Keywords: nerve regeneration, diabetes, substance P, epidermal stem cells, sensory nerve, wound healing, collagen, neural regeneration
Abstract
Exogenous substance P accelerates wound healing in diabetes, but the mechanism remains poorly understood. Here, we established a rat model by intraperitoneally injecting streptozotocin. Four wounds (1.8 cm diameter) were drilled using a self-made punch onto the back, bilateral to the vertebral column, and then treated using amniotic membrane with epidermal stem cells and/or substance P around and in the middle of the wounds. With the combined treatment the wound-healing rate was 100% at 14 days. With prolonged time, type I collagen content gradually increased, yet type III collagen content gradually diminished. Abundant protein gene product 9.5- and substance P-immunoreactive nerve fibers regenerated. Partial nerve fiber endings extended to the epidermis. The therapeutic effects of combined substance P and epidermal stem cells were better than with amniotic membrane and either factor alone. Our results suggest that the combination of substance P and epidermal stem cells effectively contributes to nerve regeneration and wound healing in diabetic rats.
Introduction
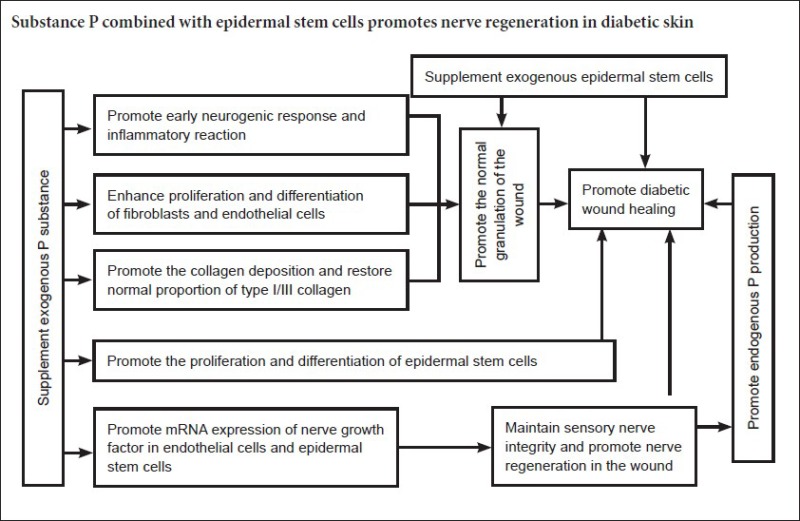
Recent studies have shown that decreased substance P content and epidermal stem cell number and activity are important factors in diabetic wound healing (Edmonds and Foster, 2006; Liu et al., 2006; Rathur and Boulton, 2007; Fiorina et al., 2010; Guo et al., 2010). Substance P belongs to the tachykinin family, and is mainly present in sensory nerve endings of skin tissue. It has neurotransmitter, neuromodulatory, and hormone effects, promoting cell division, and keratinocyte and fibroblast proliferation (O’Connor et al., 2004; Kawana et al., 2006). Moreover, exogenous substance P accelerates epidermal stem cell migration and promotes wound healing (Li et al., 2003). Epidermal stem cells effectively promote wound healing in diabetes mellitus cases. Therefore, the relationship between substance P, epidermal stem cells, and peripheral nerve regeneration is important in the diabetic environment. Substance P improves the microcirculation and promotes epidermal stem cell proliferation, differentiation, and migration. In addition, epidermal stem cells stimulate cellular secretion of substance P, while substance P and epidermal stem cells promote peripheral nerve regeneration (Guo et al., 2008; Zhong et al., 2010). Thus, this study investigated the promoting effect of substance P combined with epidermal stem cells on diabetic wound healing and nerve regeneration.
Materials and Methods
Experimental animals
Sixty pathogen-free Sprague-Dawley rats (equal number of males and females, aged 3 months old, and weighing 200–250 g) were provided by the Animal Center of the Medical Department of Nanchang University of China (Animal Certificate No. 96021) and allowed to acclimate for 1 week. All animal experiments were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). All studies were approved by the Animal Care and Use Committee of First Affiliated Hospital of Nanchang University of China. All efforts were made to minimize pain and distress of the experimental animals.
