Abstract
Background
Dietary and exercise data are frequently recorded in clinical research, but their correlation with metabolic measures needs further evaluation.
Objective
We examined the association of food and exercise habits with body size, lipid profile, and glycemia in a prospective biracial cohort.
Methods
The Pathobiology of Prediabetes in A Biracial Cohort study followed initially normoglycemic offspring of parents with type 2 diabetes (T2DM) for the occurrence of incident prediabetes, defined as impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT). At enrollment, participants underwent a 75-g OGTT, anthropometry, measurement of fasting lipids, insulin, and body fat (DEXA), and completed the Food Habits Questionnaire (FHQ), and Modifiable Activity Questionnaire (MAQ). We assessed the relationship between FHQ and MAQ scores and adiposity, cardiometabolic measures, and incident dysglycemia.
Results
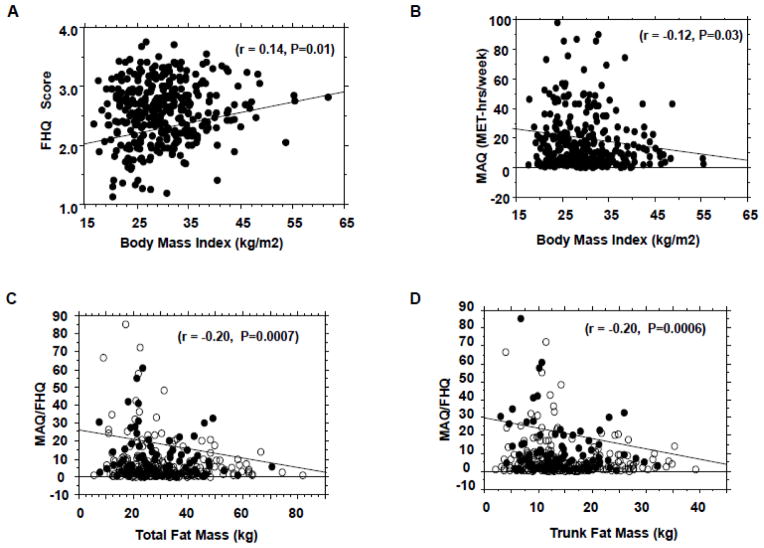
Among our cohort of 338 subjects (188 black, 150 white; mean age {± SD} 45.2 ± 10.2 y, BMI 30.3 ± 7.2 kg/m2), FHQ and MAQ scores were individually correlated with BMI (r= 0.14, −0.12; P=0.01, 0.03) and waist circumference (r= 0.19, −0.11; P=0.004, 0.05). Diet-adjusted leisure activity (MAQ/FHQ) was significantly correlated with total body fat (r= −0.20, P=0.0007), trunk fat (r= −0.20, P=0.0006), and serum triglycerides (r= −0.17, P=0.003) and HDL cholesterol (r= 0.11, P=0.04) levels. During 5.5yrs of follow-up, 111 subjects (Progressors) developed prediabetes (n=101) or diabetes (n=10) and 227 remained normoglycemic (Non-progressors). Age, BMI, MAQ and MAQ/FHQ values were significant predictors of incident prediabetes/diabetes. Progressors reported similar dietary habits (FHQ score 2.57±0.49 vs. 2.57±0.53) but 30% lower physical activity (MAQ score 15.2±20.5 vs. 22.3±30.5 MET-hr/wk, P=0.015) compared with non-progressors.
Conclusions
Among African-American and Caucasian offspring of parents with T2DM, self-reported dietary and exercise habits correlated with measures of adiposity and dyslipidemia; however, physical activity, but not dietary recall, significantly predicted incident dysglycemia during 5.5 years of follow-up.
Keywords: Prediabetes, Ethnicity, Prospective Study, Health behavior, Body Fat, Dyslipidemia
1. INTRODUCTION
Responses to standardized questionnaires constitute the primary method for assessing health behaviors in clinical research (1–3). Several types of questionnaires have been utilized for assessing dietary and exercise practices, and some have formed the basis for national recommendations (1,2, 4–6). Self-reported measures of health behavior have the advantages of being non-invasive and relatively inexpensive to administer. In addition, many questionnaires can be completed on-line, via the telephone, or by mail. Despite these advantages, self-reported questionnaires have inherent limitations, including different types of biases (e.g., recall bias and social desirability bias) (7–10).
The current epidemics of obesity and diabetes have refocused attention on the importance of lifestyle intervention, especially dietary modification and physical activity (1–3, 11, 12). Due to the increasing importance of measuring health behaviors as part of lifestyle intervention programs, it is important to determine to what extent information derived from self-reported instruments reflects objective measures. The Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) Study (13–15), a longitudinal study of incident dysglycemia among initially normoglycemic offspring of parents with type 2 diabetes (T2DM), affords a unique opportunity to assess the relationship between dietary and physical activity habits and metabolic endpoints in a high-risk population.
