Abstract
IMPORTANCE
Interstitial lung abnormalities have been associated with decreased six-minute walk distance, diffusion capacity for carbon monoxide and total lung capacity; however to our knowledge, an association with mortality has not been previously investigated.
OBJECTIVE
To investigate whether interstitial lung abnormalities are associated with increased mortality.
DESIGN, SETTING, POPULATION
Prospective cohort studies of 2633 participants from the Framingham Heart Study (FHS) (CT scans obtained 9/08–3/11), 5320 from the Age Gene/Environment Susceptibility (AGES)-Reykjavik (recruited 1/02–2/06), 2068 from COPDGene (recruited 11/07–4/10), and 1670 from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) (between 12/05–12/06).
EXPOSURES
Interstitial lung abnormality status as determined by chest CT evaluation.
MAIN OUTCOMES AND MEASURES
All cause mortality over approximately 3 to 9 year median follow up time. Cause-of-death information was also examined in the AGES-Reykjavik cohort.
RESULTS
Interstitial lung abnormalities were present in 177 (7%) of the participants from FHS, 378 (7%) from AGES-Reykjavik, 156 (8%) from COPDGene, and in 157 (9%) from ECLIPSE. Over median follow-up times of ~3–9 years there were more deaths (and a greater absolute rate of mortality) among those with interstitial lung abnormalities compared to those without interstitial lung abnormalities in each cohort; 7% compared to 1% in FHS (6% difference, 95% confidence interval [CI] 2%, 10%), 56% compared to 33% in AGES-Reykjavik (23% difference, 95% CI 18%, 28%), 16% compared to 11% in COPDGene (5% difference, 95% CI −1%, 11%) and 11% compared to 5% in ECLIPSE (6% difference, 95% CI 1%, 11%). After adjustment for covariates, interstitial lung abnormalities were associated with an increase in the risk of death in the FHS (HR=2.7, 95% CI, 1.1–65, P=0.030), AGES-Reykjavik (HR 1.3, 95% CI 1.2–1.4, P<0.001), COPDGene (HR=1.8, 95% CI, 1.1, 2.8, P=0.014), and ECLIPSE (HR=1.4, 95% CI, 1.1–2, P=0.022) cohorts. In the AGES-Reykjavik cohort the higher rate of mortality could be explained by a higher rate of death due to respiratory disease, specifically pulmonary fibrosis.
CONCLUSIONS AND RELEVANCE
In four separate research cohorts, interstitial lung abnormalities were associated with a higher risk of all-cause mortality. The clinical implications of this association require further investigation.
Keywords: Idiopathic pulmonary fibrosis, interstitial lung disease, interstitial lung abnormalities (ILA), undiagnosed, subclinical
Interstitial lung abnormalities are defined as specific patterns of increased lung density noted on chest computed tomography (CT) scans identified in participants with no prior history of interstitial lung disease. In studies of adults, interstitial lung abnormalities are present in ~2–10% of research participants,1–7 and 7% of a general population sample,6 and are associated with reductions in lung capacity,1,2,6 exercise capacity,8 gas exchange,5,6 and genetic abnormalities6,9 common to patients with familial interstitial pneumonia and idiopathic pulmonary fibrosis (IPF).10 These data suggest that interstitial lung abnormalities may, in some cases, represent an early and/or mild form of pulmonary fibrosis.
While radiologic abnormalities, worsening pulmonary function and decreased exercise tolerance are important diagnostic features of IPF,11 the most common and severe form of pulmonary fibrosis,12 IPF is associated with a high mortality rate.13–14 Although the survival rate of people with IPF appears to have increased slightly in recent years,14 the previously published median survival after the time of diagnosis is 3–5 years,13–14 which is worse than that of most malignancies.15 Given the other correlations between IPF and interstitial lung abnormalities, we hypothesized that with the presence of interstitial lung abnormalities would be associated with an increased rate of mortality.
METHODS
Study Design and Mortality Ascertainment
Protocols for participant enrollment and phenotyping in the Framingham Heart Study (FHS), the Age Gene/Environment Susceptibility (AGES)-Reykjavik study, the Genetic Epidemiology of COPD Study (COPDGene), and the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) study have been described previously.2,6,16,17 In all cohorts race was self-reported, based on fixed categories. Analyses were adjusted for race, given the known influence of race on mortality in other pulmonary diseases. In all cohorts, mortality refers to all-cause mortality unless otherwise indicated. Informed consent was obtained from all participants. The institutional review boards of the Brigham and Women’s Hospital and individual participating centers approved this study.
The FHS is a longitudinal study originally designed to identify risk factors for cardiovascular disease in the general population18. The AGES-Reykjavik study is a longitudinal birth cohort from the Reykjavik Study (established in 1967) that now includes men and women born in Reykjavik between 1907–1935 who are followed by the Icelandic Heart Association. COPDGene is a multicenter longitudinal study designed to identify the epidemiologic and genetic risk factors for COPD. Participants with known active lung diseases other than asthma, emphysema, or COPD were excluded.19 For this analysis COPDGene refers to the first 2508 participants.2 ECLIPSE is a multicenter and multinational 3-year observational study of 2164 COPD patients (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stages 2–4)20 and 582 controls aged 40–75.21 Participants with known respiratory disorders other than COPD were excluded.21 For these analyses, only the 2,164 COPD participants from ECLIPSE were included because longitudinal mortality data from control participants was not collected (see eMethods for further details on cohort study design).