Epidermal stem cell isolation and culture
Type IV collagen (Sigma, Santa Clara, CA, USA) was diluted to a concentration of 100 mg/L (6–10 μg/cm2) with PBS, evenly spread on culture bottles, and air-dried on the benchtop. Three healthy rats were intraperitoneally injected with 3% sodium pentobarbital. The skin on their back was aseptically removed and rinsed three times with D-Hank's solution containing magnamycin 800 U/mL and streptomycin 100 U/mL (5 minutes each time). Subcutaneous tissue was isolated using a blade, rinsed with D-Hank's solution (three times, 5 minutes each), and then sliced into 3.0–10.0 mm strips using eye scissors. Strips were placed in a culture bottle with 0.25% trypsin (Gibco, Carlsbad, CA, USA), and 0.02% ethylenediamine tetraacetic acid (EDTA) added at a volume ratio of 1:5, followed by incubation at 4°C for 10–13 hours (digestion time in the diabetes group was 1–2 hours shorter than the normal group). Digestion was terminated by adding fetal bovine serum. Samples were washed three times with D-Hank's solution. The cuticle layer was removed using ophthalmic forceps, and the epidermis cut up. Digestion was terminated by adding serum-free keratinocyte medium. After repeated blowing, samples were filtered through a 200-mesh filter and centrifuged at 1,000 r/min for 5 minutes. After removal of the supernatant, cells were suspended in serum-free keratinocyte medium. Type IV collagen was added and the mixture incubated in a 5% CO2 incubator at 37°C with saturated humidity. The culture medium was discarded. Twenty minutes later, the cells were resuspended. Epidermal stem cell medium containing 10% fetal bovine serum, 10 μg/L epidermal growth factor (Shanghai Y-S Biotechnology Co., Ltd., Shanghai, China), and serum-free keratinocyte medium was added, followed by incubation in a 5% CO2 incubator at 37°C with saturated humidity. K19/β1-integrin was identified by immunocytochemical staining (Sun et al., 2007). Second passage epidermal stem cells were digested with 0.25% trypsin, centrifuged at room temperature, washed with PBS, suspended with serum-free keratinocyte medium, and seeded on a feeder layer in a 5% CO2 incubator at a constant temperature of 37°C with saturated humidity (Reiisi et al., 2010; Zhong et al., 2011; Zhu et al., 2011).
Amniotic membrane construction
Fetal membrane was obtained from puerpera undergoing spontaneous deliveries. The chorion was bluntly removed with the back of a knife. The fetal membrane was flushed three times with PBS, and the amniotic membrane soaked in 0.25% trypsin and 0.02% EDTA (Gibco) digestive juice for one hour at 37°C. Epidermal cells on the amniotic membrane surface were gently wiped with the back of a knife, and rinsed in PBS solution to determine if they were still on the amniotic membrane surface. The amniotic membrane was cut into 2 cm × 2 cm blocks with a benchtop cutter, and the blocks placed flat into six-well culture plates. The epidermal surface was upwards and covered approximately 2/3 of the bottle. Culture plates were inverted in a 37°C incubator for 3–4 hours to dry the amniotic membrane. Epidermal stem cell culture medium supplemented with 10% fetal bovine serum and serum-free keratinocyte medium was added to the culture bottle overnight. For collection of fetal membrane, informed consent for puerpera was obtained from family members. The protocols were approved by the Institutional Review Board at First Affiliated Hospital of Nanchang University of China, and performed in accordance with the Declaration of Helsinki.
Second passage epidermal stem cells at 70% confluence were digested with 0.25% pancreatic enzyme at room temperature, centrifuged, and washed with PBS. Cells were added to culture medium at a concentration of 1 × 106 cells/mL. A human amniotic membrane breeding layer was prepared and placed in a constant temperature incubator. Many round bright epidermal stem cells were distributed on the amniotic membrane surface. Good cell confluence accounted for approximately 40% of the amniotic membrane (Zhong et al., 2010).
Establishment of a diabetic skin injury model and combined treatment of substance P and human amniotic membrane
Using urine indicator paper and a Johnson glucose meter (Johnson & Johnson Medical (Shanghai) Ltd., Shanghai, China), urine glucose and blood sugar in the normal range was determined in 57 rats. After fasting for 16 hours, rat models were established by intraperitoneally injecting streptozotocin (65 mg/kg; Sigma) dissolved in 0.1 M citric acid buffer solution. Forty-eight hours later, urine glucose and blood sugar were determined, once a week. The standard for establishment of a successful diabetes model is three consecutive blood sugars higher than 16.7 mM and urine glucose readings of +++–++++ (Rees and Alcolado, 2005). Five rats were eliminated: three died because of failure and two did not reach the above standard. Thus, our model success rate was 85%.
Forty-eight modeled rats were randomly taken and intraperitoneally anesthetized with 3% sodium pentobarbital. After disinfection and shaving, full-thickness dermal wounds (diameters of 1.8 cm) were made using a self-made punch. Each rat had four wounds. Bleeding was stopped.
The 48 modeled rats were randomly divided into groups A–D (Table 1). In groups A and B, the wounds were coated with human amniotic membrane incubated with epidermal stem cells. In groups C and D, the wounds were coated with human amniotic membrane without epidermal stem cells. In groups A and C, 1 × 10-7 M substance P (Sigma; PBS as a solvent) (250 μL) was injected into eight points and the center of the wound after transplantation, twice a day, for 4 consecutive days. In groups B and D, 250 μL of PBS was injected. All wounds received pressure dressings. All rats were housed at room temperature for 3 days.
Table 1.
Interventions in each group
Macroscopic observation of wound healing
Wound healing was observed and recorded at 4, 7, 10, 14, 17, and 23 days. According to the following formula, the wound healing area percentage was calculated by (initial area – measured wound area)/initial area × 100%.
Sample collection
Eight rats were randomly selected at 4, 7, 10, 14, 17, and 23 days after injury. Each group had eight wounds. Rats were sacrificed by intraperitoneal injection with sodium pentobarbital. Wound samples were harvested, fixed with 4% polyphosphate formaldehyde, embedded in paraffin, and sliced into 5 μm thick sections.
