Spinal cord injury (SCI) is a devastating pathology, with significant physical, psychosocial, and financial burdens. Despite the severity of this disease, and our deepening understanding of the pathophysiology, we remain limited in our treatment options. Recent well-publicized studies have focused on the benefit of early surgical decompression in this patient population, but fewer studies have focused on the medical management of these patients (Fehlings et al., 2012). In light of the recent controversy surrounding methylprednisolone administration, many believe that supraphysiologic mean arterial pressure (MAP) maintenance offers the best hope for improving outcomes through medical management.
The concept of MAP resuscitation in acute SCI was popularized by Vale et al. (1997). The study prospectively examined 77 SCI patients who were treated with volume resuscitation and pharmacologic blood pressure augmentation to maintain suprahysiologic MAP goals > 85 mmHg. Results indicate that incomplete SCI treated with MAP goals resulted in clinical improvement for 92% of patients studied after 12 months. Most remarkably, the authors observed improvement in 41.9% of patients who suffered complete SCIs. Based on the outcomes of the study, it was concluded that aggressive cardiopulmonary resuscitation efforts result in improved neurologic outcomes in patients with acute SCI.
The results of Vale et al. and an additional study by Levi et al. form the basis for the Level III recommendation from the American Association of Neurologic Surgeons regarding cardiopulmonary resuscitation in SCI (Levi et al., 1993; Vale et al., 1997; Ryken et al., 2013). The 2013 guidelines recommend the maintenance of MAP between 85 and 90 mmHg for the first 7 days following acute cervical SCI. Although limited by the paucity of current literature on the topic, this recommendation provides the foundation for medical management of SCI at many institutions.
At a physiologic level, it has been proposed that maintaining elevated MAP goals achieves a neuroprotective effect via two pathways. First, and arguably most importantly, the use of vasopressors serves to limit the episodes of hypotension experienced by the patient following SCI. In the days following SCI, many patients suffer from severe hemodynamic instability from a variety of factors including neurogenic shock and autonomic dysreflexia. These episodes of hypotension can place undue strain on the healing spinal cord, as decreased perfusion leads to a failure of nutrient delivery. Without appropriate perfusion, hypoxia and inadequate nutrient delivery to the injury site can serve to impair the natural healing process. In addition to avoiding hypotensive episodes, it has been proposed that overall maintenance of MAP goals also provides neuroprotection by increasing perfusion and subsequently increasing the clearance of inflammatory cytokines. By increasing cytokine clearance it may be possible to limit the inflammatory damage and secondary injury to the spinal cord following an acute SCI. Of all these mechanisms, a recent study by Hawryluk et al. (2015) suggests that avoidance of hypotension-induced hypoxia may be the most important mechanism of vasopressor neuroprotection. The presence of hypoxia at the injury site, secondary to hypotension can lead to rapid failure of the Kreb's cycle and impair the neurons’ ability to maintain ATP levels and provide the energy for cellular healing.
Maintenance of supraphysiologic MAP is not without inherent risks. In 2014, Inoue et al. (2014) reviewed the complications associated with vasopressor administration for the support of MAP goals in a cohort of 131 SCI patients. All patients were managed at a level 1 trauma center, and received at least one vasopressor for maintenance of MAP goals for an average of 120 hours. Results demonstrate high rates of vasopressor-induced complications, with 70% of patients experiencing an associated complication, including tachycardia (heart rate > 130 bpm), bradycardia (heart rate < 50 bpm), ventricular tachycardia, elevated troponins, new onset atrial fibrillation, atrial flutter, skin necrosis, electrocardiogram (ECG) ST changes consistent with ischemia, and acidosis (pH < 7.0). These clinically significant complications are noteworthy, as they likely contribute to patient instability and impact the length of stay. The data also suggest a higher rate of complications associated with dopamine administration when compared with phenylephrine. The authors conclude that vasopressor administration in SCI management yields a concerning complication rate that warrants further investigation.
