Abstract
In acute ischemic stroke, collateral circulation plays an important role in maintaining blood flow to the tissue that is at risk of progressing into ischemia, and in increasing the successful recanalization rate without hemorrhagic transformation. We have reported that well-developed collateral circulation is associated with smaller infarct volume and better long-term neurological outcome, and it disappears promptly once the effective recanalization is achieved. Contrary to the belief that collateral vessels develop over time in chronic stenotic condition, there exists a phenomenon that collateral circulation develops immediately in acute stenosis or occlusion of the arteries and it seems to be triggered by fluid shear stress, which occurs between the territories of stenotic/occluded arteries and those fed by surrounding intact arteries. We believe that this acute development of collateral circulation is a target of novel therapeutics in ischemic stroke and refer our recent attempt in enhancing collateral circulation by modulating sphingosine-1-phosphate receptor 1, which is a known shear-stress mechanosensing protein.
Keywords: ischemic stroke, collateral, S1PR1, shear stress, leptomeningeal arteries, PCA laterality, hyperintensive vessels
The treatment of acute ischemic stroke has entered a new era recently because of the consistent success in endovascular therapy with or without the administration of intravenous recombinant tissue plasminogen activator (IV rtPA). In acute ischemic stroke, tissues that remain alive despite low cerebral blood flow but that are at risk for progressing into infarction are considered to be ‘ischemic penumbra.‘ Both IV rtPA and endovascular therapies can rescue penumbral tissue by recanalizing occluded cerebral arteries. However, even after successful recanalization, some patients show little neurologic improvement, possibly because the penumbral area had already progressed into the irreversibly damaged ischemic core or because hemorrhagic transformation occurred after the recanalization therapy. Collateral circulation has the potential to protect against these ischemic injuries by maintaining cerebral blood flow, and we have focused on collateral circulation in this context.
A deterioration in the quality of the collateral circulation within 3 to 5 days after ischemic stroke reportedly is associated with infarct growth in patients without recanalization (Campbell et al., 2013), thereby suggesting the role of collateral flow in maintaining the blood supply to the penumbral area in the acute stage. Good collateral status leads to higher recanalization rate, smaller infarction volume (Bang et al., 2008), and better neurological outcome. Well-developed collateral flows can lower the rate of hemorrhagic transformation after thrombolytic and/or endovascular therapies (Bang et al., 2011). Furthermore, retrograde collateral flow may help to expose all portions of the thrombus to thrombolytic agents (Caplan et al., 1998), making collateral flow indispensable for ischemic stroke therapy. Here, we present our current opinion regarding the importance of the development of collateral circulation in acute ischemic stroke, according to findings from both clinical and basic research.
Collateral blood vessels comprise extracranial routes including the facial, maxillary, middle meningeal, and occipital arteries, as well as intracranial routes. The intracranial collateral routes are divided into two pathways: the primary pathway includes the circle of Willis, whereas secondary pathways consist of pre-existing collateral routes that do not normally feed the territory but that develop in the setting of the impaired cerebral hemodynamics after acute stroke. These secondary pathways include the ophthalmic artery and leptomeningeal anastomoses with dural arterioles (Sheth et al., 2014). Our studies have focused on leptomeningeal collaterals.
The ‘gold standard’ for evaluating the collateral circulation after ischemic stroke is digital subtraction angiography (DSA), although its invasiveness limits its application. Computed tomographic angiography (CTA) is widely available, and a positive collateral status on CTA reportedly is correlated with a favorable outcome (Miteff et al., 2009). Another common noninvasive technique is magnetic resonance angiography (MRA). We have reported that laterality of the posterior cerebral artery (PCA) on MRA (Figure 1A), in which the PCA on the ischemic side is longer than that on the intact side, is an independent prognostic marker in patients with acute middle cerebral artery (MCA) occlusion and that this marker is associated with a favorable functional outcome and increased recanalization rate after rtPA treatment (Ichijo et al., 2013). Figure 1A represents right PCA longer than the left side in a patient with right proximal MCA occlusion. Patients with ipsilateral MCA occlusion often demonstrate PCA laterality, which represents the development of collateral flow from the PCA to the MCA territory via leptomeningeal arteries, confirmed with another study with DSA (Uemura et al., 2004). The importance of our study lies in the ease with which PCA laterality is identified on magnetic resonance (MR) imaging during acute ischemic stroke and the strong correlation of this sign with long-term neurologic outcome and infarct volume.