Hematoxylin-eosin staining
Slices were dewaxed, hydrated, stained with hematoxylin for 5–10 minutes, treated with a mixture of hydrochloric acid/alcohol with 1% ammonia, stained with eosin for 2 minutes, dehydrated, and permeabilized. The edges of tissue slices were cleaned using neutral resin and covered with coverslips. Pathological change was observed by light microscopy (Olympus (China) Co., Ltd., Shanghai, China).
Masson staining
Masson staining was performed at 4, 7, and 10 days after injury. Sections were deparaffinized, hydrated, stained with hematoxylin for 5–10 minutes, washed with running water, treated with 1% hydrochloric acid, and washed again with running water for several minutes. Samples were stained with Masson composite staining solution (Zhongshan Golden Bridge Co., Ltd., Beijing, China) for 5–10 minutes, washed with distilled water, treated with 1% phosphowolframic acid for 5 minutes, directly stained with aniline blue dye for 5 minutes, treated with 1% glacial acetic acid for 1 minute, dehydrated with 95% alcohol and absolute ethanol, treated with xylene, mounted with neutral resin, and observed with a light microscope.
Immunohistochemical staining
Expression of types I and III collagen was detected at 4, 7, and 10 days after injury. At 17 and 23 days, protein gene product 9.5 (PGP9.5), a nerve fiber marker (Sun et al., 2014; Zhu et al., 2014), and substance P expression were detected. Sections (5 or 20 μm) were dewaxed, hydrated, inactivated with 3% H2O2 at room temperature, retrieved by high pressure heat, and blocked with normal goat serum. Samples were then incubated with mouse anti-type I collagen antibody (1:400; Zhongshan Golden Bridge Co., Ltd.) or mouse anti-type III collagen antibody (1:400; Zhongshan Golden Bridge Co., Ltd.) at 4°C overnight, or mouse anti-substance P antibody (1:200; Boster, Wuhan, Hubei Province, China) or mouse anti-PGP9.5 antibody (1:200; Abcam, Cambridge, UK) at 37°C for 120 minutes. All primary antibodies were followed by biotinylated goat anti-mouse IgG (ready-to-use; Zhongshan Golden Bridge Co., Ltd.) at 37°C for 20 minutes. All samples were visualized with 3,3′-diaminobenzidine at room temperature, counterstained with hematoxylin, then dehydrated, permeabilized, and mounted in neutral resin. Negative controls used PBS instead of primary antibody. Samples were observed using a light microscope (200× or 400×). Image-Pro Plus 6.0 image analysis system was used to determine type I collagen, type III collagen, PGP9.5, and substance P expression. For types I and III collagen, the results were expressed as optical density percentages. For PGP9.5 and substance P, the results were expressed as a ratio of positively stained to total area.
Statistical analysis
All data were analyzed and processed using Microsoft Office Excel 2000 (Microsoft Corporation, Redmond, WA, USA) and SPSS 16.0 software (SPSS, Chicago, IL, USA). Data were expressed as the mean ± SEM. The difference among groups was compared using one-way analysis of variance. The difference between groups was compared using Student's t-test. P-values less than 0.05 were con-sidered to be statistically significant.
Results
Substance P combined with epidermal stem cells promoted wound healing in diabetic rats
In group A, after injecting substance P, skin around the wounds was visibly wrinkled with edema present. At 4 days, there was thick granulation tissue, abundant inflammatory cell infiltration, and a severe early inflammatory reaction. Numerous epidermal cells were observed under the amniotic membrane with some cells showing a dual-nucleus mitotic phase. The wounds had visibly contracted at 7 days after injury. The number of infiltrated inflammatory cells in granulation tissue decreased. There was proliferation of a large number of fibroblasts and endothelial cells, which were densely arranged with apparent vascularization and evident epidermal growth around wounds. At 10 days, the number of capillaries was reduced, and regularly arranged fibroblasts secreted collagen. Fibroblasts and keratinocytes proliferated greatly. Keratinocytes crawled and extended from the edge to the middle of wounds. Epidermal stem cells proliferated and coated the wounds. At 14 days, the wounds had completely healed. In group D, without substance P injection, the skin was not wrinkled nor edema present around the wounds. At 4 days, granulation tissue formed and a few inflammatory cells had infiltrated. At 7 days, the wounds began to shrink. Abundant inflammatory cell infiltration was observed in granulation tissue. A few fibroblasts and endothelial cells proliferated. The epithelium extended around the wounds. At 10 days, the wounds were noticeably smaller. A few inflammatory cells had infiltrated. Fibroblasts and endothelial cells proliferated to some extent. Fibroblasts were irregularly arranged. Part of the wounds was covered by keratinocytes, which crawled and extended from the edge to the middle of the wounds. At 23 days, the wounds had completely healed. In group C, the changes in granulation tissue were similar to that of group A. Keratinocyte coverage was similar to that in group D. At 17 days, the wounds had healed completely. In group B, the changes in granulation tissue were in-between that of groups A and D. Keratinocyte coverage on the wounds was similar to that in group A. At 17 days, the wounds had healed completely (Figures 1, 2). The wound healing area percentage was significantly higher in group A than in group D (P < 0.01), and significantly higher than in the single factor groups, B and C (P < 0.01; Table 2).
