Abstract
The ubiquitin-proteasome pathway (UPP) plays an important role in regulating gene expression. Retinal pigment epithelial cells (RPE) are a major source of ocular inflammatory cytokines. In this work we determined the relationship between impairment of the UPP and expression of inflammation-related factors. The UPP could be impaired by oxidative stress or chemical inhibition. Impairment of the UPP in RPE increased the expression of several inflammatory cytokines, such as IL-6 and IL-8. However, the expression of monocyte chemoattractant protein-1 (MCP-1) and complement factor H (CFH) and was reduced upon impairment of the UPP. These data suggest that impairment of the UPP in RPE may be one of the causes of retinal inflammation and abnormal functions of monocyte and the complement system during the pathogenesis of age-related macular degeneration.
Keywords: Age-related macular degeneration; Inflammation; Ubiquitin; Proteasome; Retinal pigment epithelial cells; IL-6, IL-8, MCP-1; Complement factor H
31.1 Introduction
Age-related macular degeneration (AMD) is a multifactorial disease and a leading cause of blindness in industrialized countries. The factors that contribute to the onset and progression of AMD include aging, genetic background, cigarette smoking, and dietary patterns [1–7]. It has been proposed that oxidative damage to the retina and retinal pigment epithelial cells (RPE) is a major trigger for the onset and progress of AMD [5, 8, 9]. However, the mechanism for the relationship between oxidative damage and AMD pathogenesis remains to be elusive. Recent studies indicate that innate immunity and inflammation are related to AMD pathogenesis [4, 10]. The evidence for the involvement of innate immunity and inflammation in AMD pathogenesis includes accumulation of immunoglobulin and complement components in drusen [11–13], the association between genetic variants of complement factor H, factor B, C2, C3, factor I, and risk for AMD [14–22], and elevated serum CRP levels in AMD patients [23–25]. Emerging evidence indicates oxidative stress and inflammation is closely related. Oxidative stress may trigger inflammatory response and inflammation also exacerbates oxidative damage [26, 27]. Mechanistic investigation into the causal relationship between oxidative damage and inflammatory response will help to elucidate how oxidative damage triggers the onset and progression of AMD. This information is important for development of novel strategies for prevention or treatment of AMD.
The ubiquitin-proteasome pathway (UPP) is the major non-lysosomal proteolytic pathway within cells [28–31]. Proteins destined for degradation are first conjugated with a polyubiquitin chain by the sequential action of three classes of enzymes: E1, E2, and E3. The ubiquitin-protein conjugates are then recognized and degraded by a large protease complex called the proteasome [29, 32]. The UPP has been involved in a myriad of cellular processes [30, 31, 33], including regulation of immune response and inflammation [34, 35]. Dysfunction of the UPP has been implicated in the pathogenesis of many degenerative diseases such as Alzheimer’s disease [36], Parkinson’s disease [37], diabetic retinopathy [38], and cataract [39, 40]. A fully functional UPP is required for cells to cope with various stresses, including oxidative stress [41]. However, an extensive oxidative insult also impairs the function of critical components of the UPP [42–47]. Oxidative inactivation of the proteasome not only results in accumulation of damaged proteins [39, 40, 47], but also impairs cell signaling process [48–50]. Our previous work indicates that the proteasome is more susceptible to oxidative inactivation than other components of the UPP. Sustained physiologically relevant levels of oxidative reduce proteasome activity, but not reduce ubiquitin conjugating activities [47, 48]. Oxidative inactivation of the proteasome increases production of IL-8 in cultured RPE [50], suggesting that oxidative inactivation of the proteasome may be a mechanistic link between oxidative stress and inflammation. Since RPE is a major ocular source of pro-inflammatory mediators and a primary target of photooxidative insult, oxidative impairment of the UPP in RPE may contribute to ocular inflammation and AMD-related lesions. To further explore the relationship between proteasome inactivation and retinal inflammation, we systematically investigated the effect of impairment of the UPP and expression of several inflammation- related factors in cultured RPE. The data indicate that impairment of the UPP by photooxidation or chemical inhibition of the proteasome resulted in an increase in IL-6 and IL-8 expression, and suppressed the expression of complement factor H and MCP-1 by RPE cells, supporting the hypothesis that impairment of the UPP is a mechanistic link between oxidative stress and inflammation and the possible mechanism by which oxidative damage triggers the pathogenesis of AMD.
31.2 Materials and Methods
31.2.1 Materials
Cell culture supplies were obtained from Invitrogen (Carlsbad, CA, USA). The DuoSet ELISA kits for human MCP-1, human IL-6 and IL-8, and MG132 were obtained from R&D Systems (Minneapolis, MN, USA). Mouse monoclonal antibody (capture antibody) to human CFH was purchased from Abcam (Cambridge MA, USA) and goat-polyclonal antibody (detecting antibody) to human CFH was purchased from EMD Chemicals (Gibbstown, NJ, USA). All other reagents were obtained from Sigma Aldrich (St. Louis, MO, USA).