Figure 1.
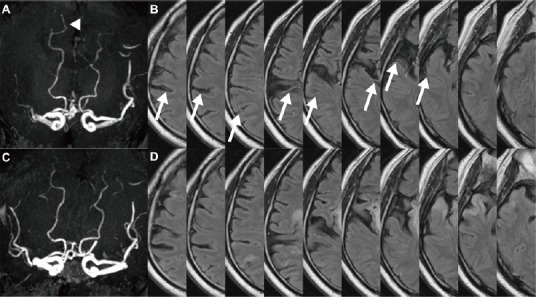
A patient with occlusion of the right proximal middle cerebral artery.
On magnetic resonance angiography, the right posterior cerebral artery (PCA) is 2 segments longer than the left PCA (arrow head); this presentation is defined as ‘PCA laterality’ (A). Hyperintensive vessels (HVs) are present along the cortex of the right middle cerebral artery perfusion territory, and are seen in 8 of 10 slices on fluid-attenuated inversion recovery magnetic resonance imaging (arrows) (B). After intravenous recombinant tissue plasminogen activator treatment, both PCA laterality (C) and HVs (D) disappeared.
In addition, the presence of hyperintense vessels (HVs) on fluid-attenuated inversion recovery (FLAIR) MR imaging (Figure 1B) is informative regarding the collateral circulation because these vessels represent slow retrograde blood flow through the leptomeningeal arteries during acute ischemic stroke (Olindo et al., 2012). Figure 1B shows HVs seen in 8 out of 10 axial slices along the cortex of right MCA perfusion territory in a patient with right MCA occlusion. We analyzed consecutive acute ischemic stroke patients in 5 years with proximal MCA occlusion treated with IV rtPA at 2 hospital stroke centers and selected 48 patients with MR imaging of pre- and post- (evaluated on average 6 to 7 days) IV rtPA treatment. By evaluating a combination of 2 MRI collateral markers, PCA laterality and HVs, we reported that patients with dramatic decrease of these signs (Figure 1C and D) after the treatment were significantly associated with a favorable outcome (Ichijo et al., 2015a). We defined this dramatic decrease or disappearance of the collateral signs as reversion of collaterals.
Recanalization is scored on DSA or MRA according to either the TICI (Thrombolysis in Cerebral Infarction) or TIMI (Thrombolysis in Myocardial Infarction) scale, to characterize the reemergence of distal MCA perfusion in cases of proximal MCA occlusion. However, despite early successful recanalization (TICI2 or 3), the neurologic outcome is inconsistent, with some patients experiencing dramatic functional recovery but others achieving no neurologic improvement. Early neurologic improvement within 24 hours after the administration of rtPA has been associated with lower baseline National Institutes of Health Stroke Scale scores and younger age (Blinzler et al., 2011), but some patients do not recover even though the time from stroke onset to treatment is short.
In one study, we found that a well-developed collateral sign before rtPA and history of atrial fibrillation were significantly associated with neurologic improvement within 24 hours of rtPA administration (Ichijo et al., 2015a). Approximately 50% of patients demonstrated reversion of collaterals, which was associated with successful recanalization on MRA, lower National Institutes of Health Stroke Scale scores at 1 and 7 days, better modified Rank in Scale scores at 3 months, and higher Alberta Stroke Program Early CT Scores (ASPECTS), indicating smaller infarct volumes. ASPECTS were significantly higher in the cortical area (M1–M6) — but not in other regions (insular cortex, basal ganglia, internal capsule) — of the patients with reversion of collaterals, thus supporting the idea that cortical areas tend to be rescued by leptomeningeal collaterals. The reversion of collateral vasculature in patients with successful recanalization is plausible because once the arterial circulation is reestablished, collateral vessels are no longer useful and, theoretically, might even cause cerebral blood overflow, leading to hemorrhagic transformation. Contrary to the belief that collateral vessels develop over time, the reversion of collaterals was not significantly correlated with stroke etiology such as cardiac emboli or large-artery sclerosis (Ichijo et al., 2015a). We attribute this situation to the idea that leptomeningeal collateral arteries in acute MCA occlusion develop because of a pressure gradient between the territories fed by the anterior cerebral artery (ACA) or PCA and one distal to the site of MCA occlusion, thus maintaining the flow to the penumbral area. We consider that these collaterals thus disappear once the gradient is normalized, that is, after successful recanalization. We believe that enhancing the acute development of collateral circulations and modulating the molecular dynamics of this event are potential targets of novel therapeutics for acute ischemic stroke.