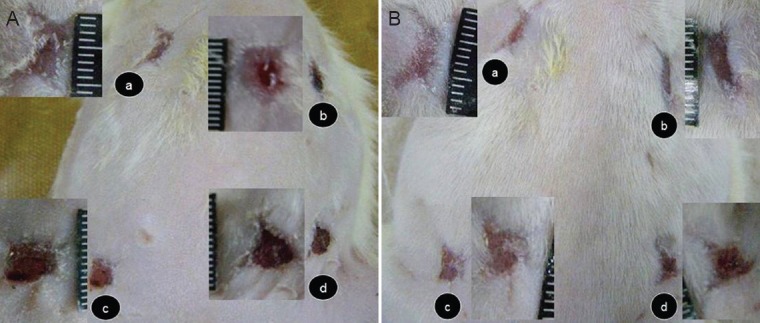
Figure 1.
Wounds in diabetic rats at 14 and 17 days after injury.
(A) At 14 days after injury, wounds had healed: the surface was stripped and the wounds flat (a) in group A; the other three groups did not heal (b–d), although the control group did. (B) At 17 days after injury, groups A, B, and C had healed. a–d: Groups A–D. Group A: Injection of substance P + human amniotic membrane incubated with epidermal stem cells around and at the center of the wound; group B: injection of 250 μL PBS + human amniotic membrane incubated with epidermal stem cells around and at the center of the wound; group C: injection of substance P + human amniotic membrane without epidermal stem cells around and at the center of the wound; group D: injection of 250 μL PBS + human amniotic membrane without epidermal stem cells around and at the center of the wound. PBS: Phosphate buffered saline.
Figure 2.

Pathological changes of wound tissue at 4, 7, 14, 17, 23 days after injury (hematoxylin-eosin staining, × 200)
The skin was visibly wrinkled and edema present after injury in all groups. With prolonged time, fibroblast and endothelial cell proliferation appeared. The wounds healed at various time points. Healing was fastest in group A and slowest in group D. Granulation changes in group C were similar to that in group A. Keratinocyte coverage on wounds in group C was similar to that in group D. Granulation changes in group B were in-between groups A and D. Keratinocyte coverage on wounds in group B was similar to that in group A. Group A: injection of substance P + human amniotic membrane incubated with epidermal stem cells around and at the center of the wound; group B: injection of 250 μL PBS + human amniotic membrane incubated with epidermal stem cells around and at the center of the wound; group C: injection of substance P + human amniotic mem-brane without epidermal stem cells around and at the center of the wound; group D: injection of 250 μL PBS + human amniotic membrane without epidermal stem cells around and at the center of the wound. PBS: Phosphate buffered saline.
Table 2.
Effect of substance P combined with epidermal stem cells on wound healing area percentage in diabetic rats
Substance P combined with epidermal stem cells promoted collagen synthesis in granulation tissue in diabetic rats
Masson staining showed that collagen synthesis was less at the site of injury in each group at 4 and 7 days after injury, therefore collagen stained could not be compared (data not shown). However, in groups A and C at 10 days, a large number of fibroblasts in granulation tissue, darkly stained collagen, a large positive area, and ordered cells were visible. Morever in group B, a few fibroblasts, lightly stained collagen, a moderate positive area, and ordered cells were observed. While in group D, abundant fibroblasts, many capillaries, lightly stained collagen, a small positive area, and disordered cells, were detected (Figure 3).
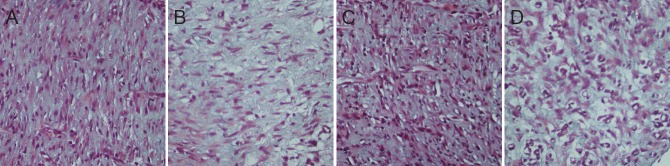
Figure 3.
Effect of substance P combined with epidermal stem cells on collagen synthesis in granulation tissue from diabetic rats at 10 days after injury (Masson staining, × 400).
(A) Group A: Darkly stained collagen, large area, and ordered arrangement; (B) group B: lightly stained collagen, large area, and ordered arrangement; (C) group C: darkly stained collagen, large area, and ordered arrangement; (D) group D: lightly stained collagen, small area, and disordered arrangement. Group A: injection of substance P + human amniotic membrane incubated with epidermal stem cells around and at the center of the wound; group B: injection of 250 μL PBS + human amni-otic membrane incubated with epidermal stem cells around and at the center of the wound; group C: injection of substance P + human amniotic membrane without epidermal stem cells around and at the center of the wound; group D: injection of 250 μL PBS + human amniotic membrane without epidermal stem cells around and at the center of the wound. PBS: Phosphate buffered saline.
Immunoreactivities of types I and III collagen
In each group, type I collagen immunoreactivity gradually increased with prolonged time. In contrast, type III collagen immunoreactivity was high in the early stage of injury, but gradually decreased with healing. The optical density percentage for types I and III collagen was significantly higher in groups A and C than in group D (P < 0.01). The optical density percentage for types I and III collagen was similar between groups B and D (P > 0.05; Figure 4, Table 3).
