Keywords: nerve regeneration, peripheral nerve injuries, acellular nerves, radiography, nerve repair, nerve tissue engineering, two-dimensional evaluation, vascularized models, angiogenesis, neural regeneration
Abstract
Vascularization of acellular nerves has been shown to contribute to nerve bridging. In this study, we used a 10-mm sciatic nerve defect model in rats to determine whether cartilage oligomeric matrix protein enhances the vascularization of injured acellular nerves. The rat nerve defects were treated with acellular nerve grafting (control group) alone or acellular nerve grafting combined with intraperitoneal injection of cartilage oligomeric matrix protein (experimental group). As shown through two-dimensional imaging, the vessels began to invade into the acellular nerve graft from both anastomotic ends at day 7 post-operation, and gradually covered the entire graft at day 21. The vascular density, vascular area, and the velocity of revascularization in the experimental group were all higher than those in the control group. These results indicate that cartilage oligomeric matrix protein enhances the vascularization of acellular nerves.
Introduction
The number of peripheral nerve injuries resulting from accidents is increasing in developing countries. Many different strategies have been investigated for developing alternative methods to promote nerve regeneration, including by focusing on how to enhance the efficiency of nerve regeneration and by successfully producing acellular nerve grafts (He et al., 2015). However, the clinical outcomes for acellular nerve grafts on long and large nerve defects, either repaired by autografts or acellular nerves, remain unsatisfactory (He et al., 2014). In one meta-analysis review of papers on upper limb peripheral nerve injuries published in the last 20 years (He et al., 2014), the analysis demonstrated that a 1 cm increase in the defect length results in a 9% decrease in the odds ratio of a good to excellent recovery, indicating a worse recovery from longer defects. Those observations are in agreement with other previous studies (Bellamkonda, 2006; Scheib and Hoke, 2013; Isaacs and Browne, 2014).
The blood supply is critically important for Schwann cells and may influence their activity (Bunge, 1994; Johnson et al., 2008; Skold et al., 2011). Thus, the blood supply may directly or indirectly affect nerve regeneration (Sondell et al., 1999; Mompeo et al., 2003; Skold et al., 2011). Revascularization of acellular nerve grafts also plays an important role in promoting nerve regeneration (Bell and Weddell, 1984; Kosaka, 1990; Best and Mackinnon, 1994; Hobson et al., 2000; Hobson, 2002; Kosacka et al., 2005).
Hobson (2002) reported that enhanced vascularization indirectly promoted axonal regeneration within a silicone nerve regeneration chamber. Vascularization can also be enhanced through growth factors. Angiopoietin-1 (Ang-1), for example, is an important angiogenic factor. Ang-1 promotes angiogenesis by activating the tyrosine kinase with Ig and epidermal growth factor homology domain 2 (Tie-2) receptor (Thurston, 2003; Cascone et al., 2005; Fagiani and Christofori, 2013). The mechanism of Ang-1/Tie-2 signaling may require phosphorylation of the Tie-2 receptor (Sato et al., 1995; Suri et al., 1996; Yancopoulos et al., 2000). Cartilage oligomeric matrix protein (COMP)-Ang-1, a variant of Ang-1, is a more potent phosphorylator of Tie-2 and Akt receptor than native Ang-1 in primary endothelial cells (Cho et al., 2004; Jeong et al., 2010; Kim et al., 2013). Jejong and Kim (Jeong et al., 1995) demonstrated the high efficiency of COMP-Ang-1 at promoting the activation of Tie-2 receptor. The aim of the present study was to determine the characteristics of vascularization of acellular nerve grafts and to determine the effects of COMP-Ang-1 on acellular nerve vascularization. To do that, radiography was used to create two-dimensional images of the microvessel development, allowing quantitative analysis of the vascularization.
Materials and Methods
Animals
Thirty-six female Sprague-Dawley (SD) rats weighing 250–300 g were purchased from the First Affiliated Hospital of Sun Yat-sen University Medical College, Guangzhou, China. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University Medical College (ethical license: [2013]A-55). The animals were housed under a 12-hour light/dark cycle and at a controlled temperature of 22°C with access to food and water ad libitum. All efforts were made to minimize the number of rats used and their pain and suffering. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1986).
