One hundred sixty participants enrolled in the study (n = 91 cohort 1, n = 69 cohort 2). 148 subjects completed the trial at week 4, and 138 completed the trial at week 12 (n = 84 cohort 1; n = 54 cohort 2). Seven participants from the taVNS group dropped from the study: five due to scheduling conflicts, one due to complete symptom relief before week 12, and one due to tinnitus enhancement resulting from incorrect manipulation of the equipment (symptoms were relieved after stopping treatment) (Figure 1). The whole study was performed between March of 2011 and December of 2013. Fifteen participants from the sham taVNS group withdrew from the study, all due to lack of a satisfactory effect. All other participants reported following the treatment instructions.
The baseline characteristics and clinical outcomes are shown in Table 1. The results showed no significant difference in age, gender, family history, HAM-D-24, SDS, and SAS between the real and sham groups at baseline. However, there was a significant difference on the HAM-A-17 level (p = 0.005).
Table 1.
Baseline subjects’ characteristics and clinical outcomes at baseline across two groups. All variables are presented as (mean (SD))
| Group | taVNS | Sham taVNS | P value |
|---|---|---|---|
| Number | 91 | 69 | |
| Age | 40.10(16.15) | 43.88(13.95) | 0.12 |
| Gender (M/F) | 23/68 | 20/49 | 0.72 |
| Marital Status (M/S) | 54/37 | 60/9 | <0.0001 |
| Family History (Y/N) | 5/86 | 5/64 | 0.75 |
| HAMD | 25.01(7.20) | 24.42(5.54) | 0.52 |
| SDS | 61.68(9.29) | 63.30(8.83) | 0.27 |
| HAMA | 16.91(7.26) | 14.16(5.09) | 0.005 |
| SAS | 52.02(9.42) | 52.67(9.67) | 0.67 |
Comparison between the taVNS and sham taVNS at week 4
We found that at week 4, the HAM-D-24 scores in both groups showed a decrease, but the reduction in the real taVNS group was significantly greater than in the sham taVNS group. In addition, we also found there was no significant interaction between group, time, and hospital interaction (p = 0.06). This trend toward significance can be explained by a difference in patients’ depression severity at baseline between the two hospitals as indicated by HAM-D-24 scores (the following analysis showed that effect size tends to be larger in patients with higher HAM-D-24 scores). Thus, we re-ran the analysis, leaving the hospital out of the model. The significant treatment effect remained (Table 3).
Table 3.
Comparisons of clinical outcome changes (week 0 – week 4) between taVNS and sham tsVNS groups combining hospitals 1 & 2. 95% indicates the treatment effect as indicated by outcome change between the real and sham taVNS (taVNS (pre-post) minus sham taVNS (pre-post).
| P-value | Lower 95% CI | Upper 95% CI | Effect size | |
|---|---|---|---|---|
| HAMD | < 0.0001 | 4.5 | 8.3 | 0.57 |
| HAMA | <0.0001 | 1.6 | 5.6 | 0.3 |
| SAS | 0.01 | 0.5 | 7.2 | 0.2 |
| SDS | <0.0001 | 5.5 | 12.4 | 0.44 |
To explore the treatment effect on the severity of patients’ depression at baseline, we divided the patients into two subgroups based on HAM-D-24 score (mild subgroup, HAM-D-24 score < 20; moderate subgroup, HAM-D-24 score ≥ 20 group) (Tang, 1984). We observed a significant treatment effect in both the mild subgroup (p = 0.04, effect size 0.4) and the moderate subgroup (p < 0.0001, effect size 0.68).
The comparison of response rates (defined by 50% reduction in the HAM-D-24 score) showed that at week 4, there were 24 responders in the taVNS group and no responders in the sham taVNS group (Table 4). There was a significant difference between the two groups at week 4 (p < 0.00001). Interestingly, there was no significant difference in the response rate between weeks 0–4 in the taVNS group and weeks 4–8 in the sham taVNS group, after the patients had shifted to taVNS (p = 0.07). Similarly, there was no significant difference between the response rates of the taVNS group in weeks 4–8 and the sham taVNS group in weeks 8–12 (Table 4).