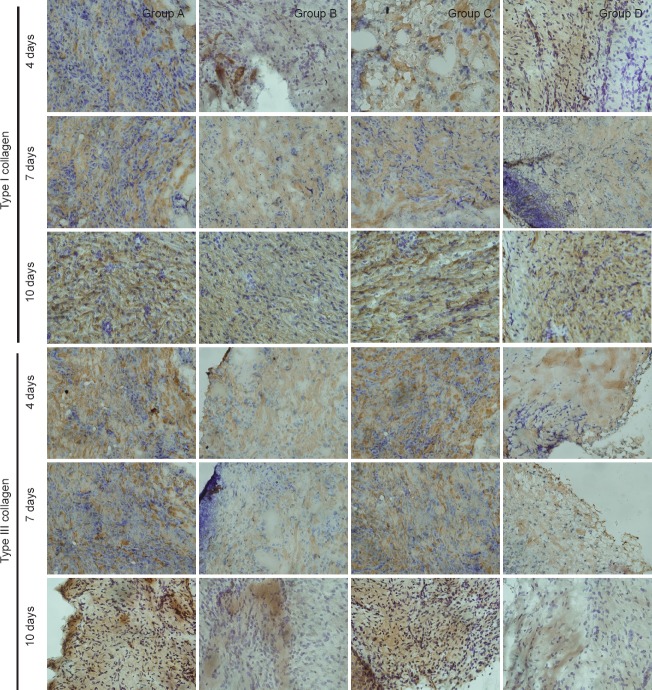
Figure 4.
Effect of substance P combined with epidermal stem cells on types I and III collagen immunoreactivities in wounds of diabetic rats at 4, 7, 10 days after injury (immunohistochemical staining, × 400)
Type I collagen immunoreactivity gradually increased with time in each group. Type III collagen immunoreactivity was high in the early stage of injury, but gradually reduced with healing. Immunoreactivity is shown by a brown color. Group A: injection of substance P + human amniotic membrane incubated with epidermal stem cells around and at the center of the wound; group B: injection of 250 μL PBS + human amniotic membrane incubated with epidermal stem cells around and at the center of the wound; group C: injection of substance P + human amniotic membrane without epidermal stem cells around and at the center of the wound; group D: injection of 250 μL PBS+ human amniotic membrane without epidermal stem cells around and at the center of the wound. PBS: Phosphate buffered saline.
Table 3.
Effect of substance P combined with epidermal stem cells on types I and III collagen immunoreactivities (optical density percentage) in wounds of diabetic rats
Substance P combined with epidermal stem cells promoted PGP9.5 and type III collagen immunoreactivity in granulation tissue of diabetic rats
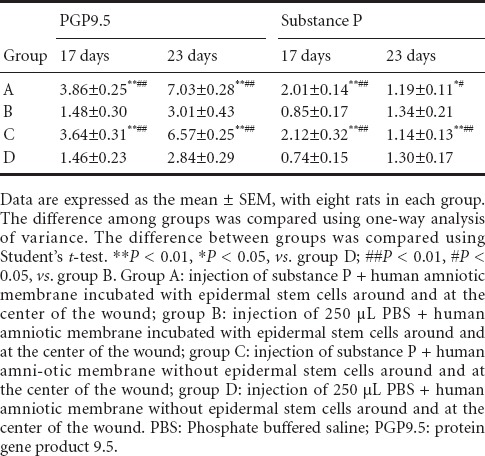
In groups A and C, immunohistochemical staining revealed that PGP9.5-immunoreactive nerve fibers had noticeably regenerated and extended towards the central injury site at 17 days after injury. Regeneration of abundant nerve fibers was visible in the deeper part of the wound. Regenerating nerve fibers extended towards the epidermis. Simultaneously, distinctly regenerating substance P-immunoreactive fibers were observed in healed wounds, partially close to the epidermis. In groups B and D, nerve fibers seldom regenerated in healed wounds and did not obviously extend. Substance P-immunoreactive fibers did not really regenerate. In groups A and C at 23 days, the number of regenerating nerves had increased, close to that in normal tissue. PGP9.5-immunoreactive fibers were identified at the dermal and epidermal junction. The number of substance P-immunoreactive fibers was markedly diminished. In groups B and D, the number of regenerating nerves had increased, and PGP9.5-immunoreactive fibers were detected at the dermal and epidermal junction. Nevertheless, these fibers were noticeably less in groups B and D than in groups A and C (Figure 5, Table 4).
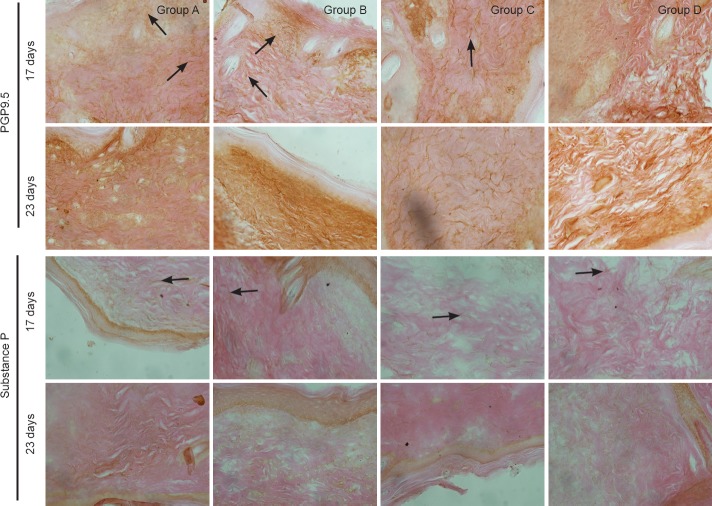
Figure 5.
