Abstract
Introduction
Paired associative stimulation (PAS), is a novel non-invasive technique where two neural substrates are employed in a temporally coordinated manner in order to modulate cortico-motor excitability within the motor cortex (M1). In swallowing, combined pharyngeal electrical and transcranial-magnetic-stimulation induced beneficial neurophysiological and behavioural effects in healthy subjects and dysphagic stroke patients. Here, we aimed to investigate the whole-brain changes in neural activation during swallowing using functional magnetic resonance imaging (fMRI) following PAS application and in parallel assess associated GABA changes with magnetic resonance spectroscopy (MRS).
Methods
Healthy adults (n = 11, 38 ± 9 years old) were randomised to receive real and sham PAS to the ‘stronger’ motor cortex pharyngeal representation, on 2 separate visits. Following PAS, event-related fMRI was performed to assess changes in brain activation in response to water and saliva swallowing and during rest. Data were analysed (SPM8) at P < .001. MRS data were acquired using MEGA-PRESS before and after the fMRI acquisitions on both visits and GABA concentrations were measured (AMARES, jMRUI).
Results
Following real PAS, BOLD signal changes (group analyses) increased at the site of stimulation during water and saliva swallowing, compared to sham PAS. It is also evident that PAS induced significant increases in BOLD signal to contralateral (to stimulation) hemispheric areas that are of importance to the swallowing neural network. Following real PAS, GABA: creatine ratio showed a trend to increase contralateral to PAS.
Conclusion
Targeted PAS applied to the human pharyngeal motor cortex induces local and remote changes in both primary and non-primary areas for water and saliva tasks. There is a possibility that changes of the inhibitory neurotransmitter, GABA, may play a role in the changes in BOLD signal. These findings provide evidence for the mechanisms underlying the beneficial effects of PAS on the brain swallowing network.
Keywords: Swallowing, Neuroplasticity, Cortex, GABA, Pharynx, Paired associative stimulation
Introduction
The swallowing neural network is diversely distributed; requiring the coordination of both cortical and brainstem regions for the safe execution of the complex sensorimotor transport of liquids and food from the lips to the stomach. Previous neuroimaging studies have shown that areas including the primary and non-primary cortex and sub-cortex are activated during swallowing (Hamdy et al., 1999; Kern et al., 2001a; Martin et al., 2001, 2004; Soros et al., 2009). Among the several brain areas involved in this swallowing network, the insula was shown to have a central integrative role in regulating this essential function. Connectivity studies showed that voxel clusters were larger from the insula than other seed regions (Lowell et al., 2012) or was functionally connected to prefrontal operculum regions (Babaei et al., 2013). Moreover, an activation likelihood estimation meta-analysis of imaging studies on swallowing showed that saliva swallowing involves a larger involvement of premotor areas when compared to water swallowing, whilst water swallowing is associated with activation in the right inferior parietal region. This is likely to be reflecting the sensory processing of intraoral water stimulation (Soros et al., 2009).
Following a focal brain lesion such as stroke, patients may experience swallowing disorders (dysphagia); a devastating complication resulting in increased risk of aspiration pneumonia (Martino et al., 2005; Broadley et al., 2003; Daniels et al., 1996). Evidence exists for the effective recovery of swallowing function after unilateral stroke, which is associated with increase in cortical excitability and cortical area map size of the unaffected hemisphere (Hamdy et al., 1998a; Li et al., 2009; Teismann et al., 2011). Consequently, numerous stimulus-driven neuroplasticity protocols are trialled in order to augment and accelerate these cortical changes in dysphagic patients (Michou and Hamdy, 2013; Martin, 2009).
One neurostimulation paradigm under investigation is paired associative stimulation (PAS). This technique induces hetero-synaptic plasticity in the motor and somatosensory cortical areas by combining peripheral stimulation to the targeted muscle with cortical stimulation over the representational area of that muscle in the motor cortex (M1) (Classen et al., 2004; Stefan et al., 2000). By combining these two modalities, peripheral and central, and by separating them with a specific time interval, M1 excitation can be induced (Jayaram and Stinear, 2008; Castel-Lacanal et al., 2009; McKay et al., 2002; Stefan et al., 2002). In the swallowing model, peripheral stimulation is delivered via application of pharyngeal electrical pulses and central stimulation with transcranial magnetic stimulation (TMS) pulses. With several studies in healthy individuals, we have verified the optimal dose, the time interval between the 2 components, the total duration, and the effects of repetition of the neurostimulation technique (Gow et al., 2004; Singh et al., 2009; Michou et al., 2012). We also observed that single PAS application to the non-lesion hemisphere of chronic stroke patients increased cortical excitability bilaterally (Michou et al., 2012, 2014). This increase was accompanied by significant functional changes in swallowing physiology and a reduction of the incidence of unsafe swallow (Michou et al., 2012, 2014).
Non-invasive measures of the endogenous brain metabolites using proton (1H) magnetic resonance spectroscopy (MRS) can detect and quantify the principal neurotransmitters in vivo (Puts and Edden, 2012). The major inhibitory and excitatory neurotransmitters, γ-aminobutyric acid (GABA) and glutamate (Glu), respectively, are considered the principal drivers underlying cortical plasticity (Arckens et al., 2000). To date, N-acetylaspartate (NAA), creatine (Cr) and choline (Cho) compounds with Glu are readily detectable with clinical MRI scanners. Recent advances in MRS have been developed to quantify GABA in vivo, with the use of spectral editing techniques such as Meshcher–Garwood Point Resolved Spectroscopy (MEGA-PRESS) (Mullins et al., 2014; Mescher et al., 1998; Rothman et al., 1993; Waddell et al., 2007).
We have previously quantified Glu changes using MRS in the PAS stimulated M1 area (Singh et al., 2009) in healthy adults. A focal and transient mean reduction of 25% in Glu was observed in the stimulated M1 following 30 min of PAS applied to the stronger pharyngeal M1 representation. The largest difference was evident immediately after PAS, with the effect declining over time. No changes in other brain metabolites (NAA, Cho) were observed at the control site, the occipital cortex. As a control for PAS, application of the central stimulation (TMS pulses) only was performed on a separate day but no changes in the other metabolites were found in the same participants.
Given the extensive evidence for the beneficial effects of PAS application in swallowing cortical excitability, behaviour (safety) of swallowing and the evidence for the neurochemical changes following stimulus-dependent rehabilitation protocols, it would be of interest to investigate the whole brain changes following real and sham PAS with fMRI. Furthermore, given that there is evidence for different levels of BOLD activation during water and saliva swallowing, we included both tasks in our fMRI protocol. The non-invasive brain stimulation paradigm, PAS, is delivered through a sensory ‘afferent’ stimulus in combination with brain stimulation and therefore it would be of interest how the different patterns of peak activations would change following neurostimulation.
We were also interested in investigating the role of GABA in mediating any potential effect in M1, which has not been measured before.
We expected that activation of cortical regions commonly involved in swallowing would be modulated following real PAS, compared to sham PAS. Finally, we hypothesised that GABA concentrations would change ipsilateral to the PAS taking into consideration the previous observations for the reduction of Glu ipsilateral to PAS stimulation. If our predictions are correct, our study could provide further evidence for the association of GABA to stimulus-induced neuroplasticity and would provide additional evidence on neurochemical grounds for the utilisation of PAS as an adjunct therapeutic neurostimulation method for patients with neurogenic dysphagia.
Materials and methods
Participants
Participants were right-handed healthy adults (n = 11, 5 women) with a mean age of 35 ± 9 years (±SD). Informed written consent was obtained prior to the study. The research protocol was approved by the Salford & Trafford Local Research Ethics Committee and was executed in accordance with the World Medical Association Declaration of Helsinki.
Methods
Transcranial magnetic stimulation
Focal TMS was performed using a flat figure-of-8 shaped magnetic coil (outer diameter, 70 mm) connected with a Magstim BiStim2 magnetic stimulator (Magstim Co, Whitland, Wales, UK), which produced maximal output of 2.2 T. The anterior–posterior direction with the plane of the coil parallel to the scalp surface and the handle/axis of the coil at 45° to midsagittal line was chosen according to previous studies. TMS was used to localise the exact resting pharyngeal M1 hotspots bilaterally.