The revascularization after vascular occlusion involves several distinct processes. ‘Angiogenesis,‘ the sprouting of endothelial cells to form capillary networks, is induced by hypoxia: defective oxygenation of cells leads to the activation of hypoxia-inducible factor 1 and downstream transcription factors such as vascular endothelial growth factor, which bind to endothelial cells, thus signaling them to proliferate, migrate, and eventually form new vessels. In post-stroke angiogenesis, endothelial cells are activated, and in accordance with smooth muscle cells and pericytes, they work for functional and mature vascular formation. However, the effect of angiogenesis on stroke recovery is still controversial and the promotion of angiogenesis by delivering vascular endothelial growth factor has been reported to aggravate vasogenic edema and hemorrhage, possibly exacerbating ischemic injury (Adamczak et al., 2015).
Another process relating to revascularization in ischemic stroke is arteriogenesis, the induced development of new vessels triggered by fluid shear stress after the stenosis or occlusion of vessels. This increased shear stress results in the formation of large collateral arteries due to the proliferation of endothelial and smooth muscle cells. Once the hemodynamically relevant stenosis or occlusion occurs, pre-existing arterioles redistribute the blood flow by connecting high-perfusion and low-perfusion regions, thus increasing the shear stress in pre-existing arterioles and leading to the development of collateral vessels (Liu et al., 2014). We consider arteriogenes rather than angiogenesis plays a role with regard to a functional collateral development. However, achieving functional vessels through arteriogenesis takes several days to weeks (Helisch et al., 2006) and therefore arteriogenesis likely contributes more to alleviating chronic artery stenosis or occlusion than to mitigating acute ischemic events.
In general, the robustness of the collateral circulation diminishes with age and other vascular comorbidities, such as hypertension and diabetes (Sheth et al., 2014). However, we have found that some collateral flow develops dramatically and rapidly after the onset of acute ischemic stroke, is independent of atherosclerosis risk factors (Ichijo et al., 2013), and disappears promptly as soon as the pressure gradient is released (Ichijo et al., 2015a). Qureshi et al. (2008) reported angiographic images of patients who underwent endovascular procedures for symptomatic stenosis of proximal MCA. During 15 to 30 seconds of balloon catheter inflation at the stenotic site, leptomeningeal arteries developed to reconstitute maximally the distal most portion of MCA territory even in those who had little or no vessels seen before the procedures, indicating that prompt occurrence of pressure gradient between MCA-ACA triggered the collateral development in acute setting (Qureshi et al., 2008). A similar phenomenon occurred in people who underwent brief (1-minute) occlusion of normal coronary arteries: 20% of subjects rapidly developed collateral flow sufficient to prevent myocardial ischemia (Wustmann et al., 2003). Whether this phenomenon primarily involves the preexisting arteries is unknown, as are the factors that contribute to it, but its initiation appears to require fluid shear stress rather than hypoxia. There are evidences that shear stress on vascular endothelial cells induces endothelial brain-derived neurotropic factor mRNA expression and protein levels (Nakahashi et al., 2000; Prigent-Tessier et al., 2013), or endothelial transforming growth factor beta-1 mRNA and its activity (Ohno et al., 1995), both of which can lead to cell proliferation, differentiation and survival, indicating the possible contribution of shear stress to neuroregenerative processes. Though shear stress has been shown to play an important role in central nervous system, the vessel's system for sensing shear stress has not been elucidated completely, but several pathways have been suggested recently. In particular, microarray data from a model of chronically elevated fluid shear stress revealed increased transcription of transient receptor potential cation channel, subfamily V, member 4 (Trpv4) on endothelial cells, and this factor was positively correlated with the intensity of fluid shear stress (Troidl et al., 2009). Furthermore, a complex composed of platelet endothelial cell adhesion molecule 1, vascular endothelial cadherin, and vascular endothelial growth factor receptor 2 is necessary to induce shear responsiveness; this complex lies upstream of integrin, which it activates to mediate shear responses (Tzima et al., 2005).