Here we report the findings of our evaluation of responses to the Food Habits Questionnaire (FHQ) (16) and the Modifiable Activity Questionnaire (MAQ) (17) against objective cardiometabolic measures, including body size, fat mass, lipid profile, energy expenditure, insulin sensitivity, and beta-cell function. We also determined whether FHQ and MAQ scores were significant predictors of incident prediabetes and T2DM during 5.5 years of follow-up.
2. SUBJECTS AND METHODS
2.1 Study Subjects
The study subjects were participants in the POP-ABC study (13–15). Healthy adults, aged 18–65 years, who are the offspring of at least one parent with T2DM were eligible for enrollment in the POP-ABC study. Other eligibility requirements included having normal fasting plasma glucose and/or normal glucose tolerance, and lack of exposure to medications that alter blood glucose, insulin sensitivity, insulin secretion, or body weight (13–15, 18). Race/ethnicity was determined by self-identification as a non-Hispanic white (European American) or a non-Hispanic black (African American) person, based on the 1990 U.S. Census questionnaire (19). All study procedures were completed at the University of Tennessee General Clinical Research Center (GCRC). The study protocol was approved by the institutional review board at the University of Tennessee Health Science Center, and all participants gave written informed consent prior the initiation of the study, which was conducted in accordance with the principles of the 1975 Declaration of Helsinki (as revised in 1983).
2.2. Procedures
2.2.1 Clinical Measurements
Participants made outpatient visits to the GCRC, after an overnight fast, for pre-specified assessments (13–15). The baseline assessments included physical examination, anthropometry, a standard 75-gram oral glucose tolerance test (OGTT), following a schedule as previously described (13–15). Blood pressure was recorded in the seated position, using an automated sphygmomanometer; the average of two readings was used for calculations. Body weight (in light outdoor clothing) was measured in duplicate on a calibrated balance beam scale. Standing height (without shoes) was determined in duplicate with a standard stadiometer. The body mass index (BMI) was calculated as weight in kilogram divided by the square of the height in meters. Waist circumference was determined to the nearest 0.1 cm at the midpoint between the highest point of the iliac crest and the lowest costal margin in the mid-axillary line, using a Gulick II tape measure (13–15). Total and trunk fat mass was measured using DEXA (Lunar-DPX-IQ Scanner, Lunar, Madison, WI). Resting energy expenditure (REE) (13) was determined by indirect calorimetry, using an automated ventilated hood system (Deltatrac II, SensorMedics, Yorba Linda, CA). In addition, the participants completed the FHQ (16) and MAQ (17).
2.2.2 Food Habits Questionnaire
Information on dietary habits was obtained using a validated FHQ (16). The FHQ contains 20 questions pertaining to the subjects’ dietary habits over the past month. The FHQ questions assess dietary habits in five subdomains, four of which focus on reduction of fat intake: 1) modifying meats to make them lower in fat, 2) avoiding fat as a seasoning, 3) substituting high fat foods with manufactured lower fat foods, 4) augmenting diet with fruits or vegetables, and 5) replacing high fat foods with low fat foods other than fruits or vegetables (16). The questions were grouped into major categories, and responses were analyzed on a scale from 1 to 4 (1=“usually or always” and 4=“rarely or never”) (16). A “not applicable” option was available if the subject did not consume the food (e.g. a vegetarian answering the question, “How often do you trim all visible fat when eating red meat?”). Lower numerical scores indicate a healthier diet compared to higher scores. Final scores for the FHQ were obtained by calculating the average of all complete responses, as is conventional for this type of survey (20).
2.2.3 Assessment of Physical Activity
We assessed physical activity using the MAQ (24). The MAQ captures leisure and occupational physical activity levels during the past year (17). For the leisure section of the MAQ, subjects were given a list of activities and asked to indicate which activities they engaged in, how many months they participated in the activity, the average number of times per month, and the average duration for each time. Each activity has an associated metabolic equivalent (MET) indicating the relative intensity compared to resting energy expenditure. MET values were used according to the moderate value listed for each activity within the NHANES Codebook and Compendium of Physical Activities (21, 22). The values were converted to the standard unit of MET-hr.wk−1 according to the equation (17):
For the occupational activity section of the MAQ, participants were asked to indicate the average hours of moderate or hard activity that they performed at work as well as the time spent walking or biking during their commute (17). Leisure and occupational activity can be summed to get a total amount of physical activity. However, due to the low percentage (<5%) of subjects who reported moderate or high occupational activity levels, the present report is limited to analysis of leisure-time physical activity.
2.2.4 Biochemical Measurements
Plasma glucose was measured with a glucose oxidase method (Yellow Spring Instruments Co., Inc., Yellow Spring, OH). Plasma insulin level was measured immunochemically in our Endocrine Research Laboratory, using commercial ELISA kits, and fasting plasma lipid profiles (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides) were measured in a contract clinical laboratory. The homeostasis model assessment of insulin resistance (HOMA-IR) and of beta-cell function (HOMA-B) were derived from fasting glucose and insulin values (23).