Table 4.
Number of patients who responded (50% reduction in HAM-D-24 score) and achieved remission (defined as a HAM-D-24 scores < 8) after treatment in two groups across different time points. Please note that patients in sham taVNS shifted to taVNS after 4 weeks of sham taVNS.
| Response N (%) | Remission N (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Week 4 | Week 8 | Week 12 | |||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| taVNS | 24 (27) |
64 (73) |
46 (53) |
40 (47) |
67 (80) |
17 (20) |
3 (3) |
85 (97) |
22 (26) |
64 (74) |
33 (39) |
51 (61) |
| Sham taVNS | 0 (0) |
60 (100) |
7 (13) |
48 (87) |
21 (39) |
33 (61) |
0 (0) |
60 (100) |
3 (5) |
52 (95) |
9 (17) |
45 (83) |
Remission (defined as a HAM-D-24 scores < 8) showed that 3 patients of the taVNS group achieved remission at week 4, while none did in the sham group (Table 4). There was no significant difference between the two groups (p = 0.148) at week 4. However, we found significant differences between weeks 0–4 and 8–12 in the taVNS group (p<= 0.0001), indicating a continuous increase in remission rate across different time points. There was no significant difference in the remission rate between weeks 0–4 in the taVNS group and weeks 4–8 in the sham taVNS group, after the patients had shifted to taVNS (p = 0.553). Similar results were also observed between weeks 4–8 in the taVNS group and weeks 8–12 in the sham taVNS group (p = 0.216) (Table 4).
Similar results were observed in secondary outcomes including SDS, SAS, and HAM-A-17, i.e., combining the two hospitals showed significant improvement at week 4 when comparing taVNS and sham taVNS (Table 3).
taVNS treatment effect at week 12
In this study, patients in the taVNS group received treatment for three months. The clinical outcomes for each month are shown in Figure 2. Data showed that symptom improvement as indicated by clinical outcomes as well as response and remission (Table 4) continued until the end of this study (week 12). Similar results were also observed in the sham group after it shifted to taVNS (Figure 3).
Figure 3.
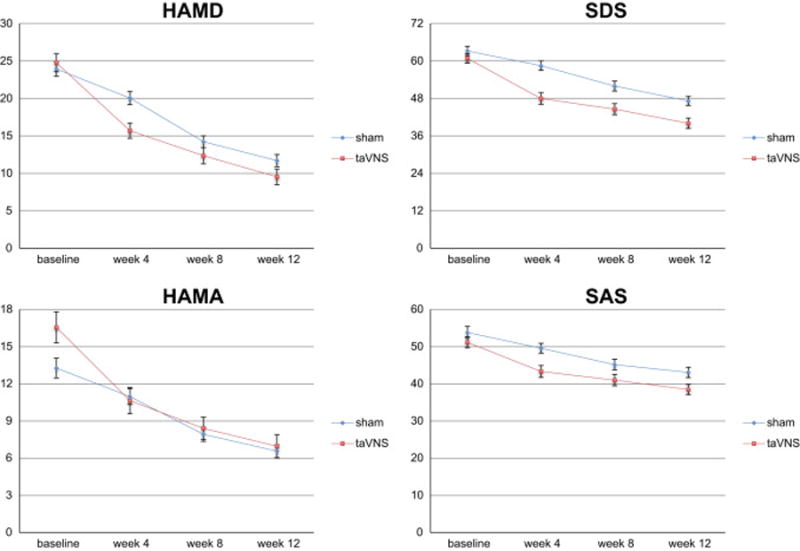
Clinical outcome measurements at different time points (bars indicate 5% confidence interval).
Safety
Based on the patients’ booklets and verbal reports, the main side effect was tinnitus, as shown in the acceleration of original tinnitus (2 in the taVNS group, 3 in the sham taVNS group). All participants recovered fully from the adverse events after stopping the treatment.
Discussion
In this study, we investigated the treatment effect of solo taVNS on patients with mild or moderate MDD. We found that taVNS could significantly reduce the symptoms of depression in the three months during which the treatments were applied. More importantly, we found that the symptom reductions were greater in the taVNS group than in the sham taVNS group for the first four weeks when sham taVNS was applied.