31.2.2 Exposure to A2E and Blue Light
ARPE-19 cells were grown to confluence and then cultured in DMEM with 10 % heat-inactivated fetal calf serum and 0.1 mM nonessential amino acid solution with or without 10 µM A2E for 14 days. The medium with fresh A2E was changed twice a week. After washing twice with PBS, cell cultures were transferred to PBS with calcium, magnesium, and glucose and were exposed to 430 nm light delivered from a tungsten halogen source (430 nm ± 20; 15 min; 2.62 mW/cm2). The cells were then incubated for an additional 6 h in DMEM with 1 % FBS. After collection of the media, cells were washed twice with cold PBS and then the dishes were placed on ice and the cells were harvested with a cell scraper. Cells that had neither accumulated A2E nor been exposed to blue light were used as controls. Cells that had accumulated A2E alone or exposed to blue light along were also tested. The control cells were treated in the same manner as the cells that were exposed to A2E and blue light. Levels of IL6 and IL-8, MCP-1, and CFH in the medium were determined by ELISA. The latter were performed according to the manufacturer’s instructions. Total RNA was also isolated from the cells for the quantitation of mRNA levels of IL-6, IL-8, MCP-1, and CFH. To determine the effects of proteasome inhibition on expression and secretion, confluent RPE were treated with 10 µM MG132 for 8 h. Levels of mRNA levels of IL-6, IL-8, MCP-1, and CFH in the cells were determined by RT-PCR and protein levels of these factors in the medium were determined by ELISA as described previously.
31.2.3 Proteasome Activity Assay
ARPE-19 cells were lysed in 25 mM Tris-HCl buffer, pH 7.6. The chymotrypsin-like activity of the proteasome was determined using the fluorogenic peptide succinyl- Leu-Leu-Val-Tyr-amidomethylcoumarin (LLVY-AMC) as a substrate, trypsin-like activity of the proteasome was determined using N-t-butyloxycarbonyl-Leu-Ser-Thr-Arg-amidomethylcoumarin (LSTR-AMC) as a substrate [51]. The mixture, containing 20 µl of cell supernatant in 25 mM Tris-HCl, pH 7.6, was incubated at 25 °C with respective peptide substrates (25 µM) in a buffer containing 50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 5 mM EDTA, 1 mM EGTA, 3 mM NaN3, and 0.04 % 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS). The final volume of the assay was 200 µl. Rates of reactions were measured in a temperature-controlled microplate fluorometric reader. Excitation/emission wavelengths were 380/440 nm. Proteasome activity was defined as the portion of peptidase activity in the cell extracts that was inhibited by 20 µM MG132, a potent proteasome inhibitor.
31.3 Results
31.3.1 Photooxidation Alters the Expression and Secretion of Inflammation-Related Factors
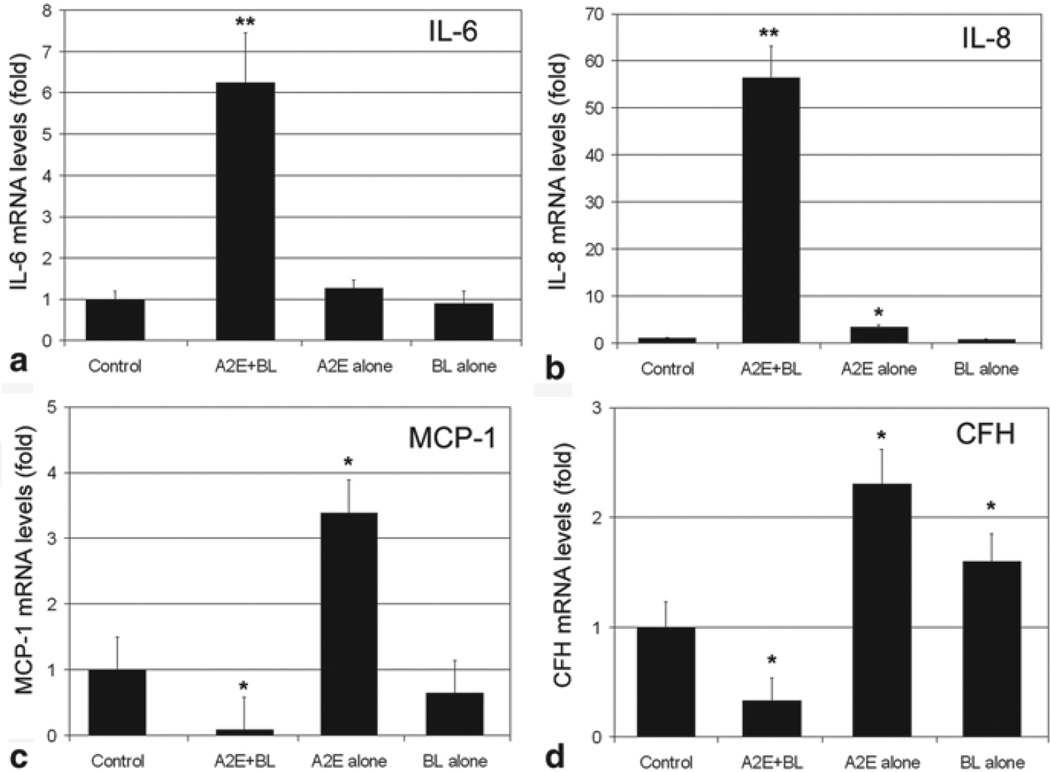
To investigate the relationship between oxidative stress and inflammatory response in cultured RPE, we used the physiologically relevant A2E-mediated photooxidation protocol [26, 47, 52, 53] to investigate the effects of oxidative stress on the expression of inflammation-related genes. We chose Il-6, IL-8, MCP-1, and CFH as targets in this study. Exposure of A2E-containing RPE cells to blue light dramatically increased the mRNA levels of IL-6 and IL-8 (Fig. 31.1a, b). The increase in mRNA levels for IL-6 was ~ 6-fold and the increase in mRNA levels for IL-8 was ~ 55-fold. In contrast, exposure of A2E-containing RPE to blue light reduced the mRNA levels for MCP-1 and CFH by ~ 90 % and ~ 70 %, respectively (Fig. 31.1c, d). Interestingly, accumulation of A2E alone increased mRNA levels of IL-8, MCP-1, and CFH by 2 to 3-fold. Exposure to blue light alone increased mRNA levels for CFH by ~ 2 fold, but did not alter the expression of other genes. These data indicate photooxidation differentially alters the expression of inflammation-related genes. The data also suggest that A2E accumulation may regulate the expression of IL-8, MCP-1, and CFH independent of photooxidation.