2.2.5 Statistical Analysis
Results are reported as means ± SD. Differences in baseline characteristics between comparison groups (Black vs. White subjects; Progressors vs. Non-progressors) were assessed using unpaired t-tests and ANOVA for continuous variables and chi squared test for categorical variables. The relationship between FHQ, MAQ, and MAQ/FHQ values and adiposity measures and metabolic variables was assessed using Pearson’s correlation coefficients. Higher order quadratic regression analyses were performed to test for curvilinear relationships among FHQ, MAQ, MAQ/FHQ and independent variables. These analyses indicated that the results were not significantly different from the linear model for the data presented in Figures 1 and 2. Univariate and multivariate logistic regression models were used to analyze FHQ, MAQ, and MAQ/FHQ scores as predictors of glycemic progression. All statistical analyses were performed with the use of SAS statistical software, version 9.3 (SAS Institute Inc., Cary, NC). P < 0.05 was accepted as statistically significant.
Figure 1.
Correlation of food habits questionnaire (FHQ) scores (A) and modifiable activity questionnaire (MAQ) scores (B) with body mass index; correlation of diet-adjusted physical activity (MAQ/FHQ) scores with total body fat (C) and trunk fat (D) mass.
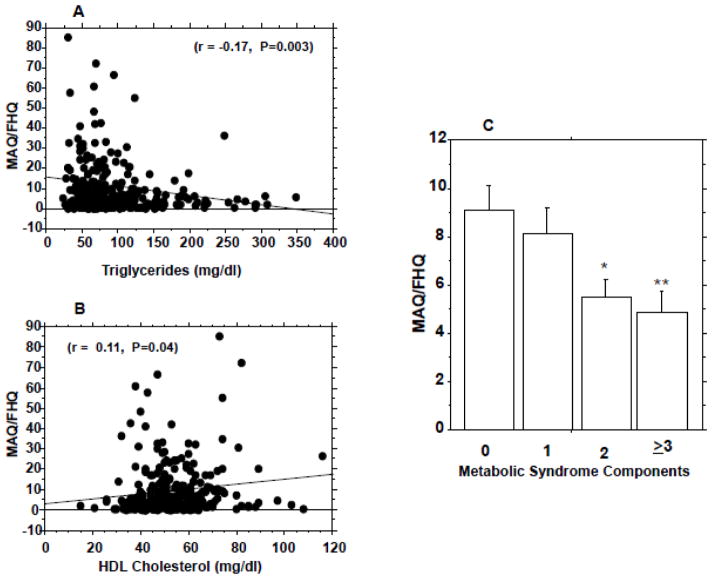
Figure 2.
Diet-adjusted physical activity (MAQ/FHQ) scores in relation to serum triglycerides (A), HDL cholesterol levels (B), and metabolic syndrome components (C). * P=0.005 vs. subjects with no components and 0.04 vs. group with 1 component. **P=0.005 vs. subjects with no components and 0.03 vs. group with 1 component. ANOVA P=0.0026 for interaction between MAQ/FHQ and metabolic syndrome components.
3. RESULTS
A total of 338 subjects (188 African-American, 150 European-American) who completed the FHQ and MAQ forms were included in this report. This group represents 98.5% of the total number of POP-ABC study participants with evaluable data (15). Table 1 shows the baseline characteristics of the study participants. Statistically significant racial differences were noted for age (p=0.0003), BMI (p=0.0002), fasting plasma glucose (p=0.017), HbA1c (P=0.001), and triglycerides (P<0.0001). The waist circumference was higher in African-American women than white women, but did not differ by race among men (Table 1). The mean FHQ score was ~8% higher in black participants than white participants (2.67 ± 0.50 vs. 2.45 ± 0.50, P<0.0001); the racial difference in FHQ scores was evident in men (P=0.002) and women (P=0.0007). Values for physical activity (MAQ scores) did not differ significantly by race (Table 1).
Table 1.