Effect of substance P combined with epidermal stem cells on PGP9.5 and substance P immunoreactivities in wounds of diabetic rats at 17 and 23 days after injury (immunohistochemical staining, × 400). In groups A and C at 17 days after injury, PGP9.5-immunoreactive fibers had regenerated and a large number of substance P-immunoreactive fibers were visible in healed wounds. In groups B and D, there were less regenerating fibers in healed wounds, and substance P-immunoreactive fibers were detected. In groups A and C at 23 days, PGP9.5-immunoreactive fibers were detected at the dermal and epidermal junction. Substance P-immunoreactive fibers had noticeably diminished. In groups B and D, the number of regenerating nerves had increased. Substance P-immunoreactive fibers were detected at the dermal and epidermal junction. Arrows show immunoreactivity. Group A: injection of substance P + human amniotic membrane in-cubated with epidermal stem cells around and at the center of the wound; group B: injection of 250 μL PBS + human amniotic membrane incubated with epidermal stem cells around and at the center of the wound; group C: injection of substance P + human amniotic membrane without epidermal stem cells around and at the center of the wound; group D: injection of 250 μL PBS + human amniotic membrane without epidermal stem cells around and at the center of the wound. PBS: Phosphate buffered saline; PGP9.5: protein gene product 9.5.
Table 4.
Effect of substance P combined with epidermal stem cells on number of PGP9.5 and substance P immunoreactive fibers (relative to total area) in wounds of diabetic rats
Discussion
Substance P promotes wound repair and epidermal stem cell migration and neovascularization in diabetic skin (Edmonds and Foster, 2006; Rathur and Boulton, 2007; Fiorina et al., 2010; Guo et al., 2010). To further study the effect of substance P and epidermal stem cells in skin wound healing and nerve regeneration following diabetes, we used streptozotocin to induce a diabetic rat model, consistent with a previous study (Zhong et al., 2011).
Our results show that in group A, wound healing ap-peared early, granulation tissue rapidly formed early, type I collagen content was high with a large positive area, and dark collagen staining. These findings indicate that epidermal stem cells have growth characteristics and extend into the dermis with substance P treatment. Indeed, the healed skin was close to a physiological state and had high resistance. It was striking that in groups A and C, abundant PGP9.5- and substance P-immunoreactive nerve fibers regenerated, with some extending close to the epidermis. In groups B and D, there were less regenerating nerve fibers in healed wounds. Substance P can promote peripheral nerve repair and regeneration, and is one of the important factors for diabetic wound healing.
The mechanism underlying the promoting effect of sub-stance P combined with epidermal stem cells on diabetic wound healing and nerve regeneration may be: (1) skin contains abundant sensory nerve fibers, which can release the neuropeptide substance P that belongs to the tachykinin family and is one of the first to appear in wound inflammation. After trauma, abundant substance P is released into local tissue to enhance the early inflammatory reaction and promote proliferation and migration of fibroblasts and endothelial cells (Behjati and Hashemi, 2009; Li and Wang, 2011), consequently forming granulation tissue. Simultaneously, substance P affects peripheral nerve repair and promotes epidermal stem cell proliferation, migration, and differentiation, thereby promoting wound healing (Ikeda et al., 2007; Wang et al., 2009). Low substance P synthesis and secretion in diabetes mellitus disrupts the entire repair process and delays wound healing. Exogenous substance P not only directly improves partial substance P content, but can also activate keratinocytes, fibroblasts, endothelial cells, and inflammatory cells to secrete endogenous substance P. Additionally, exogenous substance P further increases substance P content and compensates for the dearth of substance P in diabetic wound tissue, which enhances early neurogenic inflammatory reactions, rapidly contracts the initial wound, reduces the wound surface area, and stabilizes the in vivo surroundings. In summary, substance P combined with epidermal stem cells promotes fibroblast proliferation, migration, and differentiation, increases synthetic collagen (especially synthesis of type IV collagen), provides support for epidermal stem cell adhesion and proliferation, simultaneously promotes proliferation, migration, and differentiation of vascular endothelial cells, accelerates blood vessel and granulation tissue formation, improves vascular lesions and wound microcirculation, immediately protects wounds, and provides a good environment for epidermal cell proliferation and migration. (2) Epidermal stem cells are used as a source of skin grafts, and actively participate in wound repair (Kaur and Li, 2000; Velander et al., 2009) and promote reepithelization. Moreover, substance P binds to the substance P-related receptors, NK1 and NK2, in skin stem cells, therefore the interaction between epidermal stem cells and substance P can improve the microenvironment of epidermal stem cells. From our study, we can infer that active chemokines generated by the interaction between exogenous epidermal stem cells and substance P support nerve endings extending to the epidermis, and accelerate epithelization and wound healing. (3) Substance P plays an important role in maintaining sensory nerve integrity and regeneration. Substance P as an inflammatory neurotrophic factor increases mRNA expression of neurotrophic factors in endothelial cells and keratinocytes. Nerve growth factor is the most important nerve factor in nerve regeneration. Diabetes causes peripheral neuropathy, which prevents sensory nerve endings releasing substance P, which in turn causes a decrease in nerve growth factor and induces disordered nerves, subsequently forming a vicious cycle. In addition, diabetes as a peripheral neuropathy reduces substance P secretion. Nervous breakdown and nerve deficit also reduce substance P secretion and impairs wound healing. Thus, exogenous substance P maintains sensory nerve integrity and promotes nerve regeneration. Nerves secrete repair factors such as substance P, nerve growth factor, and cell factors, and accelerate wound healing (Dunnick et al., 1996; Matsuda et al., 1998; Scholzen et al., 1998; Kishi et al., 2006; Scott et al., 2008; Sahbaie et al., 2009).