Chest CT Analysis
The methods for chest CT characterization for interstitial lung abnormalities in the FHS and COPDGene cohorts have been reported previously2,6 and were used to characterize interstitial lung abnormalities in AGES-Reykjavik and ECLIPSE (see eMethods). In all cohorts, the chest CT scans were evaluated by up to three readers (two chest radiologists and one pulmonologist) using a sequential reading method, as previously described.22 Interstitial lung abnormalities were defined as particular patterns of increased lung density that are not limited to the posterior regions of the lung, including ground-glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing or traction bronchiectasis, affecting more than 5% of any lung zone (see eFigure 1). Chest CT images with focal or unilateral ground-glass attenuation, focal or unilateral reticulation or patchy ground glass abnormalities (<5%) were considered indeterminate (see eFigure 2). To explore the association between undiagnosed pulmonary fibrosis and mortality, an additional subset of interstitial lung abnormalities with pulmonary parenchymal architectural distortion diagnostic of fibrotic lung disease (definite fibrosis, see Figure 1A–D) was created.6 Quantitative total lung volume and emphysema (percentage below −950 Hounsfield units) where reported were measured with Airway Inspector (www.airwayinspector.org) as previously described.23 Coronary artery calcium (CAC) scores were calculated using the traditional Agatston scoring method.24
Figure 1.

Chest computed tomographic (CT) images of four participants, one from each cohort with interstitial lung abnormalities with Definite Fibrosis. Definite Fibrosis is defined as pulmonary parenchymal architectural distortion diagnostic of fibrotic lung disease. Each letter represents a different participant (A from FHS, B from AGES-Reykjavik, C from COPDGene, and D from ECLIPSE), in all panels images 1–3 are axial images, image 1 is at the level of the carina, image 2 is at the level of the right inferior pulmonary vein and image 3 is at the base of the lungs.
Statistical Analyses
In all cohorts except the FHS, association analyses between pairs of variables were conducted with Fisher’s exact tests (for categorical variables) and two-tailed t-tests (for continuous variables). In the FHS, all analyses accounted for familial relationships using generalized estimating equations as previously described.25 To evaluate the association between interstitial lung abnormalities and mortality logistic regression was used (for absolute mortality) and Cox proportional hazards models (for time-to-mortality) with robust standard errors to account for familial correlation in FHS. In Cox models, all variables were assessed, and none violated the proportional hazards assumption. Multivariable models included adjustments for age, race, gender, body-mass index (BMI), pack-years of smoking, smoking status (current vs. former), Global initiative for chronic Obstructive Lung Disease (GOLD) stage (where available). Additional covariates used included measures of coronary artery disease (CAD) (self-report of [or adjudicated in the FHS] CAD as well as CAC scores) and history of self-reported non-dermatologic malignancy. In the COPD cohorts, additional analyses were done using the Body-mass index, air flow Obstruction, Dyspnea and Exercise (BODE) index26, as an alternative measure of COPD severity. All p-values reported are two-sided and a level of 0.05 was considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC) was used for analyses in the AGES-Reykjavik, COPDGene and ECLIPSE cohorts and R version 3.1.3 was used for analyses in the FHS.