Fig. 31.1.
A2E-mediated photooxidation alters the expression of inflammation-related genes. Confluent cultured ARPE-19 cells were loaded with 10 µM A2E for 14 days. The cells were then transferred to PBS and exposed to blue light for 15 min. Cells that accumulated A2E alone, exposed to blue light alone, or neither accumulated A2E nor exposed to blue light were used as control. The cells were then cultured in fresh medium containing 1 % FBS for 6 h. Total RNA were extracted from these cells and levels of mRNA for IL-6, IL-8, MCP-1, and CFH were determined by RT-PCR. GADPH was used as a reference for relative quantitation. The relative levels of mRNA for each gene in control cells were arbitrarily designated as 1 and relative levels of mRNA for these genes in treated cells were expressed as fold of that in the control cells. The data are mean ± SD of the results from 6 samples in each group. *indicates p < 0.05 and ** indicates p < 0.001 as compared the control cells that were neither treated with A2E nor blue light
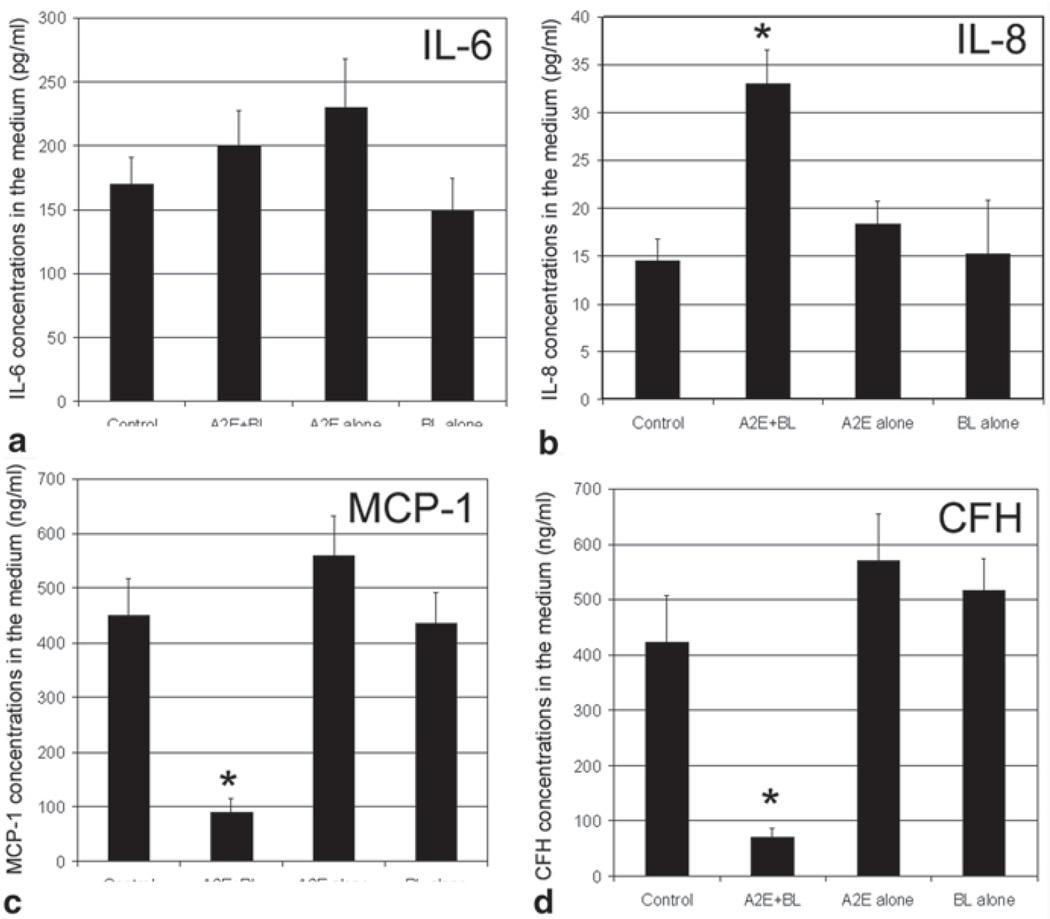
To determine whether A2E-mediated photooxidation also alters the secretion of these inflammation-related factors, we determined the levels of these factors in the medium. As shown in Fig. 31.2, exposure of A2E-containing RPE to blue light resulted in a 20 % and >2-fold increase in levels of IL-6 and IL-8 and a 70–80 % decrease in levels of MCP-1 and CFH in the medium. Accumulation of A2E alone increased the levels of IL-6, IL-8, MCP-1, and CFH marginally (Fig. 31.2), whereas exposure to blue light alone had no significant effect on levels of these inflammation- related factors in the medium.