In a previous pilot study (Hein et al., 2013), Hein and colleagues investigated the short-term (2 weeks) therapeutic effect of taVNS on patients suffering from major depression using an add-on design. They found that taVNS could significantly reduce BDI as compared with the sham condition. However, there was no significant difference on the HAMD. In this study, we found that taVNS could not only reduce HAM-D-24 and SDS, but also reduce anxiety symptoms in these patients as indicated by HAM-A-17 and SAS in comparison with sham taVNS at week 4. These results may indicate that this method may also be extended to anxiety disorders in the future.
Moreover, we further analyzed the taVNS effect in subgroups of patients with different severity. Our results showed that although the effect size is smaller, taVNS can significantly reduce the HAM-D-24 score in those with relatively mild symptoms (HAM-D-24 < 20). Despite its considerable functional impairment (Cuijpers et al., 2004; Cuijpers et al., 2013; Hwang et al., 2015), mild MDD has usually not been viewed as responsive to antidepressants. Our results suggest taVNS may be considered as a first-line treatment targeting mild and moderate MDD.
In this study, we found significant differences in the response rate at week 4 between the two groups, which is consistent with findings of HAM-D-24 score changes. Nevertheless, we did not find significant differences in the remission rate at week 4 between the two groups, and we speculate this may due to a short observation time. Future studies with longer durations of sham treatment are needed. However, we did find significant improvement of the remission rate in the taVNS group across different time points, indicating that symptom relief continued across 12 weeks of treatment.
Despite the clinical application of VNS for MDD patients, its underlying mechanism is not yet fully understood (Rush, 2003; Rush et al., 2005a; Rush et al., 2005b). Hypotheses are based on the impact of anatomy and functional changes of the vagus nerve in mood control (Mohr et al., 2011). The vagus nerve is a mixed nerve composed of approximately 80% afferent fibers. It is speculated that the antidepressant effects of VNS are attributed partially to the projection of afferent fibers to the nucleus tractus solitaries (NTS), which is further connected both directly and indirectly with brain structures such as amygdala, hypothalamus, insula, thalamus, orbitofrontal cortex, and other limbic regions responsible for mood and anxiety regulation (Conway et al., 2012; Conway et al., 2006; Lomarev et al., 2002; Mu et al., 2004). An animal study (He et al., 2013) has shown that stimulating the auricular branches of the vagus nerve can significantly suppress epileptiform activity via the NTS in rats, which further endorses the effect of taVNS.
More recently, brain-imaging tools have been applied to investigate the fMRI signal change evoked by VNS (Conway et al., 2013; Conway et al., 2012; Conway et al., 2006; Landau et al., 2015) and taVNS (Dietrich et al., 2008; Kraus et al., 2007; Kraus et al., 2013). In a previous study (Kraus et al., 2013), Kraus and colleagues investigated the anterior and posterior wall of the auditory canal separately. They found that stimulation of the anterior wall evoked significant limbic deactivation at the parahippocampal gyrus and the posterior cingulate cortex, as well as other regions including the thalamus, locus coeruleus, and solitary tract. In a more recent study (Fang et al., 2015), we found that after four weeks of taVNS in patients with MDD, resting state functional connectivity (rsFC) between the default mode network (DMN) and anterior insula and parahippocampus decreased while the rsFC between the DMN and precuneus and orbital prefrontal cortex increased compared with sham taVNS. All these FC increases are also associated with HAM-D-24 score reduction. Taken together, these studies provided the neural substrates of taVNS.
We observed only a mild side effect, tinnitus, in the study, mainly due to the mistaken belief of some patients that stronger stimulation would lead to a better effect. Patients with tinnitus fully recovered after stopping the taVNS/sham taVNS without further treatment, which suggests that taVNS is a safe therapeutic method for self-administration.