Repairing sciatic nerve defects with acellular nerve grafts combined with COMP-ANG-1
Preparation of acellular nerves
SD rats were euthanized by an intraperitoneal injection of sodium pentobarbital (0.5 mL, 60 mg/mL). The sciatic nerves were exposed bilaterally, and then 12-mm nerve segments were harvested. Acellular nerves were prepared as previously reported (Hu et al., 2012). Briefly, the nerves were agitated in distilled water for 6 hours and then incubated with 3% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 24 hours with shaking. Next, the nerves were agitated in a 4% sodium deoxycholate (Sigma-Aldrich) water solution for another 24 hours. At the beginning of each procedure, the scaffolds were rinsed three times with 10 mM PBS. After thorough washing in 10 mM PBS, the nerve segments were sterilized by 12-hour 60Co irradiation (Guangzhou Huada Biological Technology Co., Ltd., Guangzhou, China). Finally, the acellular nerves in good condition were chosen and stored in a refrigerator at 4°C until use during an operation.
Study design and intervention
Thirty-six rats were randomly divided into two groups. In the control group, a simple acellular nerve graft group (n = 18), the 10-mm segment of the sciatic nerve removed to create a defect was replaced with a 12-mm acellular nerve graft, and the animals received daily intraperitoneal injections of 0.9% NaCl solution at a dose of 5 μL/g. In the experimental group, an acellular nerve graft with COMP-Ang-1 (n =18) injections group, the 10-mm segment of the sciatic nerve removed to create a defect was replaced with a 12-mm acellular nerve graft, and the animals received daily intraperitoneal injections of COMP-Ang-1 (100 ng/mL, 0.2 μm-filtered solution in PBS containing 0.01% CHAPS; Enzo Life Sciences Farmingdale, NY, USA) at a dose of 5 μL/g.
All of the animals were anesthetized with an intraperitoneal injection of 10% chloral hydrate (Jinshan Chemical, Chengdu, China) at a dose of 0.3 mL/100 g before the operation. An incision was made on the left thigh to expose the sciatic nerve. A 10-mm segment of the nerve was excised 0.5 cm below the piriformis muscle, and a 12-mm acellular nerve was grafted in its place with the aid of a surgical microscope. The acellular nerves were sutured to the epineurium of the proximal and distal nerve stumps with 9-0 suture (Ningbo Medical Needle Co., Ltd., Ningbo, Zhejiang, China) at each junction. The overlying muscle and skin of the thigh were sutured closed with 5-0 and 3-0 polyamide sutures. Finally, all of the rats were housed in cages in a warm environment and permitted free access to food and water.
Evaluation of vascularization
Specimen preparation
Six rats from each group at each of 7, 14, and 21 days after the operation were anesthetized with an intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g) before perfusion. The left carotid artery was exposed after a small incision in the neck. The perfusion agent was prepared, and the recommended protocol for perfusion volume was used, as previously reported (Zhu et al., 2014). Briefly, the perfusion agent (10 mL/200 g body weight) was injected into the artery after arterial catheterization until discoloration of the sclera and extremities were noted. One-step injection of the perfusion agent was ensured during the entire perfusion process. Finally, the tissue was placed in a refrigerator for 2 hours, and then the nerve specimen was harvested.
Radiography of the microvessels
The harvested acellular nerve grafts were scanned using an X-ray apparatus (Guangzhou Zhongke Kaisheng Medical Technology Co., Ltd., Guangzhou, Guangdong, China), and radiography images of the microvessel networks were recorded to establish a radiography model of vascularization. Analysis of the microvessels was performed by measuring the vascular density and vascular area. The images of the acellular nerve grafts were converted to gray scale and inverted in Adobe Photoshop CS software (Abode System Incorporated, San Jose, CA, USA). Using the measure and count functions in Image Pro Plus software (Media Cybernetic Media Cybernetic, Silver Spring, MD, USA), the vascular effects and the regeneration distance of microvessels were quantified. In addition, the velocity of vascularization was assessed based on the indexes mentioned above: vascular velocity = regeneration distance of microvessels at day 7/7.
Cross-sectional scans of the harvested sciatic nerves
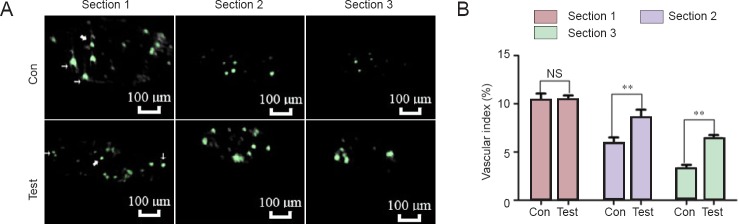
The harvested nerves were scanned by micro-CT (ZKKS-MCT-III, Guangzhou Zhongke Kaisheng Medical Technology Co., Ltd.) at a voltage of 40 kVp, and power of 45 W, with four frames every 0.72° rotational step through a total rotation range of 360°, taking a total scanning time of 28 minutes. The image reconstructions were imported into Mimics 10.0 software (Materialise Co., Ltd., Leuven, Belgium) to observe the cross-sections of the microvessels. Three cross-sections from the proximal to distal end of each nerve specimen were selected by systematic random sampling. Section 1 was taken closer to the proximal end and section 3 was taken closer to the distal end, with section 2 in between. Finally, the vascular index was calculated as: vascular index = the total area of the vascular development region/cross-sectional area of the nerves × 100%.