Baseline Characteristics of Study Participants by Ethnicity (± SD)
| Variables | Black Subjects | White Subjects | P Value |
|---|---|---|---|
| Number (F/M) | 188 (138/50) | 150 (104/46) | 0.32 |
| Age (y) | 43.3 ± 10.0 | 47.1 ± 10.3 | 0.0008 |
| BMI (kg/m2) | 31.3 ± 7.5 | 28.7 ± 6.6 | 0.0005 |
| Waist circumference (cm) | 95.9 ± 16.0 | 92.9 ± 15.0 | 0.12 |
| Men | 98.1 ± 18.0 | 98.9 ± 10.7 | 0.80 |
| Women | 94.8 ± 15.3 | 90.0 ± 15.9 | 0.02 |
| Total fat mass (kg) | 31.8 ± 13.7 | 29.3 ± 13.3 | 0.09 |
| Men | 22.6 ± 10.1 | 24.2 ± 8.10 | 0.41 |
| Women | 35.2 ± 13.3 | 31.6 ± 14.5 | 0.05 |
| FPG (mg/dL) | 90.3 ± 7.37 | 92.2 ± 7.53 | 0.015 |
| 2-hrPG (mg/dL) | 125 ± 29.2 | 125 ± 23.6 | 0.97 |
| HbA1c (%) | 5.66 ± 0.47 | 5.44 ± 0.38 | <0.001 |
| Total Cholesterol (mg/dL) | 176 ± 33.5 | 180 ± 31.3 | 0.21 |
| LDL Cholesterol (mg/dL) | 106 ± 29.8 | 105 ± 27.6 | 0.74 |
| HDL cholesterol (mg/dL) | 53.3 ± 14.3 | 51.8 ± 12.9 | 0.31 |
| Men | 48.1 ± 15.2 | 43.9 ± 8.00 | 0.08 |
| Female | 55.1 ± 13.5 | 55.3 ± 13.2 | 0.67 |
| Triglycerides (mg/dL) | 79.7 ± 39.5 | 114 ± 64.1 | <0.0001 |
| FHQ Score | 2.67 ± 0.50 | 2.45 ± 0.50 | <0.0001 |
| MAQ (MET-hr/wk) | 19.1 ± 30.2 | 20.8 ± 24.4 | 0.59 |
BMI, body mass index; FPG, fasting plasma glucose; 2hPG, 2-hour plasma glucose. To convert the values for glucose to millimoles per liter, multiply by 0.0555. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
3.1 Adiposity and Cardiometabolic Measures
FHQ and MAQ scores were both significantly correlated with BMI, waist circumference, trunk fat mass, and serum triglyceride level at enrollment (Fig. 1A, B and Table 2). There was discordance in the correlation of FHQ and MAQ scores with systolic blood pressure, total fat mass, HDL cholesterol, HOMA-IR, HOMA-B and resting energy expenditure (Table 2). We examined the correlations with FHQ and MAQ by ethnicity for the variables that displayed significant ethnic differences at baseline (BMI, waist, triglycerides, fasting glucose, HbA1c and fat mass). The findings were consistent with the correlations observed in the combined cohort (supplementary Table 2B). Neither FHQ nor MAQ scores correlated significantly with diastolic blood pressure, total cholesterol, LDL cholesterol or blood glucose values at enrollment (Table 2).
Table 2.
Correlation of Food Habits and Physical Activity Scores with Cardiometabolic Measures
| Variable | FHQ Score
|
MAQ Score
|
||
|---|---|---|---|---|
| r | P Value | r | P Value | |
| BMI (kg/m2) | 0.14 | 0.01 | −0.12 | 0.03 |
| Waist Circumference (cm) | 0.19 | 0.004 | −0.11 | 0.05 |
| Systolic blood pressure (mmHg) | 0.01 | 0.84 | −0.12 | 0.03 |
| Diastolic blood pressure (mmHg) | 0.06 | 0.24 | −0.06 | 0.29 |
| Total Fat Mass (kg) | 0.08 | 0.15 | −0.19 | 0.001 |
| Trunk Fat Mass (kg) | 0.11 | 0.06 | −0.19 | 0.001 |
| Triglycerides (mg/dL) | 0.11 | 0.03 | −0.15 | 0.007 |
| Total Cholesterol (mg/dL) | −0.006 | 0.90 | −0.08 | 0.14 |
| HDL cholesterol (mg/dL) | −0.18 | 0.0013 | 0.05 | 0.34 |
| LDL cholesterol (mg/dL) | 0.03 | 0.55 | −0.09 | 0.10 |
| Fasting plasma glucose (mg/dl) | 0.03 | 0.64 | −0.03 | 0.59 |
| 2-hr plasma glucose (mg/dl) | 0.08 | 0.12 | −0.10 | 0.07 |
| HbA1c (%) | 0.02 | 0.71 | −0.08 | 0.15 |
| HOMA-IR | 0.13 | 0.018 | −0.02 | 0.69 |
| HOMA-B | 0.11 | 0.05 | 0.02 | 0.70 |
| REE (kcal/kg FFM) | 0.19 | 0.0015 | 0.06 | 0.59 |
Plus–minus values are means ±SD. FHQ, Food Habits Questionnaire; MAQ, Modifiable Activity Questionnaire; HOMA-IR and HOMA-B denote homeostasis model of insulin resistance and beta-cell function, respectively. REE, resting energy expenditure; FFM, fat-free mass.
3.2 Diet-Adjusted Physical Activity Measure
In our study cohort, MAQ and FHQ values were weakly but significantly correlated (r=−0.13, P=0.02). To account for the opposite metabolic effects of high physical activity (high MAQ score) and poor dietary habits (high FHQ score), we derived a diet-adjusted activity measure as the MAQ score divided by the FHQ score (MAQ/FHQ), expressed as MET-hr/wk, for each subject. Compared to either FHQ or MAQ scores, the MAQ/FHQ ratio exhibited stronger correlation with total body fat (r=0.2, P=0.0007, Fig. 1C), trunk fat mass (r=0.2, P=0.0006, Fig. 1D), serum triglycerides (r=0.17, P=0.003, Fig. 2A), HDL cholesterol (r= 0.11, P=0.04, Fig. 2B), systolic blood pressure (r=0.14, P=0.015), and 2-hr plasma glucose levels during OGTT (r=0.11, P=0.05). Higher order (polynomial) regression analyses confirmed the relationships among FHQ, MAQ, FHQ/MAQ and the independent variables observed from the linear models.