RESULTS
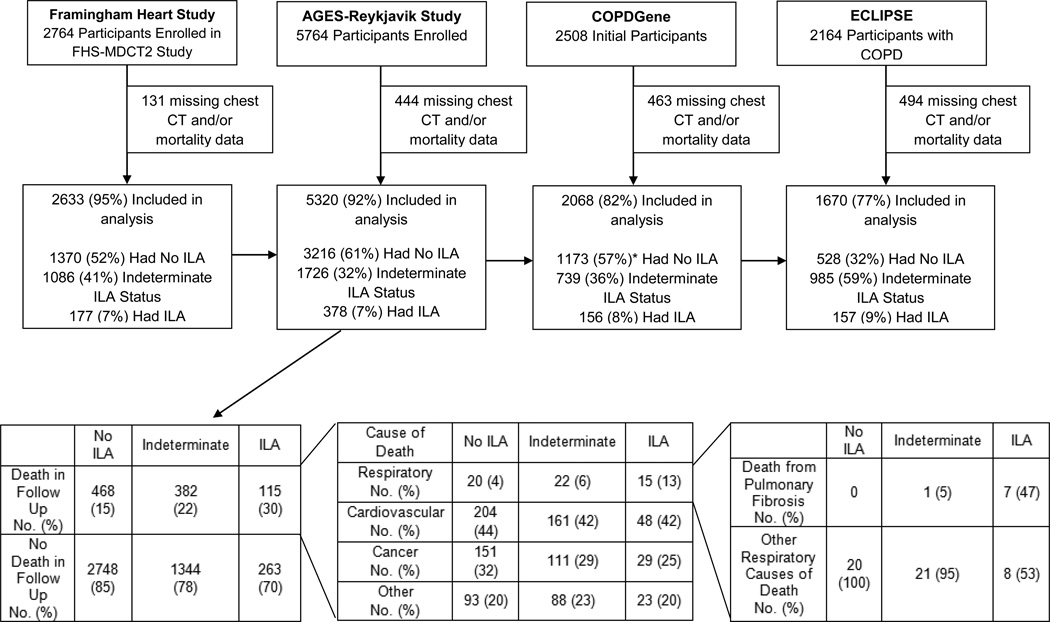
In the FHS, of the 2764 participants from the FHS-MDCT2 study between September 2008 and March 2011, 2633 (95%) had chest CT and mortality reports as of December 2013 (median follow-up time of 4.0 years) and were included. In AGES-Reykjavik, of the 5764 participants recruited between January 2002 and February 2006, 5320 (92%) had chest CT and mortality data as of December 2013 (median follow up time of 8.9 years) and were included. Additionally, cause-of-death data obtained from death certificates (ICD9 and ICD10 codes, see eMethods), was collected in December 2009 (median follow up time of 5.3 years). In COPDGene, of the first 2508 participants recruited between November 2007 and April 2010, 2068 (82%) had chest CT and mortality information as of October 2015 (median follow-up time of 6.5 years) and were included. In ECLIPSE, of the 2,164 participants recruited between December 2005 and December 2006, 1670 (77%) had both chest CT and mortality information (median follow-up time of 2.9 years) and were included.
Interstitial Lung Abnormality Prevalence
The prevalence of participants with interstitial lung abnormalities, indeterminate interstitial lung abnormality status, and without interstitial lung abnormalities in the FHS6 and COPDGene cohorts2 have been previously reported and similar percentages were noted in these subsets (in the FHS, interstitial lung abnormalities were present in 177 [7%], 1086 [41%] had indeterminate status and 1370 [52%] did not have interstitial abnormalities; in COPDGene, 156 [8%] had interstitial lung abnormalities, 739 36%] were indeterminate and 1173 57%] did not have interstitial lung abnormalities, see Table 1 and Figure 2). In the AGES-Reykjavik cohort, interstitial lung abnormalities were present in 378 (7%), 1726 (32%) had indeterminate status and 3216 (61%) had no interstitial lung abnormalities (see Table 1 and Figure 2). In ECLIPSE, interstitial lung abnormalities were present in 157 (9%), 985 (59%) had indeterminate status, and 528 (32%) had no interstitial lung abnormalities (see Table 1 and Figure 2). Additional results about reading methodology are included in the eResults.
Table 1.
Baseline characteristics of participants from Framingham Heart, COPDGene and ECLIPSE studies stratified by Interstitial Lung Abnormality (ILA) status.a
| Variable | Framingham Heart Study | AGES-Reykjavik | COPDGene | ECLIPSE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No ILA (1370) (52%)b |
ILA (177) (7%)b |
P-value | No ILA (3216) (60%)b |
ILA (378) (7%)b |
P-value | No ILA (1173) (57%)b |
ILA (156) (8%)b |
P-value | No ILA (528) (32%)b |
ILA (157) (9%)b |
P- value |
|
| Age – yr, mean (SD) | 56 (11) | 70 (12) | <.001 | 76 (5) | 78 (6) | <.001 | 60 (9) | 64 (9) | <.001 | 62 (7) | 64 (8) | <.001 |
| Female Sex – no. (%) | 675 (49) | 89 (50) | 0.81 | 1910 (59) |
172 (45) | <.001 | 564 (48) | 80 (51) | 0.50 | 182 (34) | 41 (26) | 0.05 |
| Race – no. white (%) | 1370 (100) |
177 (100) |
N/A | 3216 (100) |
378 (100) |
N/A | 929 (79) | 112 (72) | 0.04 | 515 (98) | 154 (98) | 1.0 |
| Body Mass Index, mean (SD) |
29 (6) | 28 (5) | 0.38 | 27 (4) | 27 (5) | 0.58 | 28 (6) | 30 (7) | 0.008 | 27 (6) | 26 (5) | 0.09 |
| Pack Years Smoking, median (IQR)c |
11 (4, 23) |
19 (9, 33) |
<.001 | 0 (0, 16) |
11 (0, 29) |
<.001 | 40 (30, 54) |
44 (30, 64) |
0.06 | 45 (33, 62) |
43 (30, 60) |
0.15 |
| Current Smokers – no. (%) |
73 (5) | 17 (10) | 0.04 | 374 (12) | 69 (18) | <.001 | 505 (43) | 71 (46) | 0.61 | 202 (38) | 72 (46) | 0.10 |
| FEV1 (% predicted)d, e mean (SD) |
98 (15) | 98 (17) | 0.49 | -- | -- | -- | 75 (28) | 78 (22) | 0.10 | 44 (15) | 47 (14) | 0.02 |
| FVC (% predicted)e, f mean (SD) |