Fig. 31.2.
A2E-mediated photooxidation alters the secretion of inflammation related factors. Confluent cultured ARPE-19 cells were loaded with 10 µM A2E for 14 days. The cells were then transferred to PBS and exposed to blue light for 10 min. Cells that accumulated A2E alone, exposed to blue light alone, or neither accumulated A2E nor exposed to blue light were used as control. The cells were then cultured in fresh medium containing 1 % FBS for 6 h. The medium was collected and levels of IL-6, IL-8, MCP-1, and CFH in the medium were determined by ELISA. The data are mean ± SD of the results from 6 samples in each group. * indicates p < 0.01 as compared the control cells that were neither treated with A2E nor blue light
31.3.2 Photooxidation impairs the function of the UPP
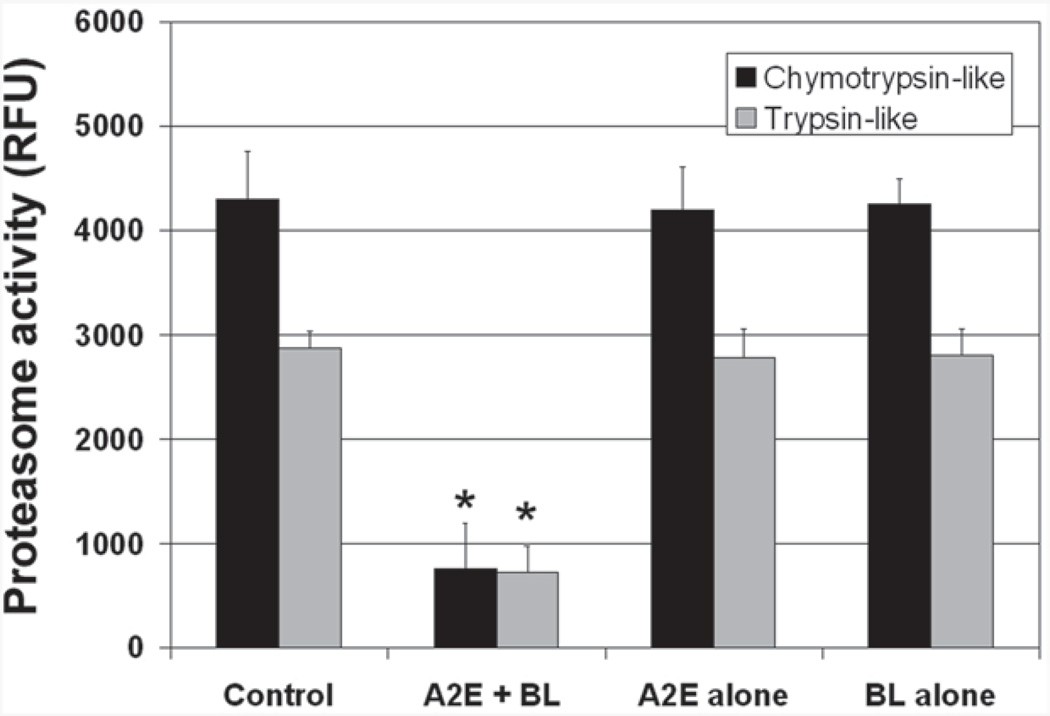
Our previous work showed that the proteasome is the most sensitive component of the UPP to oxidative inactivation [47, 48]. It is also known that the UPP is involved in regulating gene expressions by controlling signaling pathways and levels of transcript factors. It is plausible that the photooxidation induced changes in expression and secretion of the inflammation-related factors were related to the impairment of the UPP. To confirm previous results that physiologically relevant levels of oxidative stress inactivate the proteasome, we determine the effects of A2E-mediated photooxidation on the chymotrypsin-like and trypsin-like activities of the proteasome. As shown in Fig. 31.3, exposure of A2E-containing RPE to blue light resulted in a 70–80 % decrease in trypsin-like and chymotrypsin-like peptidase activities of the proteasome. Accumulation of A2E alone or exposure to blue light alone had no detectible difference in these peptidase activities of the proteasome. This data confirmed our previous results that the proteasome is a sensitive target of oxidative insults.
Fig. 31.3.