3.3. Metabolic Syndrome
We further determined the relationship between MAQ/FHQ ratio and aggregation of metabolic syndrome components among our study cohort. Metabolic syndrome components were identified using the NCEP cut-offs for waist circumference, blood pressure, HDL cholesterol and triglyceride levels (24). As shown in Fig. 2C, accumulation of metabolic syndrome components was associated with markedly lower diet-adjusted physical activity. Participants without a single component of the metabolic syndrome had a mean FHQ-adjusted MAQ score of 11.6±15.1 MET-hr/wk; the corresponding scores were 8.93±11.6 MET-hr/wk among subjects with one metabolic syndrome component, 6.25 ± 10.1 MET-hr/wk among subjects with two components, and 5.05 ± 6.20 MET-hr/wk among subjects with three or more metabolic syndrome components (ANOVA P=0.0026) (Fig. 2C).
3.4 Prediction of Glycemic Progression
During 5.5yrs of follow-up, 111 subjects (Progressors) developed prediabetes (n=101) or diabetes (n=10) and 227 remained normoglycemic (Non-progressors). Among progressors and non-progressors, black participants had significantly higher BMI and FHQ scores compared to white participants (Table 3). For the combined cohort, the mean FHQ score was similar for progressors and non-progressors, but physical activity (MAQ) score was 30% lower among progressors compared with non-progressors (15.2±20.5 MET-hr/wk vs. 22.3±30.5 MET-hr/wk, P=0.015) (Table 3). The MAQ/FHQ value also was significantly lower in progressors than non-progressors (6.47± 8.69 vs. 9.42 ± 13.2 Met-hr/wk, P=0.018). These findings were numerically consistent by race/ethnicity; however, a greater variability in MAQ scores among black subjects weakened the statistical significance in that subgroup (Table 3). Overall, progression to prediabetes/diabetes was associated with higher age and BMI, and lower MAQ and MAQ/FHQ values in the combined cohort (Table 3).
Table 3.
Food Habits and Physical Activity Scores in Persons who Developed Prediabetes (Progressors) Compared to Those who Maintained Normoglycemia (Non-Progressors)
| Combined Cohort | Black Subjects | White Subjects | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | Progressors | Non-progressors | P | Progressors | Non-progressors | P | Progressors | Non-progressors | P |
| Number | 111 | 227 | 58 | 130 | 53 | 97 | |||
| Age (yr) | 47.3±8.9 | 43.8±10.7 | 0.0018 | 45.9±8.2 | 42.0±10.5 | 0.0059 | 48.9±9.5 | 46.1±10.6 | 0.11 |
| BMI (kg/m2) | 31.4±6.9 | 29.6±7.4 | 0.0035 | 32.8±7.2 | 31.1±7.6 | 0.12 | 30.0±6.2 | 28.0±6.8 | 0.07 |
| FHQ | 2.57±0.49 | 2.57±0.53 | 0.98 | 2.68±0.46 | 2.66±0.52 | 0.80 | 2.45±0.49 | 2.45±0.51 | 0.99 |
| MAQ | 15.2±20.5 | 22.3±30.5 | 0.015 | 14.6±25.1 | 20.0±28.1 | 0.13 | 15.5±14.5 | 23.6±28.2 | 0.03 |
| MAQ/FHQ | 6.47±8.69 | 9.42±13.2 | 0.018 | 5.96±9.96 | 8.61±13.4 | 0.14 | 6.99±7.20 | 10.4±13.1 | 0.04 |
Plus–minus values are means ±SD. P values refer to comparisons between Progressors and Non-progressors for each variable. FHQ, Food Habits Questionnaire score; MAQ, Modifiable Activity Questionnaire score (MET-hr/wk).
In a step-wise logistic regression model, MAQ/FHQ values (odds ratio 0.97 [95% confidence interval 0.95–0.99], P=0.031) but not FHQ scores significantly predicted glycemic progression to prediabetes/diabetes (Table 4).
Table 4.
Logistic Regression Model for Progression
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| FHQ | 1.0 | 0.64–1.57 | 0.98 |
| MAQ/FHQ | 0.97 | 0.95–0.99 | 0.031 |
CI, confidence interval; FHQ, Food Habits Questionnaire score; MAQ, Modifiable Activity Questionnaire score.
4. DISCUSSION
In the present study, higher FHQ scores (indicative of less healthy dietary habits) and lower MAQ scores (indicative of sedentary lifestyle) were weakly but significantly correlated with higher measures of adiposity and body size as well as dyslipidemia. Adjusting the physical activity scores for habitual dietary practices (MAQ/FHQ) increased the strength of associations with measures of adiposity and cardiometabolic risk. These findings demonstrate that self-reported dietary and physical activity behaviors may be more informative when analyzed together rather than separately. The lack of association between FHQ or MAQ scores with measures of glycemia among our study subjects is not surprising, given that the POP-ABC study participants were required to have normal fasting glucose and/or normal glucose tolerance at enrollment (13–15, 18). The consequent narrow glycemic range probably decreased the chances of detecting a relationship between FHQ and MAQ scores and baseline blood glucose levels. Other workers have found significant associations between MAQ scores and fasting glucose and insulin levels in healthy subjects enrolled with a broader glycemic range (25).