Pharyngeal electromyographic measurements
Pharyngeal electromyographic measurements after single TMS pulses, termed pharyngeal motor evoked potentials (PMEPs), were recorded through a 3.2 mm-diameter intraluminal catheter (Gaeltec Ltd.; Dunvegan, Isle of Skye, Scotland) with a built-in pair of bipolar platinum ring electrodes, which was inserted either nasally (15–17 cm to pair electromyographic electrodes from the nasal flare) or orally (13–15 cm) depending on participant's preference. This allowed the recording of PMEPs at the mid-pharyngeal level and likely middle pharyngeal constrictors. The amplitude of the PMEPs following TMS single pulse amplitude was used to verify the sides of the stronger and the weaker pharyngeal hotspots. The 'stronger' pharyngeal projection was defined as the hemispheric site with the lowest resting motor threshold to evoke PMEPs, whereas the site with the highest threshold was termed the 'weaker' pharyngeal M1.
Paired associative stimulation
PAS was delivered by pairing a pharyngeal electrical stimulus (pulse = 0.2 ms) with a single TMS stimulus over the pharyngeal M1 at the intensity of motor threshold (MT) plus 20% of stimulator output for 10 min. The 2 stimuli were delivered in a controlled manner through Signal software (v2.13; Cambridge Electronic Design, Cambridge, England), with an interstimulus interval of 100 ms, based on previous investigations. The intraluminal catheter used for PMEPs above was connected to a constant current generator (model DS7; Digitimer Welwyn-Garden City, Herts, UK) to deliver pharyngeal electrical stimulation (PES). For the sham intervention, the coil was held tangentially to the skull at a 90° angle to the sagittal plane, and no PES was delivered through the pharyngeal catheter in situ.
Protocol
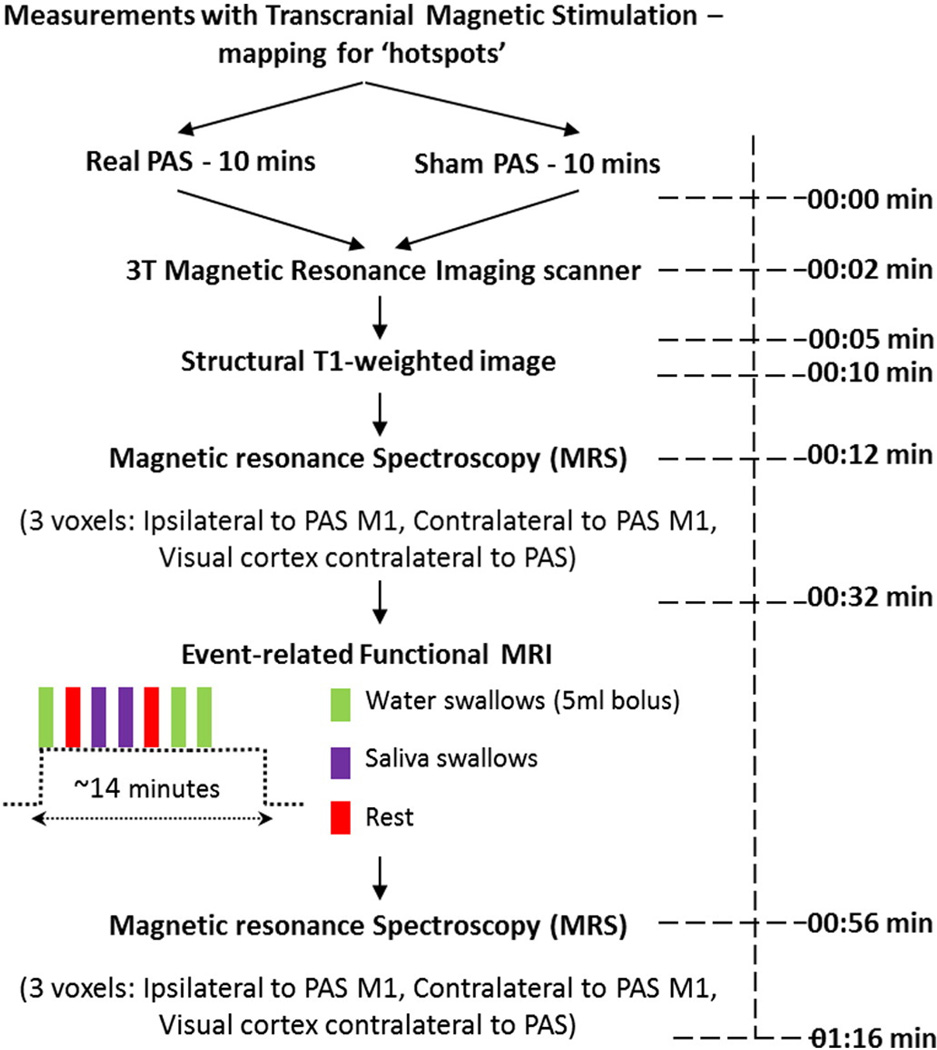
We used a double-blinded, sham-controlled methodology to quantify the effects of PAS on healthy participants. Each participant visited the laboratories twice, on two separate days with at least a week apart between the different arms (please see algorithm for the study, Fig. 1).
Fig. 1.
Presents the protocol algorithm for the study, with the respective time to complete each component.
Following electrophysiological measurements, real and sham PAS was delivered in a randomised order on two separate visits to the ‘stronger’ (largest TMS evoked PMEPs) pharyngeal M1 of the bilateral swallowing neural network as per previous studies (Singh et al., 2009; Michou et al., 2012). Before scanning, an oil capsule was taped on each site of the bilateral ‘hotspots’ of pharyngeal M1 to confirm on MR structural images that the hotspot localisation for PAS application was over the M1. Following the structural imaging (T1-weighted image), the participants underwent MRS on three sites of interest (ipsilateral to PAS, contralateral to PAS and contralateral to PAS occipital cortex: control). This was followed by an fMRI experiment, during which the participants had to perform 2 active swallowing tasks (water and saliva swallowing) and a rest condition. Immediately after the fMRI, a second MRS session took place within the same voxels localised earlier. These procedures were identical on both study days.
Magnetic resonance imaging
All MR imaging was acquired with a 3 T Achieva MRI scanner (Philips Medical Solutions, Best, The Netherlands), using an 8-channel head coil for signal collection. The participant's head was immobilized in a cradle, within the head coil, using foam padding to minimize movement.
Structural magnetic resonance imaging parameters
A T1-weighted structural image was collected using an inversion recovery fast gradient echo sequence, optimised for grey/white matter contrast with 1-mm isotropic voxels with full brain coverage. Anatomical T1 brain images were used to plan MRS voxel localisation, slice placement for the functional scans and for clinical reporting to detect abnormalities and exclude participants ineligible due to structural anomalies.
Magnetic resonance spectroscopy (MRS)
Single-voxel 1H MRS was acquired with the MEGA-PRESS sequence (Mescher et al., 1998; Mullins et al., 2014) with TR: 2000, TE: 68 ms (readout duration 1024 ms).A total of 2048 sample points were collected at a spectral width of 2 kHz. The voxel size was 3.2 × 3.2 × 3.2 cm3 (Inline Supplementary Fig. S1) and was located predominantly in the cortical grey matter of the pharyngeal M1.
Inline Supplementary Fig. S1 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
A second MRS voxel covered the contralateral pharyngeal M1. The third voxel was placed contralateral to the stimulation in the occipital cortex (control site).
We also visually inspected that the hotspots, as found with TMS procedures (also where PAS was applied) and shown by the oil capsule, were above the area covered by the voxel M1.
Some white matter or cerebrospinal fluid content within the voxels is likely, but the within-participant voxel positioning was consistently maintained throughout the study on both days so that there would be no variations in non-grey matter content. In addition, we performed further analysis of the grey/white matter segmentation for each imaging study (below). Data acquisition of each voxel lasted for 6 min 24 s, giving a total of MRS scanning time of approximately 20 min.
Functional magnetic imaging procedures: screening
Gradient echo planar imaging (GE-EPI) was used for acquiring functional images in the axial plane. The GE-EPI parameters were as follows: echo time 35 ms; repetition time TR = 2300 ms; scan resolution = 80 × 80 voxels; FOV = 230 mm. This protocol acquired 35 axial brain slices with voxel size: 1.8 × 1.8 × 3.5 mm3, and total number of 370 dynamics.