Sphingosine-1-phosphate (S1P) receptor 1 is another candidate for the trigger of the shear-stress mechanosensing pathway. The sphingolipid S1P is known to be involved in anti-apoptotic, proliferative, and inflammatory signaling. S1P binds and activates a family of five G-protein-coupled S1P receptors (S1PR1 through 5), which are widely expressed in the body, including the central nervous system. In addition, S1P signaling modulates various developmental aspects of the embryonic nervous system, including vascular maturation, neuronal progenitor migration, and astrocyte proliferation (Prager et al., 2015). Furthermore, S1PR1 stabilizes the primary vascular network in mice and is an essential factor for fluid shear-stress signaling in endothelial cells (Jung et al., 2012). In vitro, antagonism of S1PR1 blocked shear-stress-induced adherens junction assembly in human umbilical vein endothelial cells. Antagonism of S1PR1 also blocked the phosphorylation of extracellular signal-regulated kinases (ERK) and protein kinase B (Akt), and reduced the activation of endothelial nitric oxide synthase (eNOS) (Jung et al., 2012); all of these signals are known to be triggered in response to fluid shear stress (Tzima et al., 2005). In the descending aorta, which experiences constant laminar shear stress, endothelial S1PR1-knockout mice showed reduced phospho-eNOS staining, suggesting that S1PR1 mediates eNOS activation in vivo. Surprisingly, in a ligand-independent, S1P-independent manner, S1PR1 itself senses endothelial shear stress and triggers downstream signaling molecules (Jung et al., 2012).
We recently reported the upregulation of S1PR1 expression in the unilateral mouse brain cortex under prolonged shear stress after ipsilateral common carotid artery occlusion (CCAO) (Ichijo et al., 2015b). Latex perfusion revealed significantly more prominent development of the leptomeningeal collateral vessels in the ipsilateral cortex in mice with unilateral CCAO and treated with an S1PR1-selective agonist (SEW2871) compared with mice without SEW2871 or that underwent sham surgery plus SEW2871 administration. These results imply the need for shear stress to initiate the signaling that triggers enhanced development of the collateral vasculature. Furthermore, mice with CCAO and SEW2871 pretreatment followed by unilateral MCA occlusion was associated with smaller infarction volumes and better neurological function than those without SEW2871. Although the leptomeningeal arteries of SEW2871-treated CCAO mice demonstrated increased eNOS phosphorylation and improved cerebral blood flow, Ki-67 staining failed to reveal any noteworthy endothelial cell proliferation. Together, these findings prompted us to speculate that modulating S1PR1 decreases infarction volume and subsequently improves neurological outcomes owing to collateral enhancement through the eNOS vasodilation pathway rather than through cell proliferation (Ichijo et al., 2015b) (Figure 2). Furthermore, recent clinical trials have revealed that fingolimod, a known modulator of S1P receptors including S1PR1, is effective against acute ischemic stroke (Zhu et al., 2015). Specifically, in addition to its previously reported advantages in preventing inflammatory responses and protecting against excitotoxicity, fingolimod may modulate S1PR1 signals to promote the development of collateral arteries.
Figure 2.

Vessel occlusion increases fluid shear stress, consequently upregulating and activating sphingosine-1-phosphate receptor 1 (S1PR1) and leading to the growth of collateral vessels presumably through the phosphorylation of extracellular signal-regulated kinases (ERK), protein kinase B (Akt), and endothelial nitric oxide synthase (eNOS) activation.
We propose that intervening in and enhancing the acute development of collateral vessels represents opportunities for novel therapeutics for acute ischemic stroke. Since we found that well-developed collateral was related to higher recanalization rate and better functional recovery, the administration of S1PR1 modulator along with IV rtPA after stroke may theoretically increase successful recanalization rate, decrease infarction volume and lead to better outcome. Additional investigation is needed to better understand the mechanism by which fluid shear stress is sensed and to clarify the molecular events that follow, to achieve more specific and effective treatments in the future.
Eri Iwasawa gave a presentation regarding the reversion of collaterals at the 54th annual meeting of the Japanese Society of Neurology.