101 (13) | 101 (15) | 0.99 | -- | -- | -- | 87 (19) | 88 (17) | 0.49 | 79 (19) | 80 (20) | 0.78 |
| FEV1/FVC e mean (SD) | 75 (7) | 73 (7) | <.001 | -- | -- | -- | 64 (18) | 67 (14) | 0.03 | 45 (12) | 48 (11) | 0.006 |
| COPDg– no. (%) | 84 (6) | 19 (12) | 0.01 | -- | -- | -- | 561 (41) | 63 (33) | 0.02 | 528 (100) |
157 (100) |
N/A |
| GOLD Stage | ||||||||||||
| Unclassified – no. (%)h | 55 (4) | 6 (4) | 87 (7) | 17 (11) | -- | -- | ||||||
| Stage 0 – no. (%) | 983 (76) | 105 (66) | 508 (43) | 63 (41) | -- | -- | ||||||
| Stage 1 – no. (%) | 175 (14) | 29 (18) | 0.04 | -- | -- | -- | 82 (7) | 21 (14) | <.001 | -- | -- | 0.01 |
| Stage 2 – no. (%) | 80 (6) | 18 (11) | 230 (20) | 38 (25) | 241 (46) | 76 (48) | ||||||
| Stage 3 – no. (%) | 4 (0.3) | 1 (1) | 167 (14) | 11 (7) | 219 (41) | 74 (47) | ||||||
| Stage 4 – no. (%) | -- | -- | 99 (8) | 5 (3) | 68 (13) | 7 (5) | ||||||
| TLC (Liters)e,i, mean (SD) |
5.2 (1.2) | 4.5 (1.3) | <.001 | -- | -- | -- | 5.8 (1.4) | 5.2 (1.4) | <.001 | 5.9 (1.5) | 5.7 (1.4) | 0.16 |
Baseline characteristics from FHS and COPDGene are similar to what has been previously published, now limited to those with chest CT and mortality data. Data are missing for participants in the following categories: FHS: current smoking status, 3 participants (0.2%); spirometry, 91 participants (6%); total lung capacity, 93 participants (6%). COPDGene: spirometry, 1 participant (0.05%); GOLD stage, 1 participant (0.05%); and total lung capacity, 18 participants (1%)
Percentage of total population in each cohort
IQR is interquartile range
FEV1 is forced expiratory volume in 1 second.
In the AGES-Reykjavik cohort, approximately 80% of the spirometry was missing and TLC measurements were not available, as a result this information is not included in this table.
FVC forced vital capacity.
COPD refers to those with GOLD Stage 2 or higher.
GOLD Unclassified is FEV1<80%, FEV1/FVC≥0.70
TLC total lung capacity. Quantitative CT total lung capacity measurements were made using Airway Inspector (www.airwayinspector.org).
Figure 2.
The top panel of the figure is participants included in the analysis by cohort and interstitial lung abnormality status. CT stands for computed tomography, FHS-MDCT2 stands for the Framingham Heart Study Multidetector Computed Tomography 2, and ILA stands for interstitial lung abnormalities. Respiratory deaths included the following: ICD9 codes 460–519, ICD 10 codes J00-J99. Cardiovascular deaths included the following: ICD9 codes 390–459 and ICD 10 codes I00-I99. Cancer deaths included the following: ICD9 codes 140–239 and ICD 10 codes C00-D48. All causes of death not contained in these ICD9 and ICD10 codes were included in “other”. *percentages were all rounded to the nearest whole number, at times the percentages may sum to greater than 100%
Baseline Characteristics and Interstitial Lung Abnormalities
The baseline characteristics of the participants from all four cohorts, stratified by the presence or absence of interstitial lung abnormalities, are presented in Table 1. Baseline characteristics of AGES-Reykjavik and ECLIPSE including those indeterminate for interstitial lung abnormalities are included in eTable 1 and 2. Across all cohorts, interstitial lung abnormalities were associated with older age compared to the absence of interstitial lung abnormalities. As previously noted in COPDGene2 compared to, among participants in ECLIPSE23 interstitial lung abnormalities were associated with less severe airway obstruction as demonstrated by a higher forced expiratory volume in one second (FEV1) and FEV1/forced vital capacity (FVC) ratio, compared to absence of interstitial lung abnormalities. In contrast, in the FHS, interstitial lung abnormalities were associated with a higher prevalence of COPD and a lower FEV1/FVC ratio.