Functional tasks
Three different conditions were used: water swallowing, saliva swallowing and rest. All tasks were repeated 9 times within a 14 minute protocol. During the functional MR scanning, the participant swallowed a 5 ml bolus of room-temperature water delivered to the mouth through a 3 mm-diameter flexible tube, 5 m long attached to a 30 ml syringe controlled manually by the experimenter outside the scanner room. The tubing was held between the participant's lips at midline and was stabilized at the level of the participant's chin and held by the participant's right hand. The tasks were performed in response to a visual cue on a front projection screen seen via a mirror mounted above the eyes. The visual cue for water swallowing tasks was a blue circle, whilst a red circle was used for the rest condition and a yellow for the saliva swallowing. Between the task cues, no image was displayed. The order of the tasks was pseudo-randomised, and remained the same for all the scans. Participants were trained on both swallowing active tasks prior to each experimental session and were advised to restrain from movements within and between tasks. It was confirmed that all participants could clearly see the stimuli without straining or adjusting the position of their heads.
Verification of swallowing trials
The swallowing trials were verified on-line from their laryngeal movement patterns (Martin et al., 2001). Laryngeal movements were recorded (PowerLab, v4.1.1, ADInstruments) from the output signal of a pressure transducer driven from expanding magnetic resonance compatible bellows (ADInstruments, Oxfordshire, United Kingdom) positioned comfortably over the participant's thyroid cartilage. These data were used as verification of the subjects' compliance with the tasks and that there was no delay in their swallow.
Data analysis
fMRI image processing
All functional images were processed with Statistical Parametric Mapping (SPM8, University College London, UK). The ARtifact Detection Tools (ART) was employed to detect any global mean intensity and motion outliers since quality assurance protocols have been proposed previously (Johnstone et al., 2006). The full dataset was analysed twice to examine the necessity for the use of motion artefact as a covariate. However, the mean displacement was 0.96 ± 0.3 mm with the maximum absolute head movement being less than 1.8 mm in the xyz translational output, showing that the motion artefact was not a factor to use as covariate in the analysis. The ArtRepair Toolbox version 4 (Mazaika et al., 2009) was used to correct motion of the data from one participant, whose motion exceeded the 1.8 mm in z translation for dynamics number 110–200.
All data were analysed in the following order: slice-timing correction and realignment, co-registration, segmentation and normalisation to the MNI template using unified segmentation of the anatomical T1-weighted image, re-sampling to 1.8 × 1.8 × 3.5 mm isotropic voxels, and smoothed with 6 mm FWHM Gaussian kernel. In the occurrence of a physiological change in laryngeal measurements during the rest state trials, those fMRI events were discarded in the first level analysis results of each participant.
Four participants had 'stronger' pharyngeal hotspots for PAS on the right M1, whilst the rest were on the left (n = 7). In order to ensure that group analysis was carried out evenly across all individuals, during the first-level analysis those with a 'stronger' representation on the right (n = 4) had their T1-weighted image and functional images mirror-flipped prior to realignment but always following slice-timing correction, based on previously described protocols (Hammers et al., 2003; Laufs et al., 2011). Therefore, from here onwards the left was named stimulated hemisphere and the right as contralateral to PAS.
Region-of-interest analysis
We performed a region of interest (ROI) analysis of the swallowing network to enable comparison of activation between the real and sham stimulation conditions. The selection of the anatomical sections for the ROIs was based on the BOLD activation maps following the analysis of the main effects of water and saliva swallowing. These anatomical areas were included on the maximum probability maps (MPM) of the statistical Parametric Mapping Anatomy Tool box (Eickhoff et al., 2005). The mean percent BOLD signal for each ROI was extracted for each participant, prior to measuring the mean group BOLD % signal change.
MRS GABA processing
MRS analysis was performed using the jMRUI software package version 4.0 (http://www.mrui.uab.es/mrui, Stefan et al., 2009). MEGA-PRESS generates 2 sub-spectra, with the editing pulse on in one and off in the other. In the implementation on the Philips scanner the edited spectrum, which reveals GABA, is the sum of the two sub-spectra. The pre-processing of the data involved the removal of the residual water peak with the Hankel Lanczos singular value decomposition (HLSVD) toolbox (van den Boogaart et al., 1994), apodisation of the data using a 4 Hz Gaussian filter and manual phasing of the data for visualisation. The phase and reference frequency were determined from the unedited sub-spectrum, by setting the chemical shift of NAA at 2.02 ppm and this phase correction was applied to the edited (summed) spectrum prior to analysis. Spectra were fit to a Gaussian line shape, and the GABA peaks were identified at 3 ppm. The edited spectra were then analysed using AMARES to quantify GABA; a non-linear least-square fitting algorithm operating in the time domain. NAA and Cr were quantified from the unedited spectra and the GABA amplitudes were expressed as a ratio of total edited GABA (edit on)/NAA (edit off) and edited GABA (edit on)/Cr (edit off). For the unedited data, the signal was smoothed using a 5 Hz Lorentzian filter and the residual water signal was then filtered out using HLSVD as previously.
Grey/white matter segmentation
Tissue segmentation and quantification within the MRS voxels was carried out using the partial correction segmentation toolbox based in SPM8. The location and partial volume makeup of the MRS voxels were acquired. Images generated were viewed in FSL View (FMRIB Software Library, Jenkinson et al., 2012) to validate the origin of the voxel and the grey matter/white matter (GM/WM) ratio was used to investigate any difference in positioning of the voxels to the areas of interest, both for intra-participant variability (repeated MRS on the same day) and between days (real and sham).
Statistical methods
fMRI
First-level analyses of the time-series data were performed for individual participants using a general linear model (SPM8). Rather than using stimulus presentation times, swallow onset times for each condition (water, and saliva) were obtained directly using laryngeal pressure signals. We used this measurement since we performed PAS over the pharyngeal M1 and the elevation of the thyroid cartilage indicates the engagement of the pharyngeal stage of swallowing phase. The vectors of onset for each condition were convolved with the canonical hemodynamic response function (HRF) to construct the statistical model, resulting in a 6-column design matrix. Second-level analyses were initiated with F contrasts and effects of interest (EOI), reviewing the BOLD signal changes for all the tasks related activity with significance both at peak level set at P < 0.001 uncorrected, of at least k > 10 and at cluster level, at P(FWE-cor) and P(FDR-cor). Further analyses were performed for the contrast between swallow types and real vs. sham neurostimulation sessions using estimates of BOLD activation amplitude images. BOLD amplitude images were reviewed from first-level contrasts of each swallow type. T-contrasts were used to determine significant differences in the 2 active conditions (such as water and saliva swallowing–rest) following real PAS compared to sham PAS.
Region-of-interest analysis
ROI analysis was treated with non-parametric statistics, where each participant was treated as its own control and paired-wise comparison (Wilcoxon's test) between real and sham values for each of the areas under investigation was performed.
MRS
Reliability of grey/white matter segmentation ratios between and within sessions was analysed with intraclass correlation coefficient analysis. The GABA/NAA and GABA/Cr ratios were compared across visits (real and sham), across time points (before and after fMRI) and across sites (ipsilateral, contralateral, and control occipital) in a 3-way repeated measures ANOVA.
Significance was set at P < 0.05 and expressed as mean ± SD, unless otherwise stated.
Results
Paired associative stimulation
The intervention was delivered to all participants with no adverse effects on both days. The average motor threshold of all participants, measured with single TMS pulses, was 63 ± 8% of maximum magnetic stimulator output whereas pharyngeal stimulation was delivered at a mean current of 12 ± 3 mA. The sites for the application of the TMS part of PAS with respect to the vertex during both real and sham session are shown below (Inline Supplementary Fig. S2). All participants were tested in the afternoon, except for one (sub 5) who was tested in the morning.
Inline Supplementary Fig. S2 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
Functional magnetic imaging
The mean response latency from the visual cue (trigger) to the peak amplitude of the laryngeal movement signal associated with the saliva swallowing task was 2.1 ± 0.6 s. The mean latency for the water swallow was 2.5 ± 0.5 s. The latency data indicated that the participants responded promptly to the visual cues in both the saliva and water-swallowing tasks, with a response latency difference between the two tasks of approximately 0.48 s.
Main effects of real and sham PAS on water, saliva and rest conditions
The areas with group-level significant cluster and peak activations following the real and sham PAS during water and saliva swallowing are shown in supplementary material (see Supplementary Tables 1 & 2).