References
- Adamczak J, Hoehn M. Poststroke angiogenesis, con: dark side of angiogenesis. Stroke. 2015;46:e103–104. doi: 10.1161/STROKEAHA.114.007642. [DOI] [PubMed] [Google Scholar]
- UCLA Collateral Investigators. Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Viñuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UCLA-Samsung Stroke Collaborators. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- Blinzler C, Breuer L, Huttner HB, Schellinger PD, Schwab S, Kohrmann M. Characteristics and outcome of patients with early complete neurological recovery after thrombolysis for acute ischemic stroke. Cerebrovasc Dis. 2011;31:185–190. doi: 10.1159/000321869. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, Barber PA, Levi CR, Bladin C, Donnan GA, Davis SM. EPITHET Investigators (2013) Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 33:1168–1172. doi: 10.1038/jcbfm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- Helisch A, Wagner S, Kahn N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- Ichijo M, Iwasawa E, Numasawa Y, Miki K, Ishibashi S, Tomita M, Tomimitsu H, Kamata T, Fujigasaki H, Shintani S, Mizusawa H. Significance of development and reversion of collaterals on mri 0 in early neurologic improvement and long-term functional outcome after intravenous thrombolysis for ischemic stroke. AJNR Am J Neuroradiol. 2015a;36:1839–1845. doi: 10.3174/ajnr.A4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo M, Ishibashi S, Li F, Yui D, Miki K, Mizusawa H, Yokota T. Sphingosine-1-phosphate receptor-1 selective agonist enhances collateral growth and protects against subsequent stroke. PLoS One. 2015b;10:e0138029. doi: 10.1371/journal.pone.0138029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo M, Miki K, Ishibashi S, Tomita M, Kamata T, Fujigasaki H, Mizusawa H. Posterior cerebral artery laterality on magnetic resonance angiography predicts long-term functional outcome in middle cerebral artery occlusion. Stroke. 2013;44:512–515. doi: 10.1161/STROKEAHA.112.674101. [DOI] [PubMed] [Google Scholar]
- Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, Hla T. Flow-regulated endothelial S1Preceptor-1 signaling sustains vascular development. Dev Cell. 2012;23:600–610. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, Yang GY. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–156. doi: 10.1016/j.pneurobio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- Olindo S, Chausson N, Joux J, Saint - Vil M, Signate A, Edimonana-Kapute M, Jeannine S, Mejdoubi M, Aveillan M, Cabre P, Smadja D. Fluid-attenuated inversion recovery vascular hyperintensity: an early predictor of clinical outcome in proximal middle cerebral artery occlusion. Arch Neurol. 2012;69:1462–1468. doi: 10.1001/archneurol.2012.1310. [DOI] [PubMed] [Google Scholar]
- Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. J Clin Invest. 1995;95:1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager B, Spampinato SF, Ransohoff RM. Sphingosine 1-phosphate signaling at the blood-brain barrier. Trends Mol Med. 2015;21:354–363. doi: 10.1016/j.molmed.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Prigent-Tessier A, Quirie A, Maguin-Gate K, Szostak J, Mossiat C, Nappey M, Devaux S, Marie C, Demougeot C. Physical training and hypertension have opposite effects on endothelial brain-derived neurotrophic factor expression. Cardiovasc Res. 2013;100:374–382. doi: 10.1093/cvr/cvt219. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, El-Gengaihi A, Hussein HM, Suri MF, Liebeskind DS. Occurrence and variability in acute formation of leptomeningeal collaterals in proximal middle cerebral artery occlusion. J VascInterv Neurol. 2008;1:70–72. [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Liebeskind DS. Imaging Evaluation of Collaterals in the Brain: Physiology and Clinical Translation. Curr Radiol Rep. 2014;2:29. doi: 10.1007/s40134-013-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troidl C, Troidl K, Schierling W, Cai WJ, Nef H, Möllmann H, Kostin S, Schimanski S, Hammer L, Elsässer A, Schmitz-Rixen T, Schaper W. Trpv4 induces collateral vessel growth during regeneration of the arterial circulation. J Cell Mol Med. 2009;13:2613–2621. doi: 10.1111/j.1582-4934.2008.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Uemura A, O’uchi T, Kikuchi Y, Yashiro N, Ihara N, Shoji K. Prominent laterality of the posterior cerebral artery at three-dimensional time-of-flight MR angiography in M1-segment middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2004;25:88–91. [PMC free article] [PubMed] [Google Scholar]
- Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. 2003;107:2213–2220. doi: 10.1161/01.CIR.0000066321.03474.DA. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, Shi FD. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]