Recent studies have served to further obfuscate the risk-benefit ratio for vasopressor administration. Martin et al. (2015) published a study suggesting no benefit to the maintenance of supraphysiologic MAP goals. In a retrospective study of 105 patients, the authors determined that the number of hypotensive episodes was not correlated with neurologic recovery. Specifically, the authors did not find differences in improvement when using 85 mmHg as a cutoff vs. 90 mmHg. This result is in direct contrast to a 2015 study by Hawryluk et al. (2015) which used q1 minute data to demonstrate that MAP goals > 85 mmHg were associated with improved neurologic outcomes. In examining the q1 minute data, the authors determine that elevated mean MAP during the first 2–3 days following injury was associated with improved neurologic outcomes. The authors also suggest that duration of hypotension, as defined by a MAP below the threshold of 85 mmHg, may be associated with reduced neurologic recovery. The data and conclusions of this study are strengthened by the q1 minute MAP monitoring, and provide the best evidence to-date supporting the potential benefit of vasopressor administration for SCI management. It is possible that these variable results could be related to intrathecal pressure. Given that spinal cord perfusion is inversely related to intrathecal pressure, it has been proposed that elevated intrathecal pressure following injury may also lead to decreased perfusion and increased secondary injury (Leonard and Vink, 2015). While intrathecal pressure is an important variable, we believe that consideration of MAP offers the best hope for a standardized treatment protocol in this injury pattern.
Overall these studies form a foundation for the medical management of SCI. Given the devastating nature of this injury pattern, the long term sequelae of injury, and the limited number of interventions, we continue to search for alternative and improved therapies to reduce the burden of disease. While research in MAP therapy is promising, it must be viewed contextually. Throughout these studies, spinal cord injury is treated as a homogenous injury pattern, when SCIs can vary extensively in prognosis. Variables including level of injury, mechanism of injury, presence of hemorrhagic spinal cord trauma, and pattern of intraspinal injury can all impact long-term outcomes.
Applying universal standards to what must be considered a heterogeneous patient population may not be the best approach. In a 2015 study, Readdy et al. (2015) show that patients with acute traumatic central cord injury (ATCCS) who received vasopressors for MAP goal maintenance suffered high rates of cardiogenic complications (76%). Results indicate that vasopressor administration was associated with significant and preventable adverse outcomes. These complications occurred despite the fact that ATCCS typically presents with mild to moderate symptomatology when compared to SCI as a whole. Classically, the deficits are more pronounced in the upper extremities, and the prognosis is generally considered favorable versus many other injury patterns. This variability in prognosis, accompanied by the 76% complication rate demonstrated by Readdy et al. (2015) calls into question the risk-benefit ratio of vasopressor administration in this population.
It is commonly understood that patients with complete penetrating injuries (Figure 1) are unlikely to see significant neurologic improvement with current management strategies. At a pathophysiologic level, these injuries frequently result in complete cord transection, with limited likelihood of physiologic repair. The proposed mechanism of vasopressor efficacy relates to increased perfusion of the spinal cord to allow additional nutrient influx and cytokine removal, promoting healing. In penetrating injuries, there is complete severance of the axons, possibly limiting the benefit of increased perfusion. Given the poor prognostic course of complete penetrating SCI and the potential lack of benefit in perfusion augmentation for its management, the risk-benefit ratio of vasopressor administration should be re-evaluated. This concept is not novel. In the past, when methylprednisolone was commonly administered for SCI, its use was limited to blunt injuries for similar reasons (Heary et al., 1997).
Figure 1.
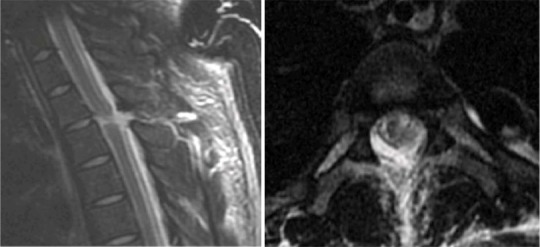
Sagittal (left) and axial (right) T2-weighted images of the upper thoracic spine demonstrate linear stab wound tract with laceration of the posterior spinal ligaments, spinal cord, and posterior longitudinal ligament at the T2–T3 vertebral level. The patient presented with a complete injury (ASIA A) and experienced no neurologic recovery during his acute hospitalization.