The present study suffers from several weaknesses inherent in questionnaire-based studies that rely on self-reported measures. Such studies are of questionable accuracy and are also prone to several biases, including the well-known social desirability response bias (7–10, 26–29). It is remarkable that, despite the numerous limitations of self-reported data, the subjective responses by our study subjects correlated significantly and congruently with several objective biological measures of cardiometabolic health. Although self-reported behavioral data are often described as “soft” data, congruent associations with objective measures have frequently been reported (30–36). For example, alcohol abuse screening questionnaire responses are associated with biological measures of alcohol withdrawal symptoms (30), self-reported smoking status is highly correlated with circulating cotinine levels (31), and dietary and physical activity habits have been shown to predict obesity and cardiometabolic risks (32–37).
The strengths of the present report include the unique study population of high-risk African-American and European-American subjects with parental diabetes, and the conjoint consideration of dietary and physical activity measures in our key analyses. Also, associations between lifestyle habits and surrogate adiposity measures (BMI and waist circumference) were corroborated with direct measurement of total body fat and trunk fat mass in the present study. The demonstrated congruent correlations argue against major systematic misrepresentations by our study participants in their responses to the food habits and physical activity questionnaires. Our findings are in accord with previous reports of significant associations between self-reported dietary and exercise data and objective metabolic measurements (32–37).
In addition to the significant correlations with measures of adiposity and dyslipidemia, leisure-time physical activity in the present study predicted the risk of incident prediabetes in our prospective cohort. Notably, participants who experienced glycemic progression during 5.5 years of follow-up reported significantly lower physical activity compared with non-progressors. Specifically, we found that diet-adjusted leisure-time physical activity (MAQ/FHQ) values were 30% lower in progressors than non-progressors. The latter finding extends previous observations of a protective effect of self-reported physical activity on diabetes risk (1, 32) by demonstrating a similar benefit at the more proximal stage of prediabetes. Our findings were numerically consistent by race/ethnicity, although marked variability in self-reported MAQ scores among black subjects reduced statistical significance in that subgroup. In the Diabetes Prevention Program, African Americans and Caucasians responded similarly to the effect of lifestyle intervention in preventing progression from prediabetes to T2DM (38). Similarly, the primary outcome data from our POP-ABC study indicated no racial/ethnic differences in incident prediabetes among initially normoglycemic offspring of parents with T2DM (15).
Our findings have important translational messages, by underscoring the importance of comprehensive lifestyle modification in the promotion of health and reduction of cardiometabolic risk. Notably, FHQ scores specifically reflect dietary habits aimed at decreasing dietary fat consumption and increasing intake of fruits and vegetables, and the MAQ scores reflect leisure-time physical activity. Thus, approaches that successfully shift dietary preferences to the healthier spectrum of the FHQ domains, while simultaneously increasing leisure-time physical activity, are likely to be more effective than fragmented approaches (38–40). Indeed, low physical activity has been identified as a major characteristic of prevalent prediabetes among individuals with parental history of T2DM (41). However, further studies are needed to assess the impact of occupational physical activity and dietary approaches not captured by the FHQ on incident prediabetes.
Supplementary Material
Acknowledgments
We are indebted to the participants who volunteered for this study and to the research nurses and staff of the University of Tennessee Clinical Research Center.
6. FUNDING
The POP-ABC study was supported by Grants 2R01 DK067269, R01 DK067269-04S1 and MO1 RR00211 from the National Institutes of Health, Grant 7-07-MN-13 from the American Diabetes Association, and State of Tennessee Clinical Research Center fund (UTHSC CRC E070166010).
6.1 Role of Funding Sources: The funding sources had no role in the design and execution of the POP-ABC study, the analysis and presentation of the data obtained from the study, or the decision to publish the results.
List of Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- DEXA
dual-energy x-ray absorptiometry
- FFM
fat-free mass
- FHQ
food habits questionnaire
- FPG
fasting plasma glucose
- GCRC
General Clinical Research Center
- HbA1c
hemoglobin A1c
- HOMA-B
homeostasis model assessment of beta-cell function
- HOMA-IR
homeostasis model assessment of insulin resistance
- 2-hrPG
2-hour plasma glucose
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- MAQ
modifiable activity questionnaire
- MAQ/FHQ
diet-adjusted physical activity
- MET
metabolic equivalent of task
- NCEP
National Cholesterol Education Program
- OGTT
oral glucose tolerance test
- POP-ABC
Pathobiology of Prediabetes in A Biracial Cohort
- REE
resting energy expenditure
- T2DM
type 2 diabetes
Footnotes
Conflicts of Interest: The authors have no relevant conflict of interest to disclose with regard to the content of this manuscript.
7. DISCLOSURE STATEMENT
The authors have no relevant conflict of interest to disclose with regard to the content of this manuscript.