During the sham PAS session BOLD peak activations were seen in several areas associated with swallowing in previous publications (Soros et al., 2009). The voluntary saliva swallow activated a number of brain regions across participants. The largest group-wise activation in voluntary saliva swallowing was located at the junction of the right (contra-stimulated hemisphere in the real PAS arm) SMA and adjacent cingulate gyrus-ACC (i.e., BA 32), bilateral precentral gyrus of the premotor area and bilateral Ml (BA 6,4). Activations during voluntary water swallowing were mostly in the bilateral precentral gyrus (BA 6) and left Ml. Right SMA was also activated during water swallowing (significant only at the peak level), as were the declive (cerebellar vermis) activations. As expected, activations during rest in sham condition were observed in the lingual gyrus and fusiform gyrus and other areas of the occipital lobe.
During the real PAS sessions, several strongly significant clusters were observed with peak activations during both water and saliva voluntary tasks. Contralateral to stimulation, the premotor cortex (BA 6) and insula showed the peak activations, alongside areas such as the bilateral declive and the ipsilateral to stimulation superior frontal gyrus. The insula seemed to be bilaterally activated (claustrum was activated within the stimulation-side) as well as the contra-stimulated SMA. During volitional saliva swallowing following PAS, the ipsistimulated superior temporal lobe (BA 22), the contra-stimulated premotor (BA 6) and Ml cortex showed peak activations, whilst SMA activations were largely associated within the contra-stimulated hemisphere. Occipital lobes were activated in both conditions, and it is where the peak activations in the rest condition were found.
The effect sizes of the responses following real vs. sham PAS seem to differ in the contralateral to stimulated Ml for saliva swallowing (see Inline Supplementary Fig. S3).
Inline Supplementary Fig. S3 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
Inline Supplementary Fig. S4 below presents the overlapping and different activations in water (A) and saliva (B) swallowing following real and sham PAS. It is evident that during both swallowing conditions, there are areas activated in both real and sham conditions (overlapping), but real stimulation engaged additional areas during the execution of the sensorimotor tasks.
Inline Supplementary Fig. S4 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
Contrasts
Based on the underlying activations during the rest conditions in the areas as shown in the Supplementary Table 1, we performed the analysis on the water–rest and saliva–rest contrast thereafter.
Effects of real and sham PAS on water swallowing (water–rest contrast)
As shown in Table 1 below, the application of real PAS to the stronger pharyngeal Ml resulted in higher BOLD signal change in the primary Ml and the claustrum, whilst the premotor areas (BA 6) of the contralateral to the application hemisphere and insula were also found. Interestingly, for the ‘water–rest contrast’ in the sham arm, the highest BOLD changes were observed in the post-central gyrus.
Table 1.
Peak bilateral activations during water swallowing following real and sham PAS in the group of participants, shown as Talairach coordinates with z and t scores of activations.
| Brain activation for the voluntary water swallowing following real and sham PAS (water-rest contrast) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Real PAS |
Sham PAS |
|||||||||
| Area (BA) | Talairach |
z scores (peak t scores) |
Area (BA) | Talairach |
z scores (peak t scores) |
|||||
| x | y | z | x | y | z | |||||
| Stimulated | Precentral gyrus (BA4) | −43.0 | −18.8 | 35.9 | 3.5 (3.7) *, ** | Postcentral gyrus, Parietal BA43 |
−54.9 | −8.8 | 20.5 | 4.81 (5.34) *,** |
| Claustrum | −37.9 | −1.1 | −0.9 | 4.33 (4.72)*, ** | ||||||
| Contra-stimulated | Precentral Gyrus BA6 | 46.6. | −13.3 | 34.4 | 4.41 (4.82) *, ** | Postcentral Gyrus, Parietal BA43 |
56.19 | −6.92 | 16.28 | 4.45 (4.86) *, ** |
| Insula | 39.7 | 4.98 | 0.92 | 3.5 (3.7)* (cluster peak) |
Precentral Gyrus BA6 | 47.7 | −9.7 | 25.7 | 4.11 (4.43)*, **(cluster level) |
|
All activations are shown here are significant for both FWE (cor, *), FDR (cor, **) for both cluster and peak level, unless otherwise stated. Other significant ‘peak-only’ activations have been omitted from this table.
Effects of real and sham PAS on saliva swallowing (saliva–rest contrast)
Table 2 presents the areas that were activated for the voluntary ‘saliva-rest’ contrast following real and sham PAS. Interestingly, bilateral activations in the premotor motor cortex were observed following real PAS. One of the most significant activations was that of the insula ipsilateral to the stimulation. As expected, activations on the primary motor cortex and premotor cortex were evident following both real and sham PAS sessions.
Table 2.
Peak bilateral activations during saliva swallowing following real and sham PAS on different days in the group of participants, shown as Talairach coordinates and z and t scores.
| Brain activation for the voluntary saliva swallowing following real and sham PAS (saliva–rest contrast) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Real PAS |
Sham PAS |
|||||||||
| Area (BA) | Talairach |
z scores (peak t scores) |
Area (BA) | Talairach |
z scores (peak t scores) |
|||||
| x | y | z | x | y | z | |||||
| Stimulated | Insula | −41.5 | 1 | −4.2 | 4.33 (4.71)*, ** | Precentral Gyrus (BA4) | −55.4 | −6.6 | 22.4 | 5.15 (5.8)*, ** |
| Precentral gyrus BA6 | −55.4 | −4.5 | 25.1 | 3.96 (4.23)*, ** | ||||||
| Contra-stimulated | Precentral gyrus BA6 | 51.4 | −7.1 | 31.6 | 5.48 (6.48) *, ** | Precentral gyrus BA6 | 52.4 | −4.5 | 25 | 4.82 (5.35) *, ** |
| Inferior frontal gyrus BA44 | 56.4 | 10.7 | 21.5 | 4.07 (4.39) *, ** | ||||||
All activations shown here are significant for both FWE (cor, *), FDR (cor, **) for both cluster and peak level, unless otherwise stated. Other significant ‘peak-only’ activations have been omitted from this table.
Region-of-interest results
Based on the mean task effects, the anatomical sections defined were the primary motor cortex (areas 4a and 4p) (Geyer et al., 1996), and the premotor cortex (BA 6) (Geyer, 2004). As anticipated from the observed BOLD signal changes following water and saliva swallowing with real and sham PAS (Table 1, Supplementary material), the ROI analysis for the bilateral M1 masks (4anterior − 4a and 4posterior − 4p) and the premotor cortices showed similar effects following the PAS application. Compared to sham PAS water swallowing activations, the contralateral-to-PAS M1, showed greater signal change in the 4p area (P < .05, z = −1.8), as did the contralateral to PAS premotor cortex (BA 6) (see Inline Supplementary Fig. S5) (P < .05, z = −1.9). For the saliva swallowing (Inline Supplementary Fig. S4), the signal change was increased in both the contralateral to stimulation and stimulated premotor areas following real PAS compared to sham stimulation (P < 0.05, z = −1.9; P < 0.01, z = −2.6 respectively).
Inline Supplementary Fig. S5 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
MRS results
We performed the segmentation of grey and white matter initially and observed that one participant (out of 11) had dramatically different ratios of tissue composition in the same voxel between real and sham sessions. Data from this participant were therefore excluded from the analysis. Median valued of the GM/WM ratios for the remaining 10 participants across the different sessions are shown in the Inline Supplementary Table S1.
Inline Supplementary Table S1 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
The ICCs for the variability across all sessions within and between days showed that there were similar levels of inclusion of grey and white matter ratios in the voxels of the stimulated and contra-stimulated MI [MCR: 0.722; CI 95% (0.268–0.922), MCL: 0.935; CI 95% (0.825–0.982)], but variance (consistency measurement) was below average standards for the control voxels overlaid onto the occipital cortex [OCC: 0.564; CI 95% (0.147–0.878)].
Inline Supplementary Fig. S6 shows an overlay of all MRS processed data for GABA from all the participants from the contra-lateral to stimulation voxel during sham PAS (no stimulation).
Inline Supplementary Fig. S6 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
Inline Supplementary Fig. S7 shows the changes in MRS voxel spectra for GABA (edited difference), Cr and NAA (edit-off) from a single participant in the stimulated and unstimulated hemispheres across real and sham stimulation, before the fMRI part of the study.