Based on experimental results, we believe that skin nerve fiber regeneration is useful in improving the quality of wound healing and functional reconstruction. In recent years, artificial skin created using tissue engineering techniques for the treatment of surgical wounds in severe burn, trauma, and diabetic ulcers is a new idea and trend (Mahjour et al., 2012; Soller et al., 2012; Biedermann et al., 2013; Blais et al., 2013; Wu et al., 2013; Du et al., 2014; Gardien et al., 2014). However at the present stage, the skin created has not yet achieved functional nerve regeneration and restoration. If the skin structure is designed from the nerve regeneration angle, and neurogenic cells, neurotrophic factors or substance P are introduced, greater effects in physiological healing and functional reconstruction of the skin may be achieved. Additionally, promoting the effect of substance P on nerve regeneration and migration may prevent and treat peripheral neuropathy in diabetes.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30560058; a grant from the Science and Technology Planning Project of Jiangxi Province, China, No. 20133BBG70026.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by James R, Haase R, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Behjati M, Hashemi M. Application of fibrocytes in the treatment of diabetic foot: as a potential new therapeutic approach. Diabetes Res Clin Pract. 2009;86:152–153. doi: 10.1016/j.diabres.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Biedermann T, Böttcher-Haberzeth S, Klar A, Pontiggia L, Schiestl C, Meuli-Simmen C, Reichmann E, Meuli M. Rebuild, restore, reinnervate: do human tissue engineered dermo-epidermal skin analogs attract host nerve fibers for innervation? Pediatr Surg Int. 2013;29:71–78. doi: 10.1007/s00383-012-3208-1. [DOI] [PubMed] [Google Scholar]
- Blais M, Parenteau-Bareil R, Cadau S, Berthod F. Concise review: tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med. 2013;2:545–551. doi: 10.5966/sctm.2012-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Gao XQ, Deng L, Chang NB, Xiong HL, Zheng Y. Transfection of the glial cell line-derived neurotrophic factor gene promotes neuronal differentiation. Neural Regen Res. 2014;9:33–40. doi: 10.4103/1673-5374.125327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick CA, Gibran NS, Heimbach DM. Substance P has a role in neurogenic mediation of human burn wound healing. J Burn Care Rehabil. 1996;17:390–396. doi: 10.1097/00004630-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Edmonds ME, Foster AV. Diabetic foot ulcers. BMJ. 2006;332:407–410. doi: 10.1136/bmj.332.7538.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorina P, Pietramaggiori G, Scherer SS, Jurewicz M, Mathews JC, Vergani A, Thomas G, Orsenigo E, Staudacher C, La Rosa S, Capella C, Carothers A, Zerwes HG, Luzi L, Abdi R, Orgill DP. The mobilization and effect of endogenous bone marrow progenitor cells in diabetic wound healing. Cell Transplant. 2010;19:1369–1381. doi: 10.3727/096368910X514288. [DOI] [PubMed] [Google Scholar]
- Gardien KL, Middelkoop E, Ulrich MM. Progress towards cell-based burn wound treatments. Regen Med. 2014;9:201–218. doi: 10.2217/rme.13.97. [DOI] [PubMed] [Google Scholar]
- Guo WY, Wang GJ, Wang P, Chen Q, Tan Y, Cai L. Acceleration of diabetic wound healing by low-dose radiation is associated with peripheral mobilization of bone marrow stem cells. Radiat Res. 2010;174:467–479. doi: 10.1667/RR1980.1. [DOI] [PubMed] [Google Scholar]
- Guo WZ, Lai XN, Wang ZG, Liu YJ, Wang LL. Application of exogenous substance P in wound healing of skin injury in diabetic rat. Chuangshang Waike Zazhi. 2008;10:251–253. [Google Scholar]
- Ikeda Y, Takei H, Matsumoto C, Mase A, Yamamoto M, Takeda S, Ishige A, Watanabe K. Administration of substance P during a primary immune response amplifies the secondary immune response via a long-lasting effect on CD8+ T lymphocytes. Arch Dermatol Res. 2007;299:345–351. doi: 10.1007/s00403-007-0767-4. [DOI] [PubMed] [Google Scholar]
- Kaur P, Li A. Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Investig Dermatol. 2000;114:413–420. doi: 10.1046/j.1523-1747.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- Kawana S, Liang Z, Nagano M, Suzuki H. Role of substance P in stress-derived degranulation of dermal mast cells in mice. J Dermatol Sci. 2006;42:47–54. doi: 10.1016/j.jdermsci.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kishi K, Ohyama K, Satoh H, Kubota Y, Tanaka T, Imanishi N, Nakajima H, Kawamura K, Nakajima T. Mutual dependence of murine fetal cutaneous regeneration and peripheral nerve regeneration. Wound Repair Regen. 2006;14:91–99. doi: 10.1111/j.1743-6109.2005.00093.x. [DOI] [PubMed] [Google Scholar]
- Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. Journal of tissue viability. 2011;20:108–120. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF, Fu XB, Sheng ZY, Sun TZ. Redistribution of epidermal stem cells in wound edge in the process of re-epithelialization. Zhonghua Yixue Zazhi. 2003;83:228–231. [PubMed] [Google Scholar]
- Liu S, Chen L, Cai HW, Chen B. Detection and significance of substance P in plasma and skin tissue of diabetic rats. Disan Junyi Daxue Xuebao. 2006;28:1038–1039. [Google Scholar]
- Mahjour SB, Ghaffarpasand F, Wang H. Hair follicle regeneration in skin grafts: current concepts and future perspectives. Tissue Eng Part B Rev. 2012;18:15–23. doi: 10.1089/ten.teb.2011.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- Rathur HM, Boulton AJ. The diabetic foot. Clin Dermatol. 2007;25:109–120. doi: 10.1016/j.clindermatol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabetic Med. 2005;22:359–370. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Reiisi S, Esmaeili F, Shirazi A. Isolation, culture and identification of epidermal stem cells from newborn mouse skin. In Vitro Cell Dev Biol Anim. 2010;46:54–59. doi: 10.1007/s11626-009-9245-y. [DOI] [PubMed] [Google Scholar]
- Sahbaie P, Shi X, Guo TZ, Qiao Y, Yeomans DC, Kingery WS, Clark JD. Role of substance P signaling in enhanced nociceptive sensitization and local cytokine production after incision. Pain. 2009;145:341–349. doi: 10.1016/j.pain.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol. 1998;7:81–96. doi: 10.1111/j.1600-0625.1998.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Scott JR, Tamura RN, Muangman P, Isik FF, Xie C, Gibran NS. Topical substance P increases inflammatory cell density in genetically diabetic murine wounds. Wound Repair Regen. 2008;16:529–533. doi: 10.1111/j.1524-475X.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller EC, Tzeranis DS, Miu K, So PT, Yannas IV. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials. 2012;33:4783–4791. doi: 10.1016/j.biomaterials.2012.03.068. [DOI] [PubMed] [Google Scholar]
- Sun XY, Fu XB, Sun TZ. Isolation, culture and identification of human epithelial stem cells. Zhonghua Shiyan Waike Zazhi. 2007;24:163–165. [Google Scholar]
- Sun Y, Zhu L, Huang X, Zhou C, Zhang X. Immunohistochemical localization of nerve fibers in the pseudocapsule of fibroids. Eur J Histochem. 2014;58:2249. doi: 10.4081/ejh.2014.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velander P, Theopold C, Bleiziffer O, Bergmann J, Svensson H, Feng Y, Eriksson E. Cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model. J Surg Res. 2009;157:14–20. doi: 10.1016/j.jss.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Wang Q, Muffley LA, Hall K, Chase M, Gibran NS. Elevated glucose and fatty acid levels impair substance P-induced dermal microvascular endothelial cell migration and proliferation in an agarose gel model system. Shock. 2009;32:491–497. doi: 10.1097/SHK.0b013e3181a1cb2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YX, Zhang JH, Ben XM. Neuronal-like cell differentiation of non-adherent bone marrow cell-derived mesenchymal stem cells. Neural Regen Res. 2013;8:2078–2085. doi: 10.3969/j.issn.1673-5374.2013.22.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong QL, Liu FR, Liu DW, Peng Y, Zhang XR. Expression of β-catenin and cyclin D1 in epidermal stem cells of diabetic rats. Mol Med Rep. 2011;4:377–381. doi: 10.3892/mmr.2011.435. [DOI] [PubMed] [Google Scholar]
- Zhong QL, Liu DW, Liu FR, Peng Y, Yu M, Xiao LL. Amniotic membrane loading epidermal stem cells accelerates wound healing in diabetic rats. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14:6010–6014. [Google Scholar]
- Zhu FB, Liu DW, Zhang HY, Peng Y, Zhong QL, Li YT. Separation, culture and characteristics of epidermal stem cells in diabetes mellitus rats. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15:96–99. [Google Scholar]
- Zhu L, Huang Q, Huang X, Zhang J, Xu H, Zhang X. Decreased nerve fibers in the oviduct isthmus of women with endometriosis. Acta Histochem. 2014;116:871–877. doi: 10.1016/j.acthis.2014.02.005. [DOI] [PubMed] [Google Scholar]