Statistical analysis
Data are expressed as the mean ± SD and were analyzed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The statistical evaluation was performed using one-way analysis of variance tests followed by the least significant difference post hoc tests to make comparisons between two groups when a significant effect was observed. P-values less than 0.05 were considered statistically significant.
Results
Vascularization patterns
Vessels began to grow into the acellular nerve grafts from the two anastomotic ends beginning at day 7 and gradually covering the entire graft at day 21 (Figure 1). The velocity of vascularization in the test group was faster than that in the control group. The cross-sectional views of the sciatic nerves (Figure 2) showed two distinct patterns of revascularization: epineurial neovascularization and endoneurial neovascularization.
Figure 1.
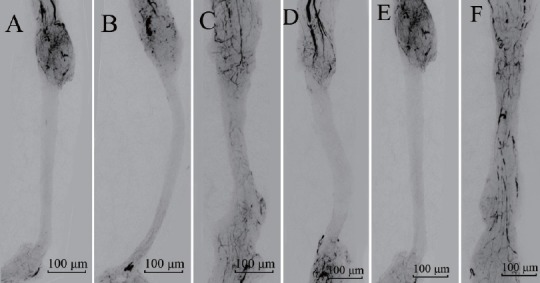
Vascularized images of acellular nerves.
In the control group, acellular nerve grafts alone were transplanted. In the test group, acellular nerve grafts were transplanted and daily intraperitoneal injections of cartilage oligomeric matrix protein-angiopoietin-1 were performed. Vessels grew into the acellular nerve graft from both anastomotic ends beginning at day 7 (A, D) and gradually covering the entire graft at day 21 (C, F) in both the control and test groups. The velocity of vascularization in the test group (D–F) was faster than that in the control group (A–C). Vessels are indicated by the black areas. Scale bars: 100 μm.
Figure 2.
Cross-sectional scans of the acellular nerve grafts at 14 days post-operation.
(A) In the control group (Con), acellular nerve grafts alone were transplanted. In the test group (Test), acellular nerve grafts were transplanted and daily intraperitoneal injections of cartilage oligomeric matrix protein-angiopoietin-1 were performed. Sections 1, 2, and 3 were selected by systematic random sampling from the proximal to the distal ends of the nerve specimen. Two distinct patterns of revascularization were found: one in the epineurium (narrow arrows) and the other in the endoneurium (broad arrows). In section 1, there was no apparent difference between the two groups. In section 2, the number of microvessels in the test group was significantly higher than that in the Con group. In section 3, the signal from the contrast agent was obvious in the test group, but it was only minimally present in the Con group. Scale bars: 100 μm. (B) Quantification of vascularization at day 14 postoperatively. The vascular index = the total area of vascular development region/cross-sectional area of the nerves × 100%. Data are expressed as the mean ± SD (n = 6). One-way analysis of variance followed by the least significant difference post hoc test was used. **P < 0.01, vs. Con group. NS: Not significant.
The effects of COMP-Ang-1 on revascularization
As the location of the cross-section of the sciatic nerve was taken closer to the distal junction of the graft, the diameter of the vessels became smaller (Figure 3; P < 0.01). In the test group, the diameter of the vessels in section 3 was significantly larger than that in the control group (P < 0.01). In the test group, the vascular diameter in the endoneurium in section 3 was also significantly larger than that in the epineurium (P < 0.01).
Figure 3.