Inline Supplementary Fig. S7 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
The average GABA/Cr and GABA/NAA values across participants are shown in Table 3 below. The 3-way ANOVA (3 voxel locations, 2 sessions (real and sham), 2 time-points (before and after)) showed only a significant effect of Site (P = .018, F = 8.2) for GABA/Cr ratio; whilst there was no major effect or an interaction for the GABA/NAA ratio. For the GABA/Cr ratio, however, in the contralateral to PAS voxels, the ratio seemed visibly larger following real PAS both in the pre fMRI and immediately post fMRI scanning compared to sham PAS session.
Table 3.
Presents the GABA/Cr and GABA/NAA ratios in the stimulated and unstimulated M1 and control site (OCC).
| Stimulated pre | Stimulated post | Contra-stimulated pre | Contra-stimulated post | Control pre | Control post | |
|---|---|---|---|---|---|---|
| GABA/Cr | ||||||
| Real | 0.19 ± 0.01 | 0.17 ± 0.01 | 0.21 ± 0.01 | 0.19 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| Sham | 0.20 ± 0.02 | 0.16 ± 0.02 | 0.175 ± 0.01 | 0.165 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| GABA/NAA | ||||||
| Real | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 |
| Sham | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 |
No significant difference in the ratios was found between the real and sham PAS.
Secondary analysis for MRS data
In this secondary analysis of the MRS dataset we attempted to elucidate the extent of the change in GABA ratios by simulating what would have happened if there had been a 20% drop in GABA under the conditions of real stimulation in the ipsilateral hemisphere. In other words, we wanted to model the likely effect size which would be needed to detect a significant change. This will guide future studies in healthy participants. The results (GABA/Cr) of PAS to the stronger pharyngeal M1 were factor-multiplied (20% power, 0.8) to adjust for the size of the GABA signal being measured and the statistical analysis was repeated. Here, we performed a 3-way rmANOVA (factors: time (pre & post fMRI) × treatment (real & sham) × voxel site).
The results showed a significant interaction of voxel site × treatment (P = .033, F1,9 = 6.33) and a significant effect of voxel site (P = .006, F1,9 = 12.8). Therefore, we proceeded with a 2-way ANOVA, without time as a factor.
Two separate 2-way ANOVAs (one for the pre-fMRI and one for post-fMRI paradigm) were performed. A significant interaction of voxel site × treatment (P = .019, F1,9 = 8) and a significant effect of voxel site (P = .003, F1,9 = 6.5) were observed for the Pre-fMRI GABA/Cr results. The 2-way ANOVA for the post-fMRI paradigm results showed no significant interaction and only significant effects of the factor Voxel Site were significant (P = .046, F1,9 = 5.2).
Therefore, a paired t-test was performed on the pre-fMRI results (or in other words, immediately post neurostimulation) between the different sites. The comparison sham vs. real in the stimulated hemisphere was significant (P = .024, t(9) = 2.7) with the stimulated hemisphere following real stimulation showing 28 ± 4% smaller response than those from the sham arm.
The exact procedures were followed for GABA/NAA ratio. However, no significant interaction or effect was observed for factor-levelled results from stimulated or contra-stimulated hemisphere.
Discussion
To our knowledge, this study is the first to elucidate the neurophysiological changes following PAS applied to the stronger (dominant) representation of pharyngeal M1. In previous studies (Singh et al., 2009; Michou et al., 2012, 2014; Michou et al., 2013) with the use of electro-physiological measurements, we observed an increase in excitability at the stimulated site following PAS. The results from our fMRI study are in accordance with the direction of the changes, since BOLD signal during water and saliva swallowing increased at the site of stimulation, compared to sham PAS. PAS also induced significant increases in BOLD signals in contralateral (to stimulation) hemispheric areas. With the use of MRS, we additionally investigated whether changes in GABA/Cr ratio could provide an underlying neurochemical mechanism. However, these changes in ratios before and after the fMRI procedure were not large enough to see significant effects.
Of interest to the discussion on our findings, is the fact that each of the components of PAS (peripheral electrical stimulation and TMS) have been shown to modulate swallowing as observed in both preclinical and clinical studies. Cerebral stimulation of the caudal pericentral cortex in monkeys can evoke a full swallow sequence (Sumi, 1969; Martin et al., 1999). Whilst single magnetic stimulation pulses cannot evoke the same result in humans, repetitive TMS stimulation, exciting a number of descending volleys from the stimulated area can produce beneficial results on healthy individuals (Jefferson et al., 2009) and stroke patients (Park et al., 2013; Michou et al., 2014; Khedr et al., 2009; Verin and Leroi, 2009). Moreover, studies in animals have shown that stimulating the pharyngeal branch of the glossopharyngeal nerve may induce, or modulate reflexive swallowing (Kitagawa et al., 2002; Ootani et al., 1995), whilst studies with pharyngeal electrical stimulation in healthy humans (Hamdy et al., 1998a,b), acute dysphagic stroke patients (Fraser et al., 2002; Jayasekeran et al., 2010), and in chronic dysphagic stroke patients (Michou et al., 2014), have shown beneficial results on accelerating swallowing recovery.
fMRI activations during water and saliva swallowing following sham PAS
Group results from the sham arm of the study, representing the pure activations during water and saliva swallowing (no intervention, Table 1 in Supplementary files), are in keeping with the previous findings (Soros et al., 2009). In published event-related functional imaging protocols (Martin, 2009; Soros et al., 2009), water and saliva functional tasks have most consistently activated areas including the primary sensorimotor cortex, sensorimotor integration areas, the insula and frontal operculum, the anterior cingulate cortex, and supplementary motor areas (SMAs). Given that differences between water and saliva swallowing have been noted in the literature (Soros et al., 2009; Malandraki et al., 2009; Humbert et al., 2009), we included both these functional tasks in our study to examine whether PAS will show similar effects on both tasks. Although comparison between water and saliva swallowing BOLD changes is not the scope of our study, it is interesting to see that our results are aligned with the results from the activation likelihood meta-analysis by Soros et al. (2009) showing that water swallowing is likely to activate sensorimotor areas, inferior parietal lobes, the insula. By comparison the saliva swallowing activation pattern included activation of premotor areas, right SMA and cingulate gyrus in our study, similar to the clusters with highest activation likelihood in the meta-analysis (Soros et al., 2009). These differences are hypothesised to reflect the additional effort required for initiation and planning of movements for saliva swallowing compared to water swallowing involving stronger muscles contractions.
fMRI activations during water and saliva swallowing following real PAS
Following PAS over the ‘stronger’ pharyngeal M1 we observed bilateral activations of the premotor cortex, the insula, and the motor cortices. During water swallowing, the activations were mostly in bilateral premotor areas, insula and cingulate gyrus. During saliva swallowing following PAS, the premotor cortex was activated bilaterally; whilst the SMA and M1 contralaterally to PAS showed the highest activations.
Our contrast analysis showed that following PAS, the premotor and M1 contralateral to stimulation had higher BOLD change compared to sham PAS, which was further verified by our ROI analysis. For saliva swallowing, bilateral BA6 activation was significantly higher following real PAS compared to sham, as well as activation of the insular areas ipsilateral to PAS. As expected, PAS modified the properties of both saliva and water swallowing networks, which show overlapping but also distinct activation patterns. Therefore it can be suggested that as a noninvasive brain technique, PAS effects are not single-task specific but show signs of ’plasticity transference’ between similar behaviours.
It is of interest to observe that following the sham stimulation, during water swallowing, peak activations were observed in bilateral postcentral gyrus; whilst after real PAS, these activations shifted to precentral gyrus, premotor areas and the insular areas bilaterally. This activation of the premotor areas was similar for the saliva swallowing following real PAS, but the peak activation during the saliva swallowing task was in the insula. It is not clear whether this is a reflection of metaplasticity in the swallowing cortical network or a shift in brain patterns as a response to stimulation, but would support the notion that PAS could remotely alter cortical networks in a functional manner.
Previously published results of an fMRI study (Fraser et al., 2002) following only pharyngeal electrical stimulation in healthy participants, showed that compared to sham, there was a bilateral sensorimotor activation with real stimulation. Here the changes following PAS were shown to be of dynamic nature and are different to the effects following the application of only the peripheral components of PAS observed previously. This is probably due to the fact that PAS provides a different dynamic input to swallowing network. PAS depends on the timely delivery of the input and on the communication between M1 and sensory cortex (S1). The first (peripheral) stimulus can evoke a motor evoked potential from M1 via activation of S1 (Gow et al., 2004). Conditioning the activation of M1 by the electrical pharyngeal stimulus with a TMS pulse on pharyngeal M1 provides a further dynamic stimulus.
The aforementioned dynamic M1 S1 inter-hemispheric communication following PAS and the subsequent shifts of peak activations to bilateral areas and insular areas observed in our fMRI study are important when considering the evidence on the dynamic cluster activations of the swallowing neural network. Earlier studies on effective connectivity of the swallowing network with principal component analysis (Mosier and Bereznaya, 2001) showed that in taking the positive BOLD clusters during swallowing, the cerebral and the insular with cerebellum clusters were interconnected through the sensorimotor–cingulate cluster. Recently, Mihai et al. (2014) using dynamic causal modelling examined the potential effective connectivity of areas such as SMA, M1 S1 and insula and showed that there is high probability that following an input delivered to either or both SMA and M1S1 simultaneously, there are bidirectional connections of the two regions for swallowing. With the dynamic causal modelling, they also observed that there is high probability that the insula receives information from M1 S1 in either an unidirectional or bidirectional manner. Accepting that more research is required to elucidate the connectivity of swallowing network, it is still interesting to see that after PAS there was an increased activation in the insular areas during swallowing tasks compared to sham PAS. There are few imaging studies showing the highest connected clusters of activations during swallowing are larger from the insula compared to other regions (Babaei et al., 2013; Lowell et al., 2012). We observed that PAS can modulate the BOLD signal in this area and induce significantly larger activations during swallowing following real PAS compared to sham.
Of interest to the swallowing network, is the shift of activations to the premotor areas following real PAS. Premotor cortices are important in swallowing for motor planning and execution as previously highlighted (Hamdy et al., 1999; Kern et al., 2001a,b; Martin et al., 2001, 2004, 2007; Mosier and Bereznaya, 2001; Mosier et al., 1999; Suzuki et al., 2003; Toogood et al., 2005). Mosier and Bereznaya (2001) showed that there are positive connections between premotor and motor cortices. Mihai et al. (2014) with temporal analysis of the fMRI study showed that the activations start at the premotor cortex, followed by SMA and M1. Hamdy et al. (1999) proposed that the premotor cortex is more likely to modulate the pharyngoesophageal components of the swallowing act. There is also extensive data for the connections between the premotor cortices and the insula (as well as somatosensory areas) (Augustine, 1996). The shift of activation to the premotor areas is of particular interest with respect to motor impairments as well. Impairments of the basic functions of premotor cortex (dorsal) such as impairments in motor planning, decision-making and execution were related to decreased premotor cortex dorsal and M1 connectivity (Hoshi and Tanji, 2007). Interestingly, a study with magnetoencephalography (MEG) in dysphagic and non-dysphagic Parkinson's patients showed that the non-dysphagic patients showed a shift of activation towards lateral motor and premotor areas at movement initiation and suggested that this premotor activation may be a compensatory mechanism for swallowing function (Suntrup et al., 2013). In the absence of imaging studies with PAS on dysphagic stroke patients, we can only tentatively suggest that the mechanism underlying the changes seen previously in stroke patients with PAS (Michou et al., 2012, 2014) were due to changes in premotor cortex–M1 communication combined with activation of insular areas, enabling better motor planning and execution of the swallow. Future studies will delineate whether this activity shift to the premotor cortices and insula is one of the potential mechanisms for beneficial changes in swallowing and dysphagia rehabilitation.
Of interest to the clinical application of PAS, we previously observed that PAS applied contralateral to a ‘virtual lesion’ induced with 1 Hz rTMS in a healthy group (PAS to the non-lesioned uninhibited site), in-creased M1 excitability bilaterally, and produced changes in behavioural responses to a swallowing reaction task (Michou et al., 2012). In addition, PAS applied to the pharyngeal M1 representation contralateral to lesioned hemispheric site in dysphagic stroke patients (Michou et al., 2012, 2014), resulted into beneficial changes of swallowing safety of dysphagic stroke patients, whilst group cortical excitability was increased bilaterally (over pharyngeal M1) compared to sham. Here we observed that the changes induced following real PAS resulted in contralateral premotor and M1 activations for water swallowing and bilateral BA6 for saliva. Therefore our data is aligned and corroborate the results observed previously with electrophysiological measurements for the bilateral interactions. The findings support the notion of “modulability” of PAS to engage transcallosal plasticity interactions between hemispheres in a synergetic rather than a competitive manner between hemispheres with PAS.
MRS changes following real and sham PAS
The results from our MRS experiment following real and sham PAS are more challenging to explain when placed in conjunction with the fMRI results. Our secondary analysis illustrating the likely effect size which would be needed to detect a significant change showed that the study did not have the fully required power. Nonetheless, it is interesting to observe that real PAS induced a change in GABA/Cr ratios compared to sham. In this proof of concept study, we observed an increase in the GABA/Cr ratio at the contralateral to PAS site immediately following real PAS and following the fMRI experiment.
Previously, it was shown that PAS applied to pharyngeal M1 resulted in reduction in glutamate ipsilateral to stimulation, not seen following sham PAS (Singh et al., 2009). Maximal reductions were seen immediately and diminished over the 2-hour follow-up period. This reduction was suggested to be an indication of Glu's role in activating the postsynaptic NMDA receptor of an already depolarized membrane as a result of PAS. This supported earlier reports for long-term potentiation (LTP) like mechanisms in inducing PAS effects.
Given the fine regulatory balance between the major inhibitory and excitatory neurotransmitters in the brain, here we were interested in extending the previous knowledge for PAS with Glu changes, with the quantification of GABA. However, comparisons between this study and previous are proven difficult since the Singh et al. (2009) study only the stimulated side was investigated with MRS. Perhaps the increase in GABA in the contralateral M1 voxel that we observed, might balance the drop in Glu in the stimulated M1 voxel observed previously and corroborate the notion that the underlying mechanism for the efficacy of PAS is LTP. The complementary results from our fMRI study, as discussed previously, showing transcallosal interactions for the effects of PAS, add credibility to this suggestion. However, this remains speculative in the absence of more definitive experiments.
Following power simulation we then observed change in the GABA/Cr in the ipsilateral to stimulation voxel (assuming a depletion of GABA by less than 20%). We were then able to see some evidence for GABAergic changes to PAS with MRS. Therefore, at this point in time we can only infer that if GABA is associated with the plastic changes following PAS, this change must be less than 20%. Perhaps, this inadequacy falls within the limitations of MRS at 3 T, which can be easily understood since the voxel size for the MRS over the M1 is large (3.2 × 3.2 × 3.2 cm) and there is high probability that not all the synapses in this area are responsible for eliciting plastic changes.
GABAergic neurotransmission is fundamental for providing a tight temporal code used by each neuron, or groups of neurons, in order to perform sophisticated computational operations (Méndez and Bacci, 2011). However, the underlying mechanisms for GABAergic plasticity are not yet completely understood. This is probably due to the diversity of inhibitory neurons embedded in cortical circuits and their equal heterogeneity of synaptic properties (Bartos et al., 2010; Kullmann and Lamsa, 2011; Kawaguchi and Kubota, 1997,1998). Any differences in GABA may reflect the individual differences in the number of GABA interneurons in certain regions, the difference in GABA concentrations per synapse or the number of synapses per neuron (Sumner et al., 2010).
Modulation of GABA with training of a novel task or after neuro-stimulation with and without training has been observed in the literature. Earlier studies showed that motor practice can increase cortico-motor excitability whilst GABA inhibition is reduced (Bütefisch et al., 2000). A number of recent studies investigated the relationship between in vivo GABA concentrations and individual's task performance to complete reaction time tasks (Boy et al., 2010; Sumner et al., 2010; Stagg et al., 2011) with MRS; whilst others used the paired pulse TMS paradigm of intracortical inhibition (ICI) to elucidate the effects (Kujirai et al., 1993; Coxon et al., 2014). Decreases in GABA appear to follow a ‘learning’ intervention (Floyer-Lea et al., 2006) and provided some insight into neurochemical factors for training and behaviour. Lastly, Stagg et al. (2011) showed a close link between anodal transcranial direct current stimulation (tDCS) and a reduction in GABA concentration with the ability of the participants to learn a novel task. The last group reported GABA/NAA changes of approximately 10–20%, similar to the simulated power analysis we performed on our dataset for GABA/Cr; however we did not observe any change in the GABA/NAA ratio either with or without PAS. All the novel motor tasks or the combined neurorehabilitation paradigms (i.e., tDCS) in the above studies differ significantly to PAS in our study. Nonetheless, given that changes in GABA following training or neurostimulation combined with training could be of clinical relevance for rehabilitation, future studies with PAS should probably include a motor training paradigm to examine and elucidate whether GABA and Glu changes are important for the changes in both functional activation.
There are limitations in our study. Our sample size was small and this might be one of the reasons for the underpowered results in MRS experiment. Similarly, the subjects had to complete 9 swallows of water and 9 swallows of saliva and 9 rest conditions, which is a number comparable to earlier imaging studies in swallowing but is still a small number of swallows. Nevertheless our results are aligned to results from other neuroimaging studies on the brain's swallowing network. One of the major reasons for the decision to complete the fMRI experiment within 14 min was the fact that we were looking into capturing the changes following PAS within a specific time-window based on the previous evidence we have gathered with electrophysiological measurements (Michou et al., 2012, 2014). Moreover, we only measured GABA in this study. It would have been more helpful to examine the concurrent changes of GABA and Glu. The scanning parameters in terms of voxel size and Echo Time (TE) and Repetition Time (TR) differ for the reliable quantification of GABA and Glu. The 1H spectrum optimised for detecting GABA was acquired by a MEGA-PRESS sequence (Rothman et al., 1984; Mescher et al., 1998) with the following parameters: TE/TR=68/2000 ms and a voxel of 3.2 ×3.2 × 3.2 cm. It has been reported in the literature that PRESS with TE ~ 30 ms achieves reliable Glu detections (Mullins et al., 2008; Hancu, 2009). Therefore, at the time of the initiation of the study, we decided to review only GABA. However, since then some groups have proposed that both neurotransmitters can be measured reliably whilst scanning with the TE of 68 ms (O'Gorman et al., 2011). Lastly, the experimental protocol would be empowered by adding imaging protocols before and after real and sham PAS on both days. However, this would have increased the complexity of the current study but in view of the results we have gathered here, such an experimental design would be a candidate for future studies, together with the combination of PAS with swallowing training, as discussed above.
Conclusions
We can conclude that PAS induced functional alterations in both targeted ipsilateral and contralateral areas of the swallowing network in health. Such changes highlight the functionality of transcallosal interactions in the swallowing network. The prominent shift of peak activations to the premotor areas and the increase in the activations in the insula during saliva and water swallowing were particularly interesting findings in our study, showing that PAS had an effect on a swallowing network level and not only on targeted M1. GABA changes were observed in the real PAS arm and are indicating that GABA neurotransmission might hold a role in the induction of changes. Our results from the “simulation for the power” approach provide the baseline for properly designed future studies for imaging the effects of neurostimulation on swallowing network with MRS and fMRI.
Supplementary Material
Acknowledgments
This study applies tools developed under NIH R01 EB016089 and P41 EB015909; RAEE also receives salary support from these grants. We would like to acknowledge Mr Barry Whitnall and Mr Neal Sherratt for their contributions during the studies.
Abbreviations
- PAS
paired associative stimulation
- M1
motor cortex
- TMS
transcranial magnetic stimulation
- MRS
magnetic resonance spectroscopy
- GABA
γ-aminobutyric acid
- NAA
N-acetylaspartate
- Cr
creatine
- Cho
choline
- OCC
occipital cortex
- PMEPs
pharyngeal motor evoked potentials
- MT
motor threshold
- PES
pharyngeal electrical stimulation
- GE-EPI
gradient echo planar imaging
- TR
repetition time
- FOV
field of view
- MPM
maximum probability maps
- HLSVD
Hankel Lanczos singular value decomposition
- HRF
hemodynamic response function
- eoi
effects of interest
- ANOVA
analysis of variance
- SMA
supplementary motor area
- rTMS
repetitive transcranial magnetic stimulation
- ACC
adjacent cingulate gyrus
- GM/WM
grey matter/white matter
- NMDA
N-methyl-d-aspartate receptor
Footnotes
Conflict of interest
Nothing to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.05.007.
References
- Arckens L, Schweigart G, Qu Y, Wouters G, Pow DV, Vandesande F, Eysel UT, Orban GA. Cooperative changes in GABA, glutamate and activity levels: the missing link in cortical plasticity. Eur. J. Neurosci. 2000 Dec;12(12):4222–4232. doi: 10.1046/j.0953-816x.2000.01328.x. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Brain Res. Rev. 1996 Oct;22(3):229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Babaei A, Ward BD, Siwiec RM, Ahmad S, Kern M, Nencka A, Li SJ, Shaker R. Functional connectivity of the cortical swallowing network in humans. NeuroImage. 2013 Aug 1;76:33–44. doi: 10.1016/j.neuroimage.2013.01.037. http://dx.doi.org/10.1016/j.neuroimage.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Alle H, Vida I. Role of microcircuit structure and input integration in hippocampal interneuron recruitment and plasticity. Neuropharmacology. 2010;60(5):730–737. doi: 10.1016/j.neuropharm.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr. Biol. 2010 Oct 12;20(19):1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley S, Croser D, Cottrell J, Creevy M, Teo E, Yiu D, Pathi R, Taylor J, Thompson PD. Predictors of prolonged dysphagia following acute stroke. J. Clin. Neurosci. 2003 May;10(3):300–305. doi: 10.1016/s0967-5868(03)00022-5. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc. Natl. Acad. Sci. U. S. A. 2000 Mar 28;97(7):3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel-Lacanal E, Marque P, Tardy J, et al. Induction of cortical plastic changes in wrist muscles by paired associative stimulation in the recovery phase of stroke patients. Neurorehabil. Neural Repair. 2009;23(4):366–372. doi: 10.1177/1545968308322841. [DOI] [PubMed] [Google Scholar]
- Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A, Kunesch E. Paired associative stimulation. Suppl. Clin. Neurophysiol. 2004;57:563–569. [PubMed] [Google Scholar]
- Coxon JP, Peat NM, Byblow WD. Primary motor cortex disinhibition during motor skill learning. J. Neurophysiol. 2014 Jul 1;112(1):156–164. doi: 10.1152/jn.00893.2013. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL, Iglesia GC, Sullivan MA. Lesion site in unilateral stroke patients with dysphagia. J. Stroke Cerebrovasc. Dis. 1996 Sep-Oct;6(1):30–34. doi: 10.1016/s1052-3057(96)80023-1. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005 May 1;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol. 2006 Mar;95(3):1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, Tyrell P, Hobson A, Williams S, Thompson D. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002 May 30;34(5):831–840. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol. 2004;174(I–VIII):1–89. doi: 10.1007/978-3-642-18910-4. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Bürgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996 Aug 29;382(6594):805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Gow D, Hobson AR, Furlong P, Hamdy S. Characterising the central mechanisms of sensory modulation in human swallowing motor cortex. Clin. Neurophysiol. 2004;115(10):2382–2390. doi: 10.1016/j.clinph.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat. Neurosci. 1998a May;1(1):64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, Tallis RC, Thompson DG. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998b Nov;115(5):1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am. J. Physiol. 1999 Jul;277(1 Pt 1):G219–G225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- Hammers A, Koepp MJ, Richardson MP, Hurlemann R, Brooks DJ, Duncan JS. Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients. Brain. 2003 Jun;126(Pt 6):1300–1318. doi: 10.1093/brain/awg138. [DOI] [PubMed] [Google Scholar]
- Hancu I. Optimized glutamate detection at 3 T. J. Magn. Reson. Imaging. 2009 Nov;30(5):1155–1162. doi: 10.1002/jmri.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr. Opin. Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, Hind J, Robbins J. Neurophysiology of swallowing: effects of age and bolus type. NeuroImage. 2009 Feb 1;44(3):982–991. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G, Stinear JW. Contralesional paired associative stimulation increases paretic lower limb motor excitability post-stroke. Exp. Brain Res. 2008 Mar;185(4):563–570. doi: 10.1007/s00221-007-1183-x. [DOI] [PubMed] [Google Scholar]
- Jayasekeran V, Singh S, Tyrrell P, Michou E, Jefferson S, Mistry S, Gamble E, Rothwell J, Thompson D, Hamdy S. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010 May;138(5):1737–1746. doi: 10.1053/j.gastro.2010.01.052. [DOI] [PubMed] [Google Scholar]