There are several limitations in this study. First of all, this is not a randomized clinical trial. We used this strategy mainly due to ethical concerns. As the first study to use taVNS alone on patients suffering from mild and moderate depression without severe suicidal intent, we thought it would be wise to test the effectiveness rather than the efficacy of taVNS on the patients first. After demonstrating that taVNS can significantly reduce patients’ symptoms, we recruited a second cohort of patients to test if the effect of taVNS was greater than that of the sham taVNS. Since the baseline characteristics are similar in the two cohorts of patients, we do not expect the design will influence the validity of this study. Nevertheless, a randomized clinical trial is needed in the future.
Secondly, this pilot study was single-blinded. We cannot exclude the possibility of evaluation bias, but we would like to emphasize that the scores on the self-rating scales (SDS and SAS) showed significant improvement alongside the observer-rating scales. We also applied a Pearson’s correlation coefficient analysis between changes in self and observer-rating scales which showed a positive result (HAM-D-24 and SDS, r = 0.59 p < 0.001, HAM-A-17 and SAS, r = 0.38 p< 0.001). This suggests that the treatment effect of taVNS is unlikely to be due to bias and should not influence the conclusion of this study. We did not formally assess the blindness at the end of the study. However, we do believe that the device was blinded since both taVNS and sham taVNS groups experienced sensations evoked by electrical stimulation. The only difference between the two groups was the stimulation location on the ear, but patients did not know if or how the location of stimulation would affect treatment. Thus, we do not think blindness is a concern in this study.
Third, a two-week washout period might not be long enough for long-acting antidepressants. Nevertheless, since only patients with mild or moderate MDD were allowed to participate in the study, few participants took antidepressants before participating. In addition, both real and sham groups used the same washout period; we thus do not expect the short washout period would influence the results and conclusion of this study. Fourth, there is no follow up for this study although patients were allowed to continue treatment after the study ended. In our study, we found constant improvement during the 3 months over which the treatments were applied. The long-term effects of this treatment remain to be investigated. Fifth, we did not apply a multiple baseline design and start the treatment until a stable baseline had been recorded. An investigator should consider incorporating this design in a future study.
Finally, all treatments were administered by the patients themselves. We cannot be sure all patients followed the experimental protocol completely. To enhance compliance, all patients were required to complete daily entries in a diary that was checked during assessments. Nevertheless, this self-administration method provides direct evidence toward the feasibility of widespread application of the method used within the study, which could significantly reduce treatment expenses.
In summary, we found that taVNS can significantly reduce the symptoms of depression in patients as compared to sham taVNS. Our results provide evidence for the application of this non-invasive, safe, and low cost therapeutic method for patients with mild and moderate depression.