Mortality and Interstitial Lung Abnormalities
The absolute mortality rates were significantly higher in participants with interstitial lung abnormalities compared to those without interstitial lung abnormalities in all cohorts (see Table 2). In the FHS 7% (12 deaths) of participants with interstitial lung abnormalities died over 4 years compared to 1% (12 deaths) of those without interstitial abnormalities; in the AGES-Reykjavik 56% (210 deaths) of participants with interstitial lung abnormalities and 33% (1065 deaths) of participants without interstitial lung abnormalities died over 8.9 years. In smokers with and without COPD from COPDGene, 16% (25 deaths) of participants with interstitial lung abnormalities died, while 11% (133 deaths) of participants without interstitial lung abnormalities died over 6.5 years. In smokers with COPD from ECLIPSE 11% (18 deaths) of participants with interstitial lung abnormalities died over 2.9 years, while 5% (27 deaths) of participants without interstitial lung abnormalities died. These findings resulted in a 6% difference (95% confidence interval [CI] 2%, 10%) in the FHS, a 23% difference (95% CI 18%, 28%) in AGES-Reykjavik, a 5% difference (95% CI −1%, 11%) in COPDGene, and a 6% difference (95% CI 1%, 11%) in ECLIPSE in the absolute mortality rates associated with interstitial lung abnormalities. The mortality rates in those with indeterminate status were 2% (24 deaths) in FHS, 43% (750 deaths) in AGES-Reykjavik, 13% (99 deaths) in COPDGene and 12% (120 deaths) in ECLIPSE.
Table 2.
Association between Interstitial Lung Abnormalities and Mortalitya
| Framingham Heart Study | AGES-Reykjavik | COPDGene | ECLIPSE | |||||
|---|---|---|---|---|---|---|---|---|
| Median Follow Up Time – yrs, (IQRb) |
4.0 (3.3, 4.6) |
8.9 (6.7, 9.9) |
6.5 (6.2, 6.7) |
2.9 (2.9, 2.9) |
||||
| Mortality – no. (%) | No ILAc 12 (1) |
ILA 12 (7) |
No ILA 1065 (33) |
ILA 210 (56) |
No ILA 133 (11) |
ILA 25 (16) |
No ILA 27 (5) |
ILA 18 (11) |
| Mortality Difference % (95% CI)d |
6 (2, 10) | 23 (18, 28) | 5 (−1, 11) | 6 (1, 11) | ||||
| Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
| Unadjusted Model | 7.7 (3.7, 16.1) |
<.001 | 1.4 (1.3, 1.6) |
<.001 | 1.5 (0.98, 2.3) |
0.08 | 1.5 (1.1, 2.1) |
0.005 |
| Adjusted Modele | 2.7 (1.1, 6.5) |
0.03 | 1.3 (1.2, 1.4) |
<.001 | 1.8 (1.1, 2.8) |
0.01 | 1.4 (1.1, 2.0) |
0.02 |
| Adjusted Model + Percentage Emphysemaf, g |
2.6 (1.03, 6.7) |
0.04 | -- | -- | 1.9 (1.2, 3.0) |
0.007 | 1.4 (1.03, 2.0) |
0.03 |
| Adjusted Model + Coronary Diseaseh,g |
2.2 (0.9, 5.9) |
0.10 | 1.2 (1.1, 1.3) |
<.001 | 1.5 (0.9, 2.0) |
0.12 | 1.7 (1.1, 2.4) |
0.008 |
| Adjusted Model + Cancer Historyh,g |
2.6 (1.1, 6.2) |
0.03 | 1.25 (1.2, 1.3) |
<.001 | 1.8 (1.1, 2.8) |
0.008 | -- | -- |
All hazard ratios are for the comparison between participants with and without interstitial lung abnormalities.
IQR is interquartile range
ILA is interstitial lung abnormalities
95% CI is 95% Confidence Interval
Adjusted hazard ratios include adjustments for age, gender, race, body-mass index, pack-years of smoking, current or former smoking status, GOLD stage of COPD (where available)
Adjusted hazard ratios include adjustments for age, gender, race, body-mass index, pack-years of smoking, current or former smoking status, GOLD stage of COPD and amount of emphysema (percentage below 950 Hounsfield units).
Data for the variables used in addition to the baseline adjusted model can be found in eTable 4
Adjusted hazard ratios include adjustments for age, gender, race, body-mass index, pack-years of smoking, current or former smoking status, GOLD stage of COPD (except in the AGES-Reykjavik where GOLD Stage was not available), history of coronary artery disease and coronary calcium score.
Adjusted hazard ratios include adjustments for age, gender, race, body-mass index, pack-years of smoking, current or former smoking status, GOLD stage of COPD (except in the AGES-Reykjavik where GOLD Stage was not available) and history of non-dermatologic malignancy in Framingham and AGES-Reykjavik, in COPDGene includes a history of lung, breast, bladder, colon and prostate cancers.