A2E-mediated photooxidation inactivates the proteasome in cultured RPE. Confluent cultured ARPE-19 cells were loaded with 10 µM A2E for 14 days. The cells were then transferred to PBS and exposed to blue light for 15 min and harvested. Cells that accumulated A2E alone, exposed to blue light alone, or neither accumulated A2E nor exposed to blue light were used as control. The chymotrypsin-like activity and trypsin-like activity of the proteasome in the cells were determined using a fluorogenic peptide as a substrate. The proteasome activity was expressed as increase in the relative fluorescence unit ( RFU) during a 5 min period. The experiments were repeated twice with triplicates each time. The data are mean ± SD of the results from 6 samples in each group. * indicates p < 0.001 as compared the control cells
31.3.3 Chemical Inhibition of the Proteasome in RPE Results in Similar Changes to that Caused by Photooxidation in Expression and Secretion of Inflammation-Related Factors
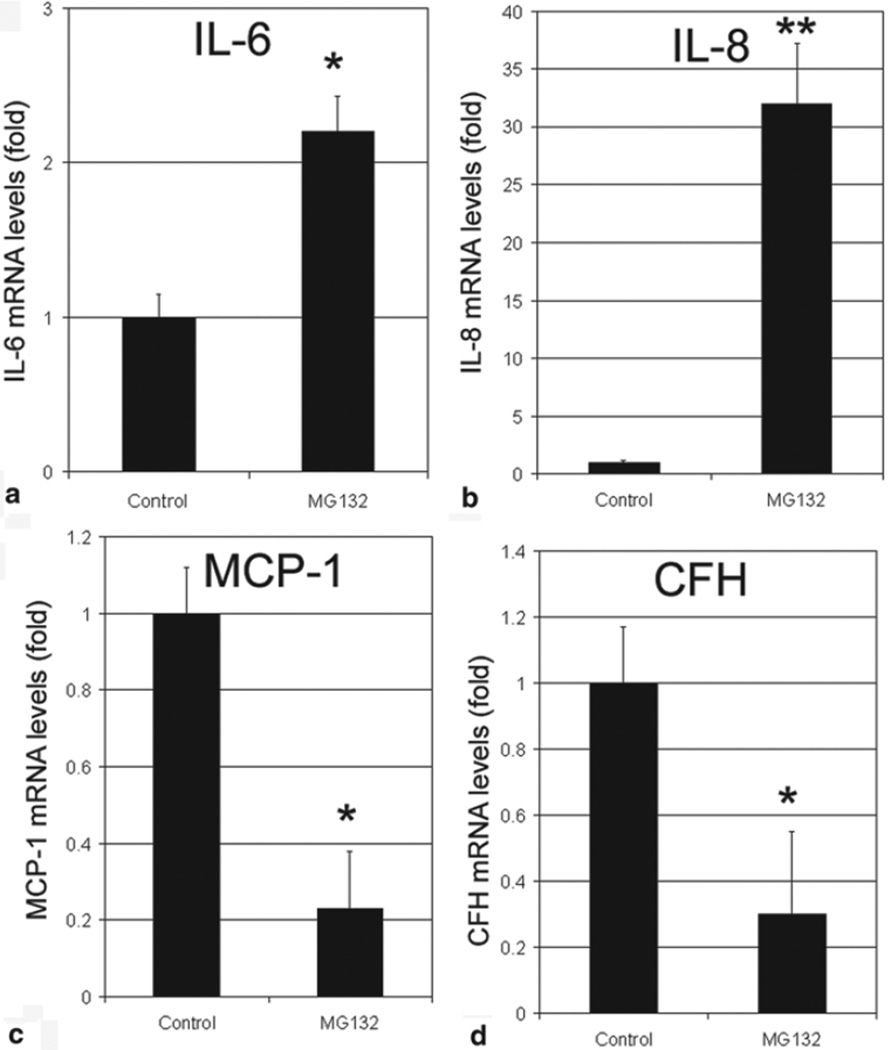
To test the hypothesis that photooxidation alters the expression and secretion of inflammation-related factors via impairment of the UPP, we inhibited proteasome activity in RPE by MG132 and determined the expression and secretion of these inflammation-related factors. We found that inhibition of proteasome resulted in a dramatic increase in mRNA levels for IL-6 and IL-8 (Fig. 31.4a, b). Levels of mRNA for IL-8 increased over 50-fold upon inhibition of the proteasome (Fig. 31.4b). Similar to photooxidation, proteasome inhibition resulted in a 70–80 % decrease in levels of mRNA for MCP-1 and CFH (Fig. 31.4c, d).
Fig. 31.4.
Inhibition of the proteasome alters the expression of inflammation-related genes. Confluent cultured ARPE-19 cells were incubated in fresh medium in the absence or presence of 10 µM MG132 for 8 h. Levels of mRNA for IL-6, IL-8, MCP-1, and CFH in the cells were determined by real-time quantitative RT-PCR using levels of mRNA for GAPDH as a reference. The relative levels of mRNA for these genes in control cells were arbitrarily designated as 1 and levels of mRNA for these genes in MG132-treated cells were expressed as fold of that in the control cells. The data are mean ± SD of the results from 4 samples in each group. * indicates p < 0.01 and ** indicates p < 0.001 as compared to the controls
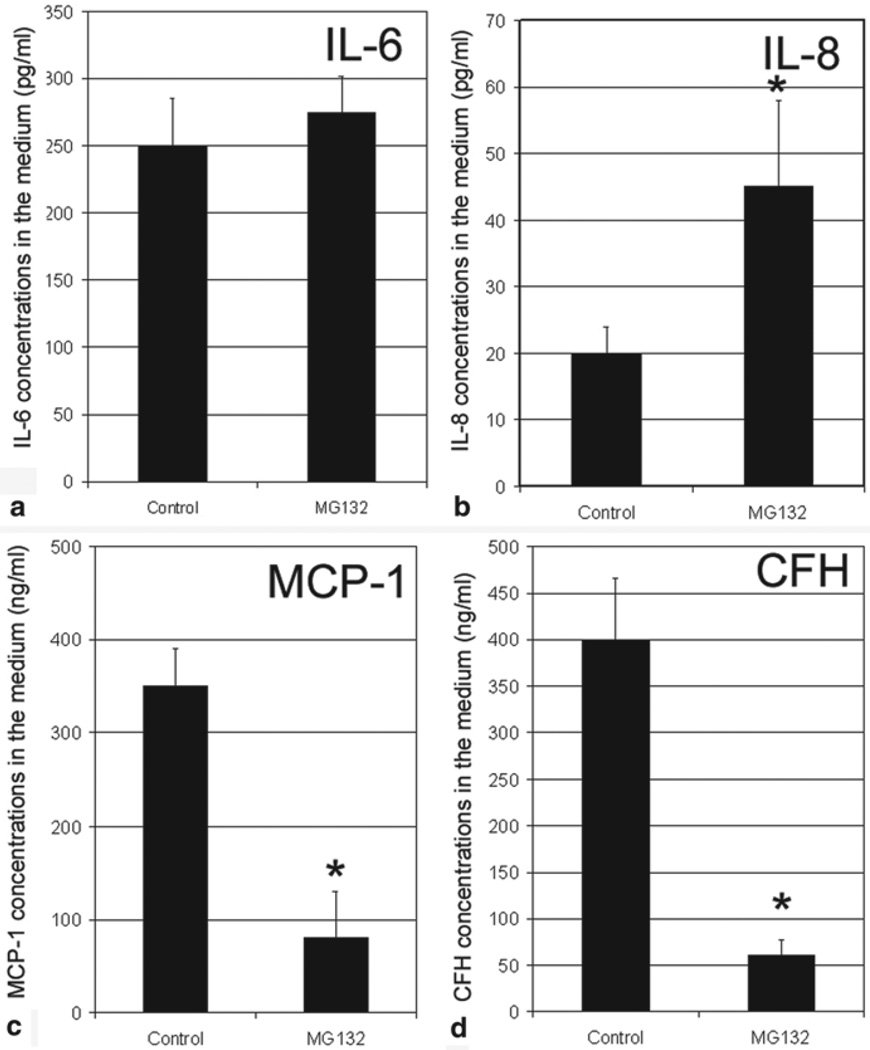
To determine whether proteasome inhibition also alter the secretion of these inflammation-related factors, we determined the levels of these factors in the medium. As shown in Fig. 31.5, inhibition of the proteasome only marginally increased the secretion of IL-6 (Fig. 31.5a), but increased the secretion of IL-8 by >2-fold (Fig. 31.5). Consistent with the decrease in mRNA levels, protein levels of MCP-1 and CFH in the medium decreased 80–90 % when the proteasome in RPE was inhibited (Fig. 31.5c, d). These data demonstrate that impairment of the UPP in RPE alters the expression and secretion of inflammation-related factors in a similar manner as photooxidative stress, suggesting oxidative inactivation of the proteasome is at least one of the mechanisms by which photooxidation alters the expression and secretion of inflammation-related factors by RPE.
Fig. 31.5.
Inhibition of the proteasome alters secretion of inflammation-related factor by cultured RPE. Confluent cultured ARPE-19 cells were incubated in fresh medium in the absence or presence of 10 µM MG132 for 8 h. The medium was collected and protein levels of IL-6, IL-8, MCP-1, and CFH in medium were determined by ELISA. The data are mean ± SD of the results from 4 samples in each group. * indicates p < 0.001 as compared to the controls