Table 2.
Scores of Clinical Outcome Scales of Three Hospitals (Mean ± SD)
| Time | Outcome | taVNS | Sham taVNS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H1 + H2 | All | H1 | H2 | H3 | H1 + H2 | ||
| Week 0 | HAMD | 23.3±7.0 | 27.6±6.2 | 24.6±8.8 | 25.1±7.0 | 25.0±7.2 | 25.1±12.4 | 26.8±3.8 | NA | 24.4±5.5 |
| Week 4 | 15.1±6.2 | 15.4±6.5 | 21.5±5.6 | 15.2±6.3 | 16.0±6.5 | 17.9±4.9 | 23.3±5.1 | NA | 20.6±5.6 | |
| Week 8 | 12.3±6.4 | 10.7±6.0 | 17.3±6.8 | 11.7±6.2 | 12.3±6.5 | 13.0±4.2 | 15.7±6.2 | NA | 14.2±4.7 | |
| Week 12 | 9.8±6.1 | 8.0±5.4 | 12.7±8.3 | 9.1±5.9 | 9.5±6.3 | 11.0±4.1 | 12.6±6.9 | NA | 11.7±4.9 | |
| Week 0 | SDS | 58.0±8.5 | 66.7±9.1 | 62.3±6.5 | 61.6±9.7 | 61.7±9.3 | 61.5±8.8 | 65.4±8.4 | NA | 63.3±8. 8 |
| Week 4 | 44.6±10.5 | 53.5±12.2 | 50.4±9.4 | 48.3±12.0 | 48.5±11.8 | 57.8±8.3 | 60.4±10.0 | NA | 59.1±9.2 | |
| Week 8 | 42.2±11.0 | 49.0±11.8 | 47.4±7.5 | 44.8±11.7 | 45.1±11.3 | 50.0±7.2 | 56.6±11.4 | NA | 52.1±9.5 | |
| Week 12 | 38.8±9.6 | 42.1±10.1 | 39.0±9.6 | 40.2±10.1 | 40.1±10.0 | 45.3±6.8 | 49.5±10.1 | NA | 47.2±8.6 | |
| Week 0 | HAMA | 14.7±6.8 | 19.0±6.9 | 20.0±7.7 | 16.4±7.1 | 16.9±7.3 | 13.1±5.4 | 15.4±4.5 | NA | 14.2±5.1 |
| Week 4 | 10.3±6.9 | 10.7±6.2 | 13.9±6.0 | 10.5±6.6 | 10.9±6.6 | 10.1±3.3 | 12.6±3.9 | NA | 11.3±3.8 | |
| Week 8 | 8.4±6.0 | 8.0±3.9 | 11.2±4.9 | 8.2±5.3 | 8.6±5.3 | 7.6±3.2 | 10.0±4.0 | NA | 8.7±3.8 | |
| Week 12 | 7.1±5.8 | 6.2±4.3 | 8.5±6.7 | 6.8±5.2 | 7.0±5.4 | 6.2±2.5 | 7.2±3.9 | NA | 6.6±3.1 | |
| Week 0 | SAS | 48.7±9.6 | 55.2±8.7 | 56.6±5.1 | 51.3±9.7 | 52.0±9.4 | 49.9±8.1 | 55.9±10.4 | NA | 52.7±9.7 |
| Week 4 | 40.9±9.8 | 47.2±11.7 | 48.3±4.9 | 43.5±11.0 | 44.1±10.6 | 47.9±6.9 | 52.2±8.3 | NA | 50.0±7.9 | |
| Week 8 | 39.8±9.6 | 43.4±11.1 | 44.6±6.4 | 41.2±10.3 | 41.7±10.0 | 42.7±6.8 | 48.3±9.5 | NA | 45.2±8.5 | |
| Week 12 | 37.4±8.1 | 39.8±7.9 | 39.6±8.3 | 38.3±8.1 | 38.5±8.1 | 40.6±6.7 | 46.2±9.3 | NA | 43.1±8.4 | |
Highlights.
We investigated effect of real and sham taVNS in MDD patients
taVNS produced greater improvement than sham taVNS.
Clinical improvements evoked by taVNS continued at least 12 weeks.
taVNS is a promising therapeutic method for MDD.
Acknowledgments
The work is supported by the Special Program of Chinese Medicine of the National Basic Research Program of China (973 Program 2012CB518503), the “Twelfth Five-year Plan” National Science and Technology Support Program of China (2012BAF14B10) and the Beijing Natural Science Foundation of China (7111007), the Acupuncture Hospital affiliated with the China Academy of Chinese Medical Sciences, and the Institutional Ethics Committee of the China Academy of Chinese Medical Sciences for their invaluable contributions to the study. Jian Kong is supported by R01AT006364 (NCCIH/NIH), R01AT008563, (NCCIH/NIH), R21AT008707 (NCCIH/NIH), and P01 AT006663 (NCCIH/NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
PJR designed the trial and was the principal clinical research investigator. PJR and JL and ZYF involved in experimental design and preparation. JL, LPW, HM, HHW, YGM, RPL involved in the patient recruitment, data collection and treatment application. PJR, JL, JK, JJZ, MV, SS, JP, SYL were responsible for data analysis and manuscript preparation/revision. All authors read and approved the final manuscript.
All authors claim no conflicts of interest.
