Thoracoabdominal aortic replacement, necessary in case of injuries, aneurysms and dissections, shows a high complication rate as a consequence of the perioperative ischemia / reperfusion-sequence (I/R). Clamping above and below the lesion leads to the spinal cord suffering from ischemia. Clamping times of less than 30 minutes show only a small risk of neurological deficit, while longer periods increase paraplegia rates disproportionately. The subsequent reperfusion as a second hit causes additional damage to the spinal cord. Up to 30 % of all patients who require being treated in the thoracoabdominal part of the aorta suffer from paraplegia within the first 24 postoperative hours (Kahn et al., 2012).
While paraplegia following ischemia can be explained by the consequent death of motor neurons, reperfusion period is still poorly studied and understood. High metabolic activity and a need for substrate of the motor neurons aggravate I/R damage to the spinal cord (Sakurai et al., 1998). One of the triggers is free radicals that consume the available buffer enzymes (Gelman, 1995). Due to the loss of cellular energy (ATP), mediated by inhibition of mitochondrial phosphorylation, membrane pumps are inhibited. This in turn leads to a disequilibrium of the Na+ / K+ balance within the cell. What follows is an intracellular hyperkalemia with cellular edema and intracellular acidosis. Via other cellular mechanisms, this results in programmed cell death, namely apoptosis (Abe et al., 1995). Currently, there is no prophylaxis to avoid this damage in clinical practice of thoracic or abdominal aortic reconstruction. Although there are different approaches to this, no method has been implemented yet (Zvara, 2002). Hence, there is a need for better experimental approaches that can support the clinical treatment, in order to reduce the complication rate for thoracoabdominal aortic replacement.
A crucial part in planning such an experimental project is to choose the best fitting model. It should be a combination of being as near to humans and to the clinical setting as possible. Furthermore it should give answers to present questions and upcoming more specific questions in the future. One animal model cannot serve all this demand so that several models are needed in order to choose the best fitting one for each experimental question. Therefore, inspired by experimental set-ups described in literature, our study group established three animal models to elucidate I/R of the spinal cord resulting in paraplegia.
The large animal model was developed to offer the opportunity to map pathophysiology of aortic clamping in a clinical relevant porcine model to test potentially protective substances during I/R of the spinal cord. In this setting pigs were anesthetized and mechanically ventilated. In order to introduce two inflatable balloon catheters, femoral arteries were prepared via inguinal surgical cut downs. One of these balloon catheters was placed at the height of the left subclavian artery and the second one directly upstream of the aortic bifurcation (Figure 1). By inflating the balloons the blood flow was stopped by means of aortic occlusion in order to mimic an aortic crossclamping.
Figure 1.
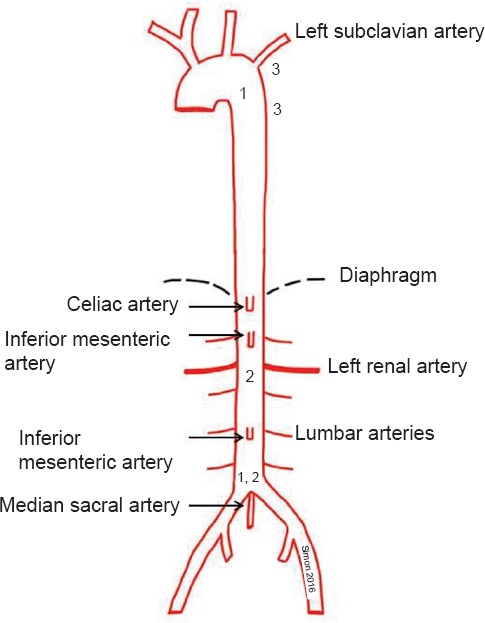
Aortic anatomy with clamping locations.
(1) Large animal model: One balloon catheter was placed at the height of the left subclavian artery and the second one directly upstream of the aortic bifurcation. (2) Medium sized animal model: Because of the strict segmental blood supply to the spinal cord in rabbits only infrarenal aortic crossclamping is needed. (3) Small animal model: Clamping of the aorta and left subclavian artery.