31.4 Discussion
Oxidative stress and inflammation are interrelated biological events [54] and both are implicated in the pathogenesis of AMD [3, 9, 27]. There is a vicious cycle in which oxidative stress triggers inflammatory responses, and inflammation also enhances the production of reactive oxygen species, all of them cause oxidative damage [55, 56]. Due to high metabolic rate and age-related accumulation of lipofuscin, RPE is a primary target of photooxidative damage in the eye [8]. RPE is also a major source of cytokines that regulate inflammatory response in the retina [50, 57, 58]. The vicious interaction between oxidative stress and inflammatory responses in RPE may contribute to the onset and progression of AMD. Any means that break the vicious cycle between oxidative stress and inflammation in RPE could be a potential strategy for prevention or treatment of AMD.
Results from this study show that photooxidative stress inactivates the proteasome in RPE and subsequently alters the expression of inflammation-related genes, such as up-regulation of IL-6 and IL-8 and down-regulation of MCP-1 and CFH. These data are consistent with our previous work which indicates that inactivation of the proteasome is a mechanistic link between oxidative stress and altered inflammatory responses [49, 50, 59].
An increasing body of evidence indicated dysregulation of inflammatory response, including improper complement activation is involved in the pathogenesis of AMD [10]. Oxidative stress is apparently one of the triggers of dysregulation of inflammatory response and the innate immune system. The data presented in the paper suggest that impairment of the UPP, particularly the proteasome, is an important step for oxidation-induced dysregulation of the inflammatory responses. However, it remains to be determined how impairment of the UPP alters the expression and secretion of these inflammation-related factors. The UPP is involved in many aspects of cellular functions. In addition to selective degradation of damaged or obsolete proteins, the UPP is involved in regulation of signal transduction and expression via controlling the levels of regulatory proteins and transcription factors. For example, UPP-mediated degradation of inhibitors of NF-κB is required for activation of the NF-κB pathway [60–62]. We found that inhibition of the proteasome in RPE suppressed the expression and secretion of MCP-1 and the suppression is related to down regulation of NF-κB signaling pathway [59]. The down-regulation of MCP-1 may have physiological consequences since MCP-1 knockout mice development of AMD-like phenotypes [63]. We also found the inactivation of the proteasome enhances the expression and secretion of IL-8 by activation of p38 signaling pathway [49, 50]. Elevated levels of IL-8 may not only promote inflammation, but also trigger neovascularization, because IL-8 is a neutrophil attractant and a strong pro-angiogenesis factor [64–67]. This study showed that inactivation of the proteasome also contributed to the down-regulation of CFH upon photooxidative stress. Although it is unknown at present how proteasome inhibition suppresses the expression of CFH, it is likely that the proteasome is involved in regulating levels of transcription factors and signaling molecules that control the expression of CFH. The down-regulation of CFH may play a role in complement activation [26] and complement attack to RPE [68] in response to oxidative stress.