8. AUTHORS’ CONTRIBUTIONS
S.D.-J., as the principal investigator and developed the study concept and design, and wrote the manuscript; A.B. collected data, reviewed and revised the manuscript; E.A.O.A. collected data, reviewed and revised the manuscript; S.E. collected data, reviewed and reviewed the manuscript; I.O. collected data, reviewed and revised the manuscript; S.E. collected data, conducted statistical analysis and reviewed the manuscript; A.K.G. reviewed and revised the manuscript; J.W. performed statistical analyses; C.E. collected data and reviewed and revised the manuscript. S.D-J. had primary responsibility for final content. All authors read and approved the final manuscript.
8. 1 POP-ABC Research Group
Current: Samuel Dagogo-Jack, MD (principal investigator); Ann Ammons; Fatoumatta Ceesay, BS; Sotonte Ebenibo, MBBS, MPH; Ashley K. Gilles, MS, RD; Ibiye Owei, MBBS, MPH; Nkiru Umekwe, MBBS.
Past members: Chimaroke Edeoga, MB.BS, MPH (2007–2013); Ebenezer Nyenwe, MBBS (2007–2013); Emmanuel Chapp-Jumbo, MBBS (2009–2011); Ruben Cuervo, MD (2006–2007); Nonso Egbuonu, MBBS (2007–2010); Nicoleta Ionica, MD (2007–2008); Dorota Malinowski, MD (2007–2008); and Gabrielle Songe, BA (2008–2010).
Consultant: Steven Haffner, MD; Data and Safety Officer: Murray Heimberg, MD, PhD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kriska AM, Edelstein SL, Hamman RF, Otto A, Bray GA, Mayer-Davis EJ, Wing RR, Horton ES, Haffner SM, Regensteiner JG. Physical activity in individuals at risk for diabetes: Diabetes Prevention Program. Med Sci Sports Exerc. 2006;38:826–32. doi: 10.1249/01.mss.0000218138.91812.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey F, Ussery-Hall A, Garcia D, McDonald G, Easton A, Kambon M, Balluz L, Garvin W, Vigeant J. Prevalence of selected risk behaviors and chronic diseases--Behavioral Risk Factor Surveillance System (BRFSS), 39 steps communities, United States, 2005. MMWR Surveill Summ. 2008;57:1–20. [PubMed] [Google Scholar]
- 4. [accessed 8 January 2015];Nutrition Resources for Health Professionals. Data and Statistics. http://www.cdc.gov/nutrition/professionals/data/index.html.
- 5.Centers for Disease Control and Prevention. [accessed 8 January 2015];What We Eat in America, NHANES 2003–2004. http://www.cdc.gov/nchs/nhanes/wweia.htm.
- 6.U.S. Department of Agriculture, U.S. Department of Health and Human Services. [accessed 8 January 2015];Dietary Guidelines for Americans. Dietary Guidelines for Americans. 2010 http://www.dietaryguidelines.gov.
- 7.Newell SA, Girgis A, Sanson-Fisher RW, Savolainen NJ. The accuracy of self-reported health behaviors and risk factors relating to cancer and cardiovascular disease in the general population: a critical review. Am J Prev Med. 1999;17:211–29. doi: 10.1016/s0749-3797(99)00069-0. [DOI] [PubMed] [Google Scholar]
- 8.Shields M, Gorber SC, Tremblay MS. Estimates of obesity based on self-report versus direct measures. Health Rep. 2008;19:61–76. [PubMed] [Google Scholar]
- 9.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110:1501–10. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. 1995;24:389–98. doi: 10.1093/ije/24.2.389. [DOI] [PubMed] [Google Scholar]
- 11.Kouki R, Schwab U, Lakka TA, Hassinen M, Savonen K, Komulainen P, Krachler B, Rauramaa R. Diet, fitness and metabolic syndrome - The DR’s EXTRA Study. Nutr Metab Cardiovasc Dis. 2012 Jul;22:553–60. doi: 10.1016/j.numecd.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Imes CC, Burke LE. The obesity epidemic: The United States as a cautionary tale for the rest of the world. Curr Epidemiol Rep. 2014;1:82–8. doi: 10.1007/s40471-014-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagogo-Jack S, Edeoga C, Nyenwe E, Chapp-Jumbo E, Wan J. Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC): design and methods. Ethn Dis. 2011;21:33–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Dagogo-Jack S, Edeoga E, Ebenibo S, Chapp-Jumbo E. Pathobiology of Prediabetes in a Bi-racial Cohort (POP-ABC) Study: Baseline characteristics of enrolled subjects. J Clin Endocrinol Metab. 2013;98:120–8. doi: 10.1210/jc.2012-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagogo-Jack S, Edeoga C, Ebenibo S, Nyenwe E, Wan J for the Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Research Group. Lack of racial disparity in incident prediabetes and glycemic progression among black and white offspring of parents with type 2 Diabetes: The Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Study. J Clin Endocrinol Metab. 2014;99:E1078–87. doi: 10.1210/jc.2014-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristal AR, Shattuck AL, Henry HJ. Patterns of dietary behavior associated with selecting diets low in fat: reliability and validity of a behavioral approach to dietary assessment. J Am Diet Assoc. 1990;90:214–20. [PubMed] [Google Scholar]
- 17.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 18.Ebenibo S, Edeoga E, Ammons A, Egbuonu N, Dagogo-Jack S. Recruitment strategies and yields for the Pathobiology of Prediabetes in a Biracial Cohort: A prospective natural history study of incident dysglycemia. BMC Med Res Methodol. 2013 May 10;13:64. doi: 10.1186/1471-2288-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bureau of the Census. Census of the Population, 1990. Washington, DC: U.S. Government Printing Office; 1990. [Google Scholar]
- 20.Watters JL, Satia JA. Psychosocial correlates of dietary fat intake in African-American adults: a cross-sectional study. Nutr J. 2009 Mar 25;8:15. doi: 10.1186/1475-2891-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Health and Nutrition Examination Survey. [accessed 8 January 2015];2005 – 2006 Data documentation, codebook, and frequencies. http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/PAQIAF_D.htm#Appendix_1_Physical_Activity_Codes.