When compared to those without interstitial lung abnormalities in multivariable Cox proportional hazards models adjusting for age, sex, race, body mass index, pack-years of smoking, current smoking status and GOLD stage (where available), interstitial lung abnormalities were associated with a higher risk of death in the FHS (hazard ratio [HR]=2.7, 95% confidence interval [CI], 1.1–6.5, P=0.030), AGES-Reykjavik (HR=1.3, 95% CI 1.2–1.4, P<.001), COPDGene (HR=1.8, 95% CI 1.1, 2.8, P=0.014) and ECLIPSE (HR=1.4, 95% CI, 1.1–2.0, P=0.022) (see Figure 3). Similar results were seen, with higher odds of death, when using multivariable logistic regression (see eTable 3). For further analyses with respect to definite fibrosis and those indeterminate for interstitial lung abnormalities see the eResults, eTables 5 and 6 and eFigure 3.
Figure 3.

Curves showing percent mortality, comparing participants with and without interstitial lung abnormalities (ILA). P-values included in each panel are those associated with the hazard ratios from the adjusted Cox proportional hazards model, including adjustments for age, gender, race, body-mass index, pack-years of smoking, current or former smoking status and GOLD stage of COPD (except in AGES-Reykjavik where GOLD stage was not available). The results are for the comparison between participants with interstitial lung abnormalities to participants without interstitial lung abnormalities.
Mortality, Interstitial Lung Abnormalities and Never-Smokers
To determine if unmeasured differences in smoking behavior among smokers could explain the associations between interstitial lung abnormalities and mortality, associations between interstitial lung abnormalities and mortality in never-smokers from both the FHS and AGES-Reykjavik cohorts were analyzed. In the FHS, 6% of never smokers with interstitial lung abnormalities died, compared to 0.3% of never smokers without interstitial abnormalities, a difference of 5.7% (95% CI 1%, 12%), (HR 19.9, 95% CI 5.1–78.1, P<0.001). In AGES-Reykjavik 52% of never smokers with interstitial lung abnormalities died compared to 31% of those without interstitial lung abnormalities, a difference of 21%, (95% CI 11%, 31%), (HR=1.3, 95% CI 1.1–1.4, P=0.002).
Mortality, Interstitial Lung Abnormalities, COPD, CAD, and Cancer
To determine if the presence of other chronic diseases could explain the associations between interstitial lung abnormalities and mortality, analyses were performed in each cohort additionally adjusting for the percentage of emphysematous lung, measures of CAD or reports of malignancy (where available). The association between interstitial lung abnormalities and mortality remained statistically significant after additional adjustments for disease specific measures (see Table 2), except in the FHS and COPDGene, in which additional adjustment for adjudicated CAD and CAC scores resulted in no association (see Table 2). Similar associations between interstitial lung abnormalities and mortality were seen in COPDGene and ECLIPSE when adjusting for BODE Index (see eResults). Additionally, the absolute mortality rates of each GOLD stage were consistently greater in participants with interstitial lung abnormalities compared to those without interstitial lung abnormalities (see eFigure 4).
Mortality, Interstitial Lung Abnormalities and Cause-of-Death
To determine the causes-of-death among those with interstitial lung abnormalities, data from the AGES-Reykjavik cohort was assessed where causes-of-death were available on death certificates from an interim follow up date (December 31, 2009, median follow-up of 5.4 years). Participants with interstitial lung abnormalities in the AGES-Reykjavik cohort were more likely to die from a respiratory cause (13%) compared to those without interstitial lung abnormalities (4%) or those with indeterminate status (6%, see Figure 2). After adjusting for covariates (age, sex, race, body mass index, pack-years of smoking, current smoking status), those with interstitial lung abnormalities had higher odds of death from a respiratory cause (Odds Ratio 2.4, 95% CI 1.7–3.4, P<0.001) compared to those without interstitial lung abnormalities. Results were similar when comparing those with interstitial lung abnormalities to those indeterminate for interstitial lung abnormalities (see eResults). After adjusting for covariates there was no association between interstitial lung disease status and death due to cardiovascular disease, cancer, or other deaths. Among participants who died from a respiratory cause, interstitial lung abnormalities were associated with an increased rate of dying from pulmonary fibrosis (47%], 7 of the 15 respiratory deaths among those with interstitial lung abnormalities were due to pulmonary fibrosis, see Figure 2). Of the eight deaths due to pulmonary fibrosis, 5 participants had evidence of definite fibrosis on chest CT, 2 had interstitial lung abnormalities without definite fibrosis, and 1 participant was indeterminate for interstitial lung abnormality status. Only one of these participants had previously diagnosed pulmonary fibrosis at the time of the CT scan.
Discussion
In this study, interstitial lung abnormalities, a set of imaging abnormalities noted among ~7% adults,6 were associated with a higher rate of all-cause mortality. The associations between interstitial lung abnormalities and mortality were not attenuated after adjustment by smoking, cancer, or COPD or CAD. Among an older population from Iceland, the higher rate of mortality in those with interstitial lung abnormalities was associated with a higher rate of death from respiratory failure and pulmonary fibrosis. These findings, in conjunction with those previously published,2,6,8 demonstrate that despite often being undiagnosed and asymptomatic,2,6 interstitial lung abnormalities may be associated with lower survival rates among older persons.