Together, these results show that impairment of the UPP in RPE alters the expression and secretion of inflammation-related factors, suggest that inactivation of the proteasome by oxidation stress may be a mechanistic link between oxidative stress and dysregulation of inflammatory responses. Therefore, to preserve or to improve UPP activity in RPE may be a valid strategy for AMD prevention or treatment.
Acknowledgments
This work is supported by USDA AFRI Award 2009–35200-05014, NIH grant EY 011717, USDA contract 1950-510000-060-01A.
Contributor Information
Zhenzhen Liu, Email: 723179127@qq.com, Laboratory for Nutrition and Vision Research, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, USA.
Tingyu Qin, Email: tingyuqin@126.com, Laboratory for Nutrition and Vision Research, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, USA.
Jilin Zhou, Email: jz219@columbia.edu, Department of Ophthalmology, Columbia University, New York, USA.
Allen Taylor, Email: allen.taylor@tufts.edu, Laboratory for Nutrition and Vision Research, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, USA.
Janet R. Sparrow, Email: jrs88@columbia.edu, Department of Ophthalmology, Columbia University, New York, USA.
Fu Shang, Email: fu.shang@tufts.edu, Laboratory for Nutrition and Vision Research, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, USA.
References
- 1.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385(1):28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 2.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 3.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7(11):860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 4.Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30(2):97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandhadia S, Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev Mol Med. 2010;12:e34. doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 6.Chiu CJ, Taylor A. Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res. 2007;84(2):229–245. doi: 10.1016/j.exer.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Chiu CJ, Taylor A. Nutritional antioxidants, dietary carbohydrates, and age-related maculopathy and cataract. In: Bendich A, Deckelbaum R, editors. Preventive nutrition. 4. Humana Press; 2010. pp. 501–543. [Google Scholar]
- 8.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80(5):595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29(2):95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umeda S, Suzuki MT, Okamoto H, Ono F, Mizota A, Terao K, et al. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis) Faseb J. 2005;19(12):1683–1685. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- 12.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51(2):137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souied EH, Leveziel N, Richard F, Dragon-Durey MA, Coscas G, Soubrane G, et al. Y402H complement factor H polymorphism associated with exudative age-related macular degeneration in the French population. Mol Vis. 2005;11:1135–1140. [PubMed] [Google Scholar]
- 15.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 17.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 18.Postel EA, Agarwal A, Caldwell J, Gallins P, Toth C, Schmidt S, et al. Complement factor H increases risk for atrophic age-related macular degeneration. Ophthalmology. 2006;113(9):1504–1507. doi: 10.1016/j.ophtha.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 19.Despriet DD, Klaver CC, Witteman JC, Bergen AA, Kardys I, de Maat MP, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. Jama. 2006;296(3):301–309. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 20.Simonelli F, Frisso G, Testa F, di Fiore R, Vitale DF, Manitto MP, et al. Polymorphism p.402Y > H in the complement factor H protein is a risk factor for age related macular degeneration in an Italian population. Br J Ophthalmol. 2006;90(9):1142–1145. doi: 10.1136/bjo.2006.096487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau LI, Chen SJ, Cheng CY, Yen MY, Lee FL, Lin MW, et al. Association of the Y402H polymorphism in complement factor H gene and neovascular age-related macular degeneration in Chinese patients. Invest Ophthalmol Vis Sci. 2006;47(8):3242–3246. doi: 10.1167/iovs.05-1532. [DOI] [PubMed] [Google Scholar]
- 22.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaumberg DA, Christen WG, Buring JE, Glynn RJ, Rifai N, Ridker PM. High-sensitivity C-reactive protein, other markers of inflammation, and the incidence of macular degeneration in women. Arch Ophthalmol. 2007;125(3):300–305. doi: 10.1001/archopht.125.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. Jama. 2004;291(6):704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 25.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123(6):774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2006;103(44):16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14(2):194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciechanover A. The ubiquitin proteolytic system and pathogenesis of human diseases: a novel platform for mechanism-based drug targeting. Biochem Soc Trans. 2003;31(2):474–481. doi: 10.1042/bst0310474. [DOI] [PubMed] [Google Scholar]
- 29.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 30.Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med. 2011;51(1):5–16. doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang F, Taylor A. Roles for the ubiquitin-proteasome pathway in protein quality control and signaling in the retina: Implications in the pathogenesis of age-related macular degeneration. Mol Aspects Med. 2012;33(4):446–466. doi: 10.1016/j.mam.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 33.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6(8):599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi N, Vogel SN, Van Way C, 3rd, Papasian CJ, Qureshi AA, Morrison DC. The proteasome: a central regulator of inflammation and macrophage function. Immunol Res. 2005;31(3):243–260. doi: 10.1385/IR:31:3:243. [DOI] [PubMed] [Google Scholar]
- 35.Kloetzel PM. Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII. Nat Immunol. 2004;5(7):661–669. doi: 10.1038/ni1090. [DOI] [PubMed] [Google Scholar]