References
- Aihua L, Lu S, Liping L, Xiuru W, Hua L, Yuping W. A controlled trial of transcutaneous vagus nerve stimulation for the treatment of pharmacoresistant epilepsy. Epilepsy Behav. 2014;39:105–110. doi: 10.1016/j.yebeh.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Conway CR, Chibnall JT, Gebara MA, Price JL, Snyder AZ, Mintun MA, Craig AD, Cornell ME, Perantie DC, Giuffra LA, Bucholz RD, Sheline YI. Association of cerebral metabolic activity changes with vagus nerve stimulation antidepressant response in treatment-resistant depression. Brain Stimul. 2013;6:788–797. doi: 10.1016/j.brs.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CR, Sheline YI, Chibnall JT, Bucholz RD, Price JL, Gangwani S, Mintun MA. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul. 2012;5:163–171. doi: 10.1016/j.brs.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res. 2006;146:179–184. doi: 10.1016/j.pscychresns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79:71–79. doi: 10.1016/S0165-0327(02)00348-8. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202:22–27. doi: 10.1192/bjp.bp.112.112169. [DOI] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Cruz N, Vieta E. Safety and efficacy of Vagus Nerve Stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110:1–15. doi: 10.1016/j.jad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Gaynes BN, Warden D, Morris DW, Luther JF, Farabaugh A, Cook I, Rush AJ. Health-related quality of life in depression: a STAR*D report. Ann Clin Psychiatry. 2010;22:43–55. [PubMed] [Google Scholar]
- Dietrich S, Smith J, Scherzinger C, Hofmann-Preiss K, Freitag T, Eisenkolb A, Ringler R. A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed Tech (Berl) 2008;53:104–111. doi: 10.1515/BMT.2008.022. [DOI] [PubMed] [Google Scholar]
- Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, Zhang G, Chen X, Shi S, Wang L, Liu R, Hwang J, Li Z, Tao J, Wang Y, Zhu B, Kong J. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.025. pii: S0006-3223(0015)00274-00277. doi: 00210.01016/j.biopsych.02015.00203.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB. Non-pharmacological biological treatment approaches to difficult-to-treat depression. Med J Aust. 2013;199:S48–51. doi: 10.5694/mja12.10509. [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux LJ, Van Noord M, Mager U, Gaynes BN, Thieda P, Strobelberger M, Lloyd S, Reichenpfader U, Lohr KN. Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. Rockville (MD): 2011. [PubMed] [Google Scholar]
- George MS, Nahas Z, Bohning DE, Kozel FA, Anderson B, Chae JH, Li XB, Mu QW. Potential mechanisms of action of vagus nerve stimulaiton for depression. In: Schachter SC, Schmidt D, editors. Vagus nerve stimulaiton. Martin Dunitz; London: 2003. pp. 68–83. [Google Scholar]
- He W, Jing XH, Zhu B, Zhu XL, Li L, Bai WZ, Ben H. The auriculo-vagal afferent pathway and its role in seizure suppression in rats. BMC Neurosci. 2013;14:85. doi: 10.1186/1471-2202-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, Kraus T. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm. 2013;120:821–827. doi: 10.1007/s00702-012-0908-6. [DOI] [PubMed] [Google Scholar]
- Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59:S3–14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- Huang F, Dong J, Kong J, Wang H, Meng H, Spaeth RB, Camhi S, Liao X, Li X, Zhai X, Li S, Zhu B, Rong P. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement Altern Med. 2014;14:203. doi: 10.1186/1472-6882-14-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Egorova N, Yang XQ, Zhang WY, Chen J, Yang XY, Hu LJ, Sun S, Bao T, Kong J. Subthreshold depression is associated with impaired resting state functional connectivity of the cognitive control network Translational Psychiatry. 2015;5 doi: 10.1038/tp.2015.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Riphagen JM, Razat CM, Wiese S, Sack AT. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol Aging. 2015;36:1860–1867. doi: 10.1016/j.neurobiolaging.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Kraus T, Hosl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- Kraus T, Kiess O, Hosl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal - a pilot study. Brain Stimul. 2013;6:798–804. doi: 10.1016/j.brs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- La Marca R, Nedeljkovic M, Yuan L, Maercker A, Elhert U. Effects of auricular electrical stimulation on vagal activity in healthy men: evidence from a three-armed randomized trial. Clin Sci (Lond) 2010;118:537–546. doi: 10.1042/CS20090264. [DOI] [PubMed] [Google Scholar]