This study builds on prior studies7, demonstrating that interstitial lung abnormalities were associated with older age, smoking, and a restrictive lung deficit. The findings in ECLIPSE are similar to those previously reported in COPDGene2 which demonstrate that among smokers with COPD, interstitial lung abnormalities were identified in those with more preserved FEV1/FVC ratios. Although COPD was associated with interstitial lung abnormalities in the FHS, this association may be related to older age and history of smoking that are common in both COPD and interstitial lung abnormalities.
It is important to consider the higher mortality rates associated with interstitial lung abnormalities in context; the mortality rates associated with interstitial lung abnormalities are less than the well documented mortality rates associated with clinically identified IPF.13–14 In addition, although data from the AGES-Reykjavik cohort demonstrated that interstitial lung abnormalities were associated with death due to respiratory failure and pulmonary fibrosis, respiratory failure death is more common in patients with IPF.11,27
The absolute mortality rates differed between the cohorts. This was due in part to differences in recruitment criteria and follow-up time. Compared to the FHS (which included a general population sample of adults), the higher absolute mortality rates in COPDGene and ECLIPSE are likely explained by a longer follow-up time (COPDGene) and the inclusion of greater numbers of COPD patients (COPDGene and ECLIPSE). Although the FHS and AGES-Reykjavik were both recruited from among community dwelling men and women, the higher mortality rates in the AGES-Reykjavik cohort are likely explained by the older age and longer follow-up times of the average participants in this cohort.
This study has a number of limitations. First, participants with interstitial lung abnormalities were older than those without interstitial lung abnormalities7. Further study will be required to determine the prognostic significance of interstitial lung abnormalities in younger age groups. Even among older populations, studies evaluating the longitudinal CT imaging progression of interstitial lung abnormalities may be required to distinguish imaging characteristics that could reflect a normal variant of the aging lung28 from an early stage of a progressive interstitial lung disease. Second, interstitial lung abnormalities were associated with a higher risk of death among never-smokers from two cohorts; however the large hazard of mortality associated with interstitial lung abnormalities in the FHS among never-smokers was driven by a small number of deaths. Third, although an association between interstitial lung abnormalities and increased risk of respiratory death was identified in the AGES-Reykjavik study, data on the cause of death was not available from other cohorts. Fourth, despite the correlations presented between research participants with interstitial lung abnormalities and patients with IPF (as well as other forms of interstitial lung disease) this study cannot explain the large discrepancy between the prevalence of interstitial lung abnormalities (7% in general population samples,6 see Table 1) and the reported prevalence of IPF (~0.002% to 0.04% of the general population)29–31 and interstitial lung disease. Of note, the prevalence of definite fibrosis in each cohort (~1.6–2.4%) is similar to the prevalence of IPF noted in an autopsy study of 510 cases from New Mexico (1.8%) even though IPF was suspected as a cause of death in less than a tenth of these cases.32 Fifth, unmeasured confounders could explain these findings. Sixth, there are differences in the estimates of the association of interstitial lung abnormalities on mortality in unadjusted and adjusted models in the FHS. Seventh, although data on inter-observer variability in interstitial lung abnormality scoring is presented, data on intra-observer variability in interstitial lung abnormality scoring was not recorded.
Follow-up studies should determine the risk factors for, and the events that lead to, death among persons with interstitial lung abnormalities. Given the ability to treat more advanced stages pulmonary fibrosis33,34 future clinical trials attempting to reduce the overall mortality associated with pulmonary fibrosis should consider including early stages of the disease as well.
Conclusions
In four separate research cohorts, interstitial lung abnormalities were associated with a higher risk of all-cause mortality. The clinical implications of this association require further investigation.
Supplementary Material
Acknowledgments
Funding Support: Dr. Putman is supported by NIH Grant Number T32 HL007633. Dr Gudmundsson is supported by project grant 141513-051 from the Icelandic Research Fund and Landspitali Scientific Fund A-2015-030. Dr. Nishino is supported by NCI grant number 1K23CA157631. Dr. Cho is supported by NIH Grants: K08 HL097029 and R01 HL113264. Dr. El-Chemaly is supported by NIH Grant R21 HL119902. Dr. San Jose Estepar is supported by NIH Grants: K25 HL104085, R01 HL116931 and R01 HL116473. Dr. Diaz is supported by NIH Grant K01 HL118714. This work was partially supported by the NHLBI’s Framingham Heart Study contract: N01-HC-25195. COPDGene is supported by NIH Grant Numbers R01 HL089897 and R01 HL089856. The ECLIPSE study (NCT00292552; GSK code SCO104960) was sponsored by GlaxoSmithKline. The Age, Gene/Environment Susceptibility-Reykjavik Study was supported by NIA grant: 27120120022C, NIH contracts N01-AG-1-2100 and HHSN27120120022C, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Dr. Gudnason is supported by NIA grant: 27120120022C and project grant 141513-051 from the Icelandic Research Fund. Dr. Silverman is supported by NIH Grants: R01 HL089856, P01 HL105339 and P01 HL114501. Dr. Washko is supported by NIH Grants: R01 HL107246, R01 HL116473 and R01 HL122464. Dr. Rosas is supported by NIH Grant P01 HL114501. Dr. Hunninghake and this study are supported by NIH grants: P01 HL114501, and R01 HL111024, and project grant 141513-051 from the Icelandic Research Fund.