When using such a model permission by the local authorities to let animals wake up again is not necessarily given, so other solutions need to be found in order to be able to control spinal cord function during anesthesia. Therefore motor evoked potentials (MEP) were recorded, reflex status was tested and histological staining was performed after harvesting of the spinal cord. The recording of the MEPs of the lower extremities presented potential damage of the spinal cord while the upper limbs served as technical proof. Corresponding to the low ischemic tolerance of the spinal cord, aortic occlusion was limited to 30 minutes and the follow-up period was extended up to 10 hours. Tissue damage was evaluated using hematoxylin and eosin, Nissl, and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining. In addition several plasma parameters for inflammation and oxidative stress were taken during the experiments (Simon et al., 2008; Simon et al., 2011). The advantages of such an animal model are plain to see, because there are two complementary aspects. On the one hand, anatomy and physiology of these animals are close enough to mimic the clinical situation of human patients and on the other hand one can use clinical equipment without special adjustments because of animal size. As anatomy is a very important factor in animal studies the pig is one of the most ideal animals to study ischemia and reperfusion of the spinal cord. Like humans, these animals provide blood to the spinal cord via many vessels of different origin. In humans the spinal cord receives blood supply through the unpaired anterior spinal artery and the two posterior spinal arteries. Both originate from the vertebral arteries. The anterior spinal artery also receives blood from segmental arteries of the aorta. Perfusion in pigs differs slightly from humans as the anterior spinal artery gets additional blood influx by an anastomosis with the median sacral artery that is much more pronounced in pigs than in humans. Therefore, as pigs are concerned, terminology is “aortic trifurcation” rather than bifurcation. Another advantage of a large animal model is that enough tissue and/or blood samples can be taken for several examinations whereas in small animal models these options are much more limited. At the same time, there are also clear disadvantages when pigs or sheep are being used for experimental models. Expenses for animals and for experimental set-up are much higher and it takes a lot more of manpower to realise such elaborate projects. Compared to mouse models costs can be several hundred times higher for each experiment.
The required aortic occlusion near to the heart causes major hemodynamic imbalances and ischemia of all abdominal organs. Therefore, the large animal model should be carried out as a terminal test. For a better observation of the postoperative outcome and the effectiveness of various neuroprotective substances a rabbit model was established in which postoperative observation times up to 96 hours were performed. Even longer observation times are possible when needed for answering specific questions. Due to its special anatomy with strict segmental blood supply to the spinal cord the rabbit is an easy to use experimental model for studying clinical spinal ischemia-reperfusion injury (DeGirolami et al., 1982). Because of this segmental blood supply only infrarenal aortic crossclamping is needed to gain neuronal damage in the spinal cord level comparable to the thoracic crossclamping of the aorta in humans and pigs (Figure 1). The surgical impact can therefore be reduced to the size of a laparotomy. Due to the limited surgical trauma and the avoided ischemia of the kidney and visceral organs blood pressure could be kept stable and postoperative awakening was possible due to intact visceral organ function. The neurological examinations were done at 0, 6, 24, 36, 48, 60, 72, 84 and 96 hours postoperatively, using a modified Tarlov score. After 96 hours, the spinal cord was harvested for histopathological examination like hematoxylin-eosin staining. In literature different clamping times were mentioned, so, in this case, clamping times between 17 and 25 minutes were tested. Here, 22 minutes of aortic clamping was found to be the perfect ischemia duration to gain reproducible paraplegia. The rabbit model provides, in contrast to terminal experiments on large animals, important statements concerning the clinical effectiveness of pharmacological conditioning. Clear benefits of such a model using medium sized animals are the possibility for long reperfusion observation, relatively low costs in combination with reduced manpower needed. This model offers the opportunity to gain enough tissue and/or blood for examinations. Another positive effect is that administration of drugs that have to be given intravenously can be applied much easier than in animals of smaller size. Best vein in most of the cases is certainly one of the ear veins that can be cannulised with human peripheral venous catheter. Furthermore, more abstract results can be found than in a large animal model. Reasons for this can certainly be found at DNA level. One can see the differences among the species already in macroscopic anatomy as already described above, e.g. of the blood supply of the spinal cord. Additionally, when wanting to perform immunohistological stainings, it is very complicated to find working antibodies. The reason is that many antibodies are produced in rabbits and therefore are not specific working on rabbit tissue. So laboratory possibilities are limited by using this kind of animal model (Simon et al., 2015).
Mouse models have some important advantages. The vascular anatomy in mice is more similar to humans than in other animals such as rabbits. In mice there is one anterior and two posterior spinal arteries responsible for the spinal cord blood supply (Lang-Lazdunski et al., 2000) while e.g., in rabbits there is a strict segmental blood supply. The anatomical vessel structure in mice is the reason for the clamping of the thoracic aorta that induces an ischemia of the associated spinal cord sections, accordingly to humans and the clinical problems. Despite in large animal models clamping procedure in mice only needs 7 minutes to produce paraplegia, which causes less damage to the visceral organs compared to 30 minutes occlusion procedure in pigs. Due to the size of the animals only direct clamping of the aorta via a thoracotomy is possible, since murine blood vessels are too small for balloon occlusion (Figure 1). After the clamping procedure (including closing chest and skin), it is possible to awaken these animals and to observe the experiment for several days. Furthermore, mouse models offer also the advantage of the variety of genetically altered mouse strains. This is a clear advantage of mouse models for human diseases compared to rabbits or pigs and, therefore, should be taken into account. An additional factor in planning experimental models, although of only from a financial viewpoint, is, that animal models in mice are ten times cheaper and even more when considering following costs like keeping of animals. A single mouse (weight 20–25 g; C57BL/6J mice) costs approx. € 24, while a New Zealand White Rabbit (weight 2.5–3 kg) of the same company costs about €204 each. Finally, it is rather obvious that small animal models have model typical disadvantages. As the animal is of smallest size your experiment is limited if it is based on many or large amounts of blood or tissue samples. As already mentioned above, a small animal size needs special equipment or procedures. For example when performing a thoracotomy, intubation and ventilation is a challenge for the inexperienced scientist and takes time to be learned. Special equipment is not only needed for doing anesthesia like e.g., special inhaling units etc., but also offers a broad spectrum of experimental possibilities like e.g., whole body laser doppler imaging, which becomes the more difficult the larger the animal is.