- Landau AM, Dyve S, Jakobsen S, Alstrup AK, Gjedde A, Doudet DJ. Acute Vagal Nerve Stimulation Lowers alpha2 Adrenoceptor Availability: Possible Mechanism of Therapeutic Action. Brain Stimul. 2015 doi: 10.1016/j.brs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, Bohning DE. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36:219–227. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Michaud CM, Murray CJ, Bloom BR. Burden of disease–implications for future research. Jama. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- Mohr P, Rodriguez M, Slavickova A, Hanka J. The application of vagus nerve stimulation and deep brain stimulation in depression. Neuropsychobiology. 2011;64:170–181. doi: 10.1159/000325225. [DOI] [PubMed] [Google Scholar]
- Mu Q, Bohning DE, Nahas Z, Walker J, Anderson B, Johnson KA, Denslow S, Lomarev M, Moghadam P, Chae JH, George MS. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol Psychiatry. 2004;55:816–825. doi: 10.1016/j.biopsych.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–37. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- Rong P, Liu A, Zhang J, Wang Y, He W, Yang A, Li L, Ben H, Liu H, Wu P, Liu R, Zhao Y, Huang F, Li X, Zhu B. Transcutaneous vagus nerve stimulation for refractory epilepsy: a randomized controlled trial. Clin Sci (Lond) 2014a;127:300–304. doi: 10.1042/CS20130518. [DOI] [PubMed] [Google Scholar]
- Rong P, Liu A, Zhang J, Wang Y, Yang A, Li L, Ben H, Li L, Liu R, He W, Liu H, Huang F, Li X, Wu P, Zhu B. An alternative therapy for drug-resistant epilepsy: transcutaneous auricular vagus nerve stimulation. Chin Med J (Engl) 2014b;127:300–304. [PubMed] [Google Scholar]
- Rong PJ, Fang JL, Wang LP, Meng H, Liu J, Ma YG, Ben H, Li L, Liu RP, Huang ZX, Zhao YF, Li X, Zhu B, Kong J. Transcutaneous vagus nerve stimulation for the treatment of depression: a study protocol for a double blinded randomized clinical trial. BMC Complement Altern Med. 2012;12:255. doi: 10.1186/1472-6882-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ. Vagus nerve stimulation: clinical results in depression. In: Schachter SC, Schmidt D, editors. Vagus nerve stimulaiton. Martin Dunitz; London: 2003. pp. 85–112. [Google Scholar]
- Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, Howland R, Kling MA, Rittberg BR, Burke WJ, Rapaport MH, Zajecka J, Nierenberg AA, Husain MM, Ginsberg D, Cooke RG. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005a;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, Lavori P, Howland R, Kling MA, Rittberg B, Carpenter L, Ninan P, Moreno F, Schwartz T, Conway C, Burke M, Barry JJ. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005b;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Lisanby SH. physical treatments in psychiatry. In: Weissman MM, editor. Treatment of depression: bridging the 21 st century. American psychiatric press; Washington, DC: 2001. pp. 151–172. [Google Scholar]
- Shiozawa P, Silva ME, Carvalho TC, Cordeiro Q, Brunoni AR, Fregni F. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: a systematic review. Arq Neuropsiquiatr. 2014;72:542–547. doi: 10.1590/0004-282x20140061. [DOI] [PubMed] [Google Scholar]
- Stefan H, Kreiselmeyer G, Kerling F, Kurzbuch K, Rauch C, Heers M, Kasper BS, Hammen T, Rzonsa M, Pauli E, Ellrich J, Graf W, Hopfengartner R. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: A proof of concept trial. Epilepsia. 2012;53:e115–118. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
- Tang YH. Hamilton Depression Rating Scale. The Shanghai Archives of Psychiatry. 1984;2:61–64. [Google Scholar]
- Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst. 2000;16:101–102. doi: 10.1007/s003810050021. [DOI] [PubMed] [Google Scholar]
- Wang XY, Shang HY, He W, Shi H, Jing XH, Zhu B. Effects of transcutaneous electrostimulation of auricular concha at different stimulating frequencies and duration on acute seizures in epilepsy rats. Zhen Ci Yan Jiu. 2012;37:447–452. 457. [PubMed] [Google Scholar]