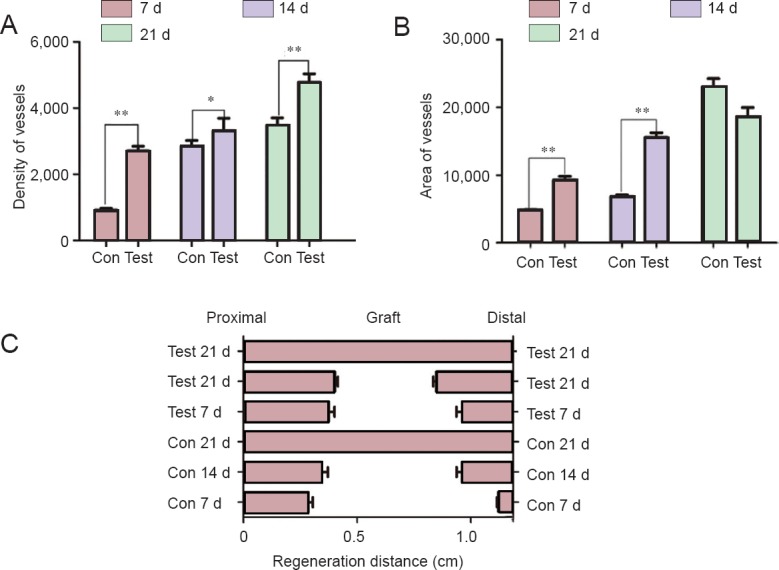
Density and area of the vessels in the acellular nerves and vascular regeneration distance.
In the control group (Con), acellular nerve grafts alone were transplanted. In the test group (Test), acellular nerve grafts were transplanted and daily intraperitoneal injections of cartilage oligomeric matrix protein-angiopoietin-1 were performed. (A) The vascular density was higher in the test group than in the Con group at days 7, 14, and 21. (B) The vascular area was larger in the test group than in the Con group at days 7 and 14. However, there was no difference between the two groups at day 21. Data are expressed as the mean ± SD (n = 6). One-way analysis of variance followed by the least significant difference post hoc tests was used. *P < 0.05 and **P < 0.01, vs. Con group. (C) Microvessels began to enter into the graft from the proximal and distal nerve junctions. As time passed, the graft was gradually covered with microvessels. Vascular recanalization was found in both groups at day 21. Vascular outgrowth was more obvious at the proximal junction than at the distal junction. d: Days.
Vascular density and vascular area
As shown in Figure 3, the vascular density was higher in the test group than in the control group at days 7 (P = 0.003), 14 (P = 0.021), and 21 (P = 0.003). The vascular area (Figure 3B) was also larger in the test group than in the control group at days 7 (P = 0.007) and 14 (P = 0.009). However, there was no significant difference in the vascular area between the two groups at day 21 (P = 0.056).
Regeneration distance of the microvessels
As shown in Figures 3, 4, the microvessels began to enter into the grafts from the proximal and distal nerve junctions. As time passed, the graft was gradually covered with microvessels. Vascular recanalization was found in both groups at day 21. Vascular outgrowth was more obvious at the proximal junction than the distal junction.
Figure 4.
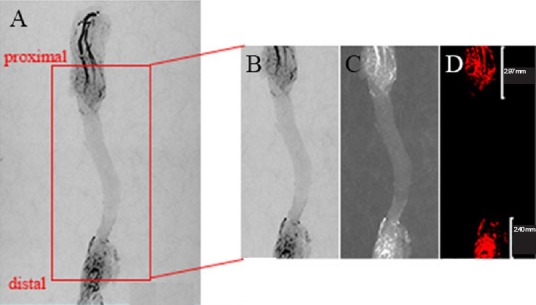
Micro-CT assessments of vascular density and vascular area at day 7 postoperatively.
A CT image of the acellular nerve graft from the proximal to the distal junction was cropped from the whole vascular graft image in Photoshop (A and B). The graft segment was converted to gray scale and inverted with Image Pro Plus software (C). The visual images were created by selecting the color level using the measure function tool in Image Pro Plus (D). The vascular density and vascular area were automatically calculated. Finally, the regeneration distance was measured on the visual images.
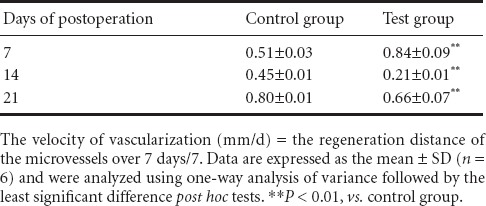
Velocity of vascularization
The velocity of vascularization in the test group was higher than that in the control group at day 7 (Table 1). During the next 7 days (day 7 to day 14), the velocity of vascularization declined significantly compared with that before day 7 (P < 0.01). From day 14 to 21, the velocity increased again.
Table 1.
Velocity of vascularization
Vascular index
As shown in Figure 5, the vascular index was not statistically different between the control group and test group in section 1 (P = 0.84). In section 2, the vascular index in the control group was less than that in the test group (P = 0.001). In section 3, the vascular index in the control group was also less than that in the test group (P < 0.001).
Figure 5.