- 36.Hope AD, de Silva R, Fischer DF, Hol EM, van Leeuwen FW, Lees AJ. Alzheimer’s associated variant ubiquitin causes inhibition of the 26S proteasome and chaperone expression. J Neurochem. 2003;86(2):394–404. doi: 10.1046/j.1471-4159.2003.01844.x. [DOI] [PubMed] [Google Scholar]
- 37.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 38.Fernandes R, Ramalho J, Pereira P. Oxidative stress upregulates ubiquitin proteasome pathway in retinal endothelial cells. Mol Vis. 2006;12:1526–1535. [PubMed] [Google Scholar]
- 39.Dudek EJ, Shang F, Valverde P, Liu Q, Hobbs M, Taylor A. Selectivity of the ubiquitin pathway for oxidatively modified proteins: relevance to protein precipitation diseases. Faseb J. 2005;19(12):1707–1709. doi: 10.1096/fj.05-4049fje. [DOI] [PubMed] [Google Scholar]
- 40.Shang F, Nowell TR, Jr, Taylor A. Removal of oxidatively damaged proteins from lens cells by the ubiquitin-proteasome pathway. Exp Eye Res. 2001;73(2):229–238. doi: 10.1006/exer.2001.1029. [DOI] [PubMed] [Google Scholar]
- 41.Shang F, Deng G, Liu Q, Guo W, Haas AL, Crosas B, et al. Lys6-modified ubiquitin inhibits ubiquitin-dependent protein degradation. J Biol Chem. 2005;280(21):20365–20374. doi: 10.1074/jbc.M414356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jahngen-Hodge J, Obin M, Gong X, Shang F, Nowell T, Gong J, et al. Regulation of ubiquitin conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- 43.Obin M, Shang F, Gong X, Handelman G, Blumberg J, Taylor A. Redox regulation of ubiquitin-conjugating enzymes: mechanistic insights using the thiol-specific oxidant diamide. FASEB J. 1998;12(7):561–569. doi: 10.1096/fasebj.12.7.561. [DOI] [PubMed] [Google Scholar]
- 44.Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 2004;101(29):10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishii T, Sakurai T, Usami H, Uchida K. Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 s proteasome. BioChemistry. 2005;44(42):13893–13901. doi: 10.1021/bi051336u. [DOI] [PubMed] [Google Scholar]
- 46.Caballero M, Liton PB, Epstein DL, Gonzalez P. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308(2):346–352. doi: 10.1016/s0006-291x(03)01385-8. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Zhou J, Fernandes AF, Sparrow JR, Pereira P, Taylor A, et al. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49(8):3622–3630. doi: 10.1167/iovs.07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu M, Bian Q, Liu Y, Fernandes AF, Taylor A, Pereira P, et al. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic Biol Med. 2009;46(1):62–69. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandes AF, Bian Q, Jiang JK, Thomas CJ, Taylor A, Pereira P, et al. Proteasome inactivation promotes p38 mitogen-activated protein kinase-dependent phosphatidylinositol 3-kinase activation and increases interleukin-8 production in retinal pigment epithelial cells. Mol Biol Cell. 2009;20(16):3690–3699. doi: 10.1091/mbc.E08-10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes AF, Zhou J, Zhang X, Bian Q, Sparrow J, Taylor A, et al. Oxidative inactivation of the proteasome in retinal pigment epithelial cells. A potential link between oxidative stress and up-regulation of interleukin-8. J Biol Chem. 2008;283(30):20745–20753. doi: 10.1074/jbc.M800268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, et al. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276(32):30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 52.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40(12):2988–2995. [PubMed] [Google Scholar]
- 53.Zhou J, Cai B, Jang YP, Pachydaki S, Schmidt AM, Sparrow JR. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp Eye Res. 2005;80(4):567–580. doi: 10.1016/j.exer.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Nicholls SJ. The complex intersection of inflammation and oxidation: implications for atheroprotection. J Am Coll Cardiol. 2008;52(17):1379–1380. doi: 10.1016/j.jacc.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 55.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med. 2010;48(9):1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, et al. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. 2010;13(7):1033–1049. doi: 10.1089/ars.2009.2930. [DOI] [PubMed] [Google Scholar]
- 57.Larrayoz IM, Huang JD, Lee JW, Pascual I. Rodriguez IR. 7-ketocholesterol-induced inflammation: involvement of multiple kinase signaling pathways via NFkappaB but independently of reactive oxygen species formation. Invest Ophthalmol Vis Sci. 2010;51(10):4942–4955. doi: 10.1167/iovs.09-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 59.Fernandes AF, Guo W, Zhang X, Gallagher M, Ivan M, Taylor A, et al. Proteasome-dependent regulation of signal transduction in retinal pigment epithelial cells. Exp Eye Res. 2006;83(6):1472–1481. doi: 10.1016/j.exer.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing of NF-kB procursor protein and the activation of NF-kB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 61.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412(6844):346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 62.Yaron A, Gonen H, Alkalay I, Hatzubai A, Jung S, Beyth S, et al. Inhibition of NF-kappa-B cellular function via specific targeting of the I-kappa-B-ubiquitin ligase. Embo J. 1997;16(21):6486–6494. doi: 10.1093/emboj/16.21.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9(11):1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 64.Simonini A, Moscucci M, Muller DW, Bates ER, Pagani FD, Burdick MD, et al. IL-8 is an angiogenic factor in human coronary atherectomy tissue. Circulation. 2000;101(13):1519–1526. doi: 10.1161/01.cir.101.13.1519. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, et al. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17(7):4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170(6):3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 67.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278(10):8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 68.Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284(25):16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]