All together, there are several aspects one should consider if thinking about building up an animal model. First of all it is important to understand that experimental models are only an attempt to mimic clinical situation and require a lot of discussion when conclusions are to be drawn with a view to humans. There will never be an ideal model. For a deeper understanding of a clinic problem or side-effect several models are needed so that the best fitting one can be chosen. This indicates that a precise formulation of the question to be answered is needed in advance. It requires a rather big budget, a lot of time and manpower to realise a large animal model that should take place in an animal intensive care. At the same time this has the benefit of more clinical relevant data acquisition. For those needing a simple model that is easy to learn and only needs an animal operation theatre but no animal intensive care unit, the rabbit model might be the right choice. It combines the small surgical impact with the possibility of long-time postoperative observation. When looking at questions dealing with DNA focus or needing animals of genetically altered strains the mouse model will fit best as it offers these advantages that normally cannot be found in other animal models. Eventually, when building up a new model one should take into account that research in literature will not replace teamwork with already experienced scientists in this field. Results will always differ from literature of other groups, because micromanagement differs. So each new experiment, especially when using a new animal model, needs a learning curve for building up one's own know-how.
References
- Abe A, Aoki M. Ischemic delayed neuronal death: a mitochondrial hypothesis. Stroke. 1995;26:1478–1489. doi: 10.1161/01.str.26.8.1478. [DOI] [PubMed] [Google Scholar]
- DeGirolami U, Zivin JA. Neuropathology of experimental spinal cord ischemia in the rabbit. J Neuropathol Exp Neurol. 1982;41:129–149. doi: 10.1097/00005072-198203000-00004. [DOI] [PubMed] [Google Scholar]
- Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82:1026–1060. doi: 10.1097/00000542-199504000-00027. [DOI] [PubMed] [Google Scholar]
- Kahn SN, Stansby G. Cerebrospinal fluid drainage for thoracic and thoracoabdominal aortic aneurysm Surgery. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD003635.pub3. CD003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang-Lazdunski L, Matsushita K, Hirt L, Waeber C, Vonsattel JP, Moskowitz MA, Dietrich WD. Spinal cord ischemia. Development of a model in the mouse. Stroke. 2000;31:208–213. doi: 10.1161/01.str.31.1.208. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Hayashi T, Abe K, Aoki M, Sadahiro M, Tabayashi K. Enhancement of heat shock protein expression after transient ischemia in the preconditioned spinal cord of rabbits. J Vasc Surg. 1998;27:720–725. doi: 10.1016/s0741-5214(98)70238-1. [DOI] [PubMed] [Google Scholar]
- Simon F, Scheuerle A, Calzia E, Bassi G, Oter S, Duy CN, Kick J, Brückner UB, Radermacher P, Schelzig H. Erythropoietin during porcine aortic balloon occlusion-induced ischemia/reperfusion injury. Crit Care Med. 2008;36:2143–2150. doi: 10.1097/CCM.0b013e31817d7912. [DOI] [PubMed] [Google Scholar]
- Simon F, Scheuerle A, Gröger M, Vcelar B, McCook O, Möller P, Georgieff M, Calzia E, Radermacher P, Schelzig H. Comparison of carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury. Intensive Care Med. 2011;37:1525–1533. doi: 10.1007/s00134-011-2303-4. [DOI] [PubMed] [Google Scholar]
- Simon F, Erhart P, Vcelar B, Scheuerle A, Schelzig H, Oberhuber A. Erythropoietin preconditioning improves clinical and histologic outcome in an acute spinal cord ischemia and reperfusion rabbit model. J Vasc Surg. 2015;S0741-5214(15):02047–9. doi: 10.1016/j.jvs.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Zvara DA. Thoracoabdominal aneurysm surgery and the risk of paraplegia: contemporary practice and future directions. J Extra Corpor Technol. 2002;34:11–17. [PubMed] [Google Scholar]