- 22.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med Sci Sports Exerc. 2011;43:1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 23.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Kriska AM, Hanley AJG, Harris SB, Zinman B. Physical activity, physical fitness, and insulin and glucose concentrations in an isolated Native Canadian population experiencing rapid lifestyle change. Diabetes Care. 2001;24:1787–92. doi: 10.2337/diacare.24.10.1787. [DOI] [PubMed] [Google Scholar]
- 26.Worsley A, Baghurst KI, Leitch DR. Social desirability response bias and dietary inventory responses. Hum Nutr Appl Nutr. 1984;38:29–35. [PubMed] [Google Scholar]
- 27.Gillum RF, Sempos CT. Ethnic variation in validity of classification of overweight and obesity using self-reported weight and height in American women and men: the Third National Health and Nutrition Examination Survey. Nutr J. 2005;4:27. doi: 10.1186/1475-2891-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoeller DA, Thomas D, Archer E, Heymsfield SB, Blair SN, Goran MI, Hill JO, Atkinson RL, Corkey BE, Foreyt J, et al. Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr. 2013;97:1413–5. doi: 10.3945/ajcn.113.062125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40:515–9. doi: 10.1093/alcalc/agh189. [DOI] [PubMed] [Google Scholar]
- 31.Yeager DS, Krosnick JA. The validity of self-reported nicotine product use in the 2001–2008 National Health and Nutrition Examination Survey. Med Care. 2010;48:1128–32. doi: 10.1097/MLR.0b013e3181ef9948. [DOI] [PubMed] [Google Scholar]
- 32.Hu FB1, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 33.Berg CM, Lappas G, Strandhagen E, Wolk A, Torén K, Rosengren A, Aires N, Thelle DS, Lissner L. Food patterns and cardiovascular disease risk factors: the Swedish INTERGENE research program. Am J Clin Nutr. 2008;8:289–97. doi: 10.1093/ajcn/88.2.289. [DOI] [PubMed] [Google Scholar]
- 34.Stefler D, Pikhart H, Jankovic N, Kubinova R, Pajak A, Malyutina S, Simonova G, Feskens EJ, Peasey A, Bobak M. Healthy diet indicator and mortality in Eastern European populations: prospective evidence from the HAPIEE cohort. Eur J Clin Nutr. 2014;68:1346–52. doi: 10.1038/ejcn.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fung TT, Hu FB, Yu J, Chu NF, Spiegelman D, Tofler GH, Willett WC, Rimm EB. Leisure-time physical activity, television watching, and plasma biomarkers of obesity and cardiovascular disease risk. Am J Epidemiol. 2000;152:1171–8. doi: 10.1093/aje/152.12.1171. [DOI] [PubMed] [Google Scholar]
- 36.Altmaier E, Kastenmuller G, Romisch-Margl W, Thorand B, Weinberger KM, Illig T, Adamski J, Doring A, Suhre K. Questionnaire-based self-reported nutrition habits associate with serum metabolism as revealed by quantitative targeted metabolomics. Eur J Epidemiol. 2011;26:145–56. doi: 10.1007/s10654-010-9524-7. [DOI] [PubMed] [Google Scholar]
- 37.Boggs DA, Palmer JR, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Dietary patterns and 14-y weight gain in African American women. Am J Clin Nutr. 2011;94:86–94. doi: 10.3945/ajcn.111.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagogo-Jack S, Egbuonu N, Edeoga C. Principles and practice of nonpharmacological interventions to reduce cardiometabolic risk. Med Princ Pract. 2010;19:167–75. doi: 10.1159/000285280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kontogianni MD, Liatis S, Grammatikou S, Perrea D, Katsilambros N, Makrilakis K. Changes in dietary habits and their association with metabolic markers after a non-intensive, community-based lifestyle intervention to prevent type 2 diabetes, in Greece. The DEPLAN study. Diabetes Res Clin Pract. 2012;95:207–14. doi: 10.1016/j.diabres.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Cha E1, Umpierrez G, Kim KH, Bello MK, Dunbar SB. Characteristics of American young adults with increased risk for type 2 diabetes: a pilot study. Diabetes Educ. 2013;39:454–63. doi: 10.1177/0145721713486199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.