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Budoff has received grant support from General Electric. Dr. Celli has received research grant support from Astra Zeneca and has served on advisory boards for GlaxoSmithKline, Boehringer-Ingelheim, Astra Zeneca, Almirall and Takeda. Dr. Coxson has received grant support from GlaxoSmithKline, has served on the advisory boards of GlaxoSmithKline and Samsung. Dr. Diaz has served as a speaker for Novartis. Dr. Hatabu has received research grant support from Canon USA Inc. and Toshiba Medical Inc. Dr. Hunninghake has done consulting work for Medna LLC and the George Lehman Group; and has served on the board of advisors for Patients Like Me and Genentech. Dr. Nishino has received grant support from Canon Inc, and has served as a consultant for Bristol Myers Squibb. In the past three years Dr. Silverman has received honoraria and consulting fees from Merck, grant support and consulting fees from GlaxoSmithKline and honoraria from Novartis. Dr. Washko has done consulting work for Merck and GlaxoSmithKline.
Footnotes
This paper is subject to the NIH public access policy: http://www.nih.gov/about/publicaccess/Finalpublicaccessimplementation031505.htm.
Author Contributions:
Study Design: Celli, Coxson, Hunninghake, O’Connor, Rosas, Silverman, Washko
Acquisition, analysis or interpretation of the data: Araki, Budoff, Harmouche, Hatabu, Hoffmann, Hokanson, Hunninghake, Kinney, MacNee, Murchison, Nishino, O’Donnell, Okajima, Putman, Ross, San Jose Estepar, Williams, Zazueta
Critical revision of the manuscript for important intellectual content: Araki, Aspelund, Budoff, Celli, Cho, Coxson, Diaz, Dupuis, Eiríksdottír, El-Chemaly, Fernandez, Gao, E. Gudmundsson, G. Gudmundsson, Gudnason, Harmouche, Harris, Hatabu, Hoffmann, Hokanson, Hunninghake, Kinney, Latourelle, Launer, MacNee, Murchison, Nishino, O’Connor, O’Donnell, Okajima, Putman, Rosas, Ross, San José Estépar, Sigurdsson, Silverman, Washko, Williams, Zazueta
Statistical analysis: Dupuis, Gao, Hunninghake, Latourelle, Putman
Obtained funding: G. Gudmundsson, Hunninghake, Rosas
Access to Data: Dr. Hunninghake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures:
No other disclosures are reported.
REFERENCES
- 1.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sverzellati N, Guerci L, Randi G, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 4.Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsushima K, Sone S, Yoshikawa S, Yokoyama T, Suzuki T, Kubo K. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respir Med. 2010;104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Hunninghake GM, Hatabu H, Okajima Y, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:770–778. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle TJ, Washko GR, Fernandez IE, et al. Interstitial Lung Abnormalities and Reduced Exercise Capacity. Am J Respir Crit Care Med. 2012;185(7):756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kropski JA, Pritchett JM, Zoz DF, et al. Extensive Phenotyping of Individuals At-risk for Familial Interstitial Pneumonia Reveals Clues to the Pathogenesis of Interstitial Lung Disease. Am J Respir Crit Care Med. 2015;191(4):417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 13.King TE, Jr, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 14.Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. The Lancet Respiratory Medicine. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 15.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 16.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 17.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management and prevention of COPD. [accessed, August 1st, 2015]; (updated 2007). http://www.goldcopd.org. [Google Scholar]
- 21.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31:869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 22.Washko GR, Lynch DA, Matsuoka S, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross JC, Estepar RS, Diaz A, et al. Lung extraction, lobe segmentation and hierarchical region assessment for quantitative analysis on high resolution computed tomography images. Med Image Comput Comput Assist Interv. 2009;12:690–698. doi: 10.1007/978-3-642-04271-3_84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 25.Uh HW, Wijk HJ, Houwing-Duistermaat JJ. Testing for genetic association taking into account phenotypic information of relatives. BMC Proc. 2009;3(Suppl 7):S123. doi: 10.1186/1753-6561-3-s7-s123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 27.Panos RJ, Mortenson RL, Niccoli SA, King TE., Jr Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med. 1990;88:396–404. doi: 10.1016/0002-9343(90)90495-y. [DOI] [PubMed] [Google Scholar]
- 28.Copley SJ, Wells AU, Hawtin KE, et al. Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old. Radiology. 2009;251(2):566–573. doi: 10.1148/radiol.2512081242. [DOI] [PubMed] [Google Scholar]
- 29.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 30.Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y. Idiopathic pulmonary fibrosis. Epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med. 1994;150:670–675. doi: 10.1164/ajrccm.150.3.8087336. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez Perez ER, Daniels CE, Schroeder DR, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 137:129–137. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 33.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 34.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.