- Jefferson S, Mistry S, Michou E, Singh S, Rothwell JC, Hamdy S. Reversal of a virtual lesion in human pharyngeal motor cortex by high frequency contralesional brain stimulation. Gastroenterology. 2009 Sep;137(3):841–849. doi: 10.1053/j.gastro.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum. Brain Mapp. 2006 Oct;27(10):779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex. 1997;7(6):476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85(3):677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2001a Mar;280(3):G354–G360. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am. J. Physiol. Gastrointest. Liver Physiol. 2001b Apr;280(4):G531–G538. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol. Scand. 2009 Mar;119(3):155–161. doi: 10.1111/j.1600-0404.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa J, Shingai T, Takahashi Y, Yamada Y. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002 May;282(5):R1342–R1347. doi: 10.1152/ajpregu.00556.2001. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J. Physiol. 1993 Nov;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. LTP and LTD in cortical GABAergic interneurons: emerging rules and roles. Neuropharmacology. 2011;60(5):712–719. doi: 10.1016/j.neuropharm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Laufs H, Richardson MP, Salek-Haddadi A, Vollmar C, Duncan JS, Gale K, Lemieux L, Löscher W, Koepp MJ. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology. 2011 Aug 30;77(9):904–910. doi: 10.1212/WNL.0b013e31822c90f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Luo C, Yu B, Yan B, Gong Q, He C, He L, Huang X, Yao D, Lui S, Tang H, Chen Q, Zeng Y, Zhou D. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J. Neurol. Neurosurg. Psychiatry. 2009 Dec;80(12):1320–1329. doi: 10.1136/jnnp.2009.176214. [DOI] [PubMed] [Google Scholar]
- Lowell SY, Reynolds RC, Chen G, Horwitz B, Ludlow CL. Functional connectivity and laterality of the motor and sensory components in the volitional swallowing network. Exp. Brain Res. 2012 May;219(1):85–96. doi: 10.1007/s00221-012-3069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum. Brain Mapp. 2009 Oct;30(10):3209–3226. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RE. Neuroplasticity and swallowing. Dysphagia. 2009 Jun;24(2):218–229. doi: 10.1007/s00455-008-9193-9. [DOI] [PubMed] [Google Scholar]
- Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis) J. Neurophysiol. 1999 Sep;82(3):1529–1541. doi: 10.1152/jn.1999.82.3.1529. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J. Neurophysiol. 2001 Feb;85(2):938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J. Neurophysiol. 2004 Oct;92(4):2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- Martin R, Barr A, MacIntosh B, Smith R, Stevens T, Taves D, Gati J, Menon R, Hachinski V. Cerebral cortical processing of swallowing in older adults. Exp. Brain Res. 2007 Jan;176(1):12–22. doi: 10.1007/s00221-006-0592-6. [DOI] [PubMed] [Google Scholar]
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005 Dec;36(12):2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- Mazaika PK, et al. Methods and software for fMRI analysis of clinical subjects. NeuroImage. 2009;47(Suppl. 1) [Google Scholar]
- McKay DR, Ridding MC, Thompson PD, Miles TS. Induction of persistent changes in the organisation of the human motor cortex. Exp. Brain Res. 2002;143(3):342–349. doi: 10.1007/s00221-001-0995-3. [DOI] [PubMed] [Google Scholar]
- Méndez P, Bacci A. Assortment of GABAergic plasticity in the cortical interneuron melting pot. Neural Plast. 2011:976856. doi: 10.1155/2011/976856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Michou E, Hamdy S. Neurostimulation as an approach to dysphagia rehabilitation: current evidence. Curr. Phys. Med. Rehabil. Rep. 2013 Dec;1(4):257–266. [Google Scholar]
- Michou E, Mistry S, Jefferson S, et al. Targeting unlesioned pharyngeal motor cortex improves swallowing in healthy individuals and after dysphagic stroke. Gastroenterology. 2012 Jan;142(1):29–38. doi: 10.1053/j.gastro.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michou E, Mistry S, Rothwell J, Hamdy S. Priming pharyngeal motor cortex by repeated paired associative stimulation: implications for dysphagia neurorehabilitation. Neurorehabil. Neural Repair. 2013 May;27(4):355–362. doi: 10.1177/1545968312469837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michou E, Mistry S, Jefferson S, Tyrrell P, Hamdy S. Characterizing the mechanisms of central and peripheral forms of neurostimulation in chronic dysphagic stroke patients. Brain Stimul. 2014 Jan-Feb;7(1):66–73. doi: 10.1016/j.brs.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihai PG, Otto M, Platz T, Eickhoff SB, Lotze M. Sequential evolution of cortical activity and effective connectivity of swallowing using fMRI. Exp. Brain Res. 2014 Dec;35(12):5962–5973. doi: 10.1002/hbm.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp. Brain Res. 2001 Oct;140(3):280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR Am J. Neuroradiol. 1999 Sep;20(8):1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn. Reson. Med. 2008 Oct;60(4):964–969. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O'Gorman RL, Puts NA, Vidyasagar R, Evans CJ. Cardiff Symposium on MRS of GABA, Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014 Feb 1;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J. Magn. Reson. Imaging. 2011 May;33(5):1262–1267. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootani S, Umezaki T, Shin T, Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res. Bull. 1995;37(4):397–404. doi: 10.1016/0361-9230(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Park JW, Oh JC, Lee JW, Yeo JS, Ryu KH. The effect of 5Hz high-frequency rTMS over contralesional pharyngeal motor cortex in post-stroke oropharyngeal dysphagia: a randomized controlled study. Neurogastroenterol Motil. 2013 Apr;25(4):324–e250. doi: 10.1111/nmo.12063. [DOI] [PubMed] [Google Scholar]
- Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012 Jan;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc. Natl. Acad. Sci. U. S. A. 1993 Jun 15;90(12):5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Mistry S, Jefferson S, et al. A magnetic resonance spectroscopy study of brain glutamate in a model of plasticity in human pharyngeal motor cortex. Gastroenterology. 2009;136(2):417–424. doi: 10.1053/j.gastro.2008.10.087. [DOI] [PubMed] [Google Scholar]
- Soros P, Inamoto Y, Martin R. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum. Brain Mapp. 2009;30:2426–2439. doi: 10.1002/hbm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R, Woolrich M, Near J, Johansen-Berg H, Rothwell JC. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. 2011 Dec 1;589(Pt 23):5845–5855. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J. Physiol. 2002;543(Pt 2):699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan D, Di Cesare F, Andrasescu A, Popa E, Lazariev A, Vescovo E, Graveron-Demilly D. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas. Sci. Technol. 2009;20(10):104035. [Google Scholar]
- Sumi T. Some properties of cortically-evoked swallowing and chewing in rabbits. Brain Res. 1969 Sep;15(1):107–120. doi: 10.1016/0006-8993(69)90313-8. [DOI] [PubMed] [Google Scholar]
- Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat. Neurosci. 2010 Jul;13(7):825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- Suntrup S, Teismann I, Bejer J, Suttrup I, Winkels M, Mehler D, Pantev C, Dziewas R, Warnecke T. Evidence for adaptive cortical changes in swallowing in Parkinson's disease. Brain. 2013 Mar;136(Pt 3):726–738. doi: 10.1093/brain/awt004. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003 Spring;18(2):71–77. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- Teismann IK, Suntrup S, Warnecke T, Steinsträter O, Fischer M, Flöel A, Ringelstein EB, Pantev C, Dziewas R. Cortical swallowing processing in early subacute stroke. BMC Neurol. 2011 Mar 11;11:34. doi: 10.1186/1471-2377-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp. Brain Res. 2005 Feb;161(1):81–90. doi: 10.1007/s00221-004-2048-1. [DOI] [PubMed] [Google Scholar]
- van den Boogaart A, van Ormondt D, Pijnappel WWF, De Beer R, Ala-Korpela M. Removal of the water resonance from 1H magnetic resonance spectra. In: McWhirter JG, editor. Mathematics in Signal Processing III. Oxford, UK: Clarendon Press; 1994. pp. 175–195. [Google Scholar]
- Verin E, Leroi AM. Poststroke dysphagia rehabilitation by repetitive transcranial magnetic stimulation: a noncontrolled pilot study. Dysphagia. 2009 Jun;24(2):204–210. doi: 10.1007/s00455-008-9195-7. [DOI] [PubMed] [Google Scholar]
- Waddell KW, Avison MJ, Joers JM, Gore JC. A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn. Reson. Imaging. 2007;25(7):1032–1038. doi: 10.1016/j.mri.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.