It is our belief that these discrepancies highlight the continued importance of clinical decision-making. The implementation of the 2013 AANS Guidelines on the administration of vasopressors in acute SCI shows promise, but clinicians must continue to exercise situational judgment. Universal application of guidelines to the heterogeneous mixture of spinal cord injury patients may result in concerning risk-benefit ratios in certain patient populations. Additionally, a multi-center study is needed to elucidate the proper management of acute SCI. To date, a major limitation in the development of such a study has been the incongruence amongst SCI data recording methods across centers. We recommend that members of the clinical SCI research community begin to adapt a standardized data collection approach, such as the National Institute of Health/National Institute of Neurologic Disorders and Stroke (NIH/NINDS) Common Data Elements to facilitate multi-center studies. Expanding the current single-institutional studies to larger multi-institutional collaborations is essential, given the limited incidence of acute SCI. Multi-center studies will provide the statistical power to permit researchers to analyze the risks and benefits of vasopressor administration in subpopulations of SCI, such as ATCCS, penetrating SCI, and others. These subpopulation analyses will allow for clearer, personalized guidelines while also elucidating the true risk-benefit ratio of vasopressor administration in each group.
The authors would like to thank Iqra Farooqi for her role in developing and editing this manuscript.
References
- Fehlings MG, Vaccaro A, Wilson JR, Singh A, D WC, Harrop JS, Aarabi B, Shaffrey C, Dvorak M, Fisher C, Arnold P, Massicotte EM, Lewis S, Rampersaud R. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk G, Whetstone W, Saigal R, Ferguson A, Talbott J, Bresnahan J, Dhall S, Pan J, Beattie M, Manley G. Mean arterial blood pressure correlates with neurological recovery after human spinal cord injury: Analysis of high frequency physiologic data. J Neurotrauma. 2015;32:1958–1967. doi: 10.1089/neu.2014.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heary RF, Vaccaro AR, Mesa JJ, Northrup BE, Albert TJ, Balderston RA, Cotler JM. Steroids and gunshot wounds to the spine. Neurosurgery. 1997;41:576–583. doi: 10.1097/00006123-199709000-00013. discussion 583-574. [DOI] [PubMed] [Google Scholar]
- Inoue T, Manley GT, Patel N, Whetstone WD. Medical and surgical management after spinal cord injury: vasopressor usage, early surgerys, and complications. J Neurotrauma. 2014;31:284–291. doi: 10.1089/neu.2013.3061. [DOI] [PubMed] [Google Scholar]
- Leonard AV, Vink R. Reducing intrathecal pressure after traumatic spinal cord injury: a potential clinical target to promote tissue survival. Neural Regen Res. 2015;10:380–382. doi: 10.4103/1673-5374.153683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi L, Wolf A, Belzberg H. Hemodynamic parameters in patients with acute cervical cord trauma: description, intervention, and prediction of outcome. Neurosurgery. 1993;33:1007–1016. [PubMed] [Google Scholar]
- Martin ND, Kepler C, Zubair M, Sayadipour A, Cohen M, Weinstein M. Increased mean arterial pressure goals after spinal cord injury and functional outcome. J Emerg Trauma Shock. 2015;8:94–98. doi: 10.4103/0974-2700.155507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readdy WJ, Whetstone WD, Ferguson AR, Talbott JF, Inoue T, Saigal R, Bresnahan JC, Beattie MS, Pan JZ, Manley GT, Dhall SS. Complications and outcomes of vasopressor usage in acute traumatic central cord syndrome. J Neurosurg Spine. 2015:1–7. doi: 10.3171/2015.2.SPINE14746. [DOI] [PubMed] [Google Scholar]
- Ryken TC, Hurlbert RJ, Hadley MN, Aarabi B, Dhall SS, Gelb DE, Rozzelle CJ, Theodore N, Walters BC. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery. 2013;2:84–92. doi: 10.1227/NEU.0b013e318276ee16. [DOI] [PubMed] [Google Scholar]
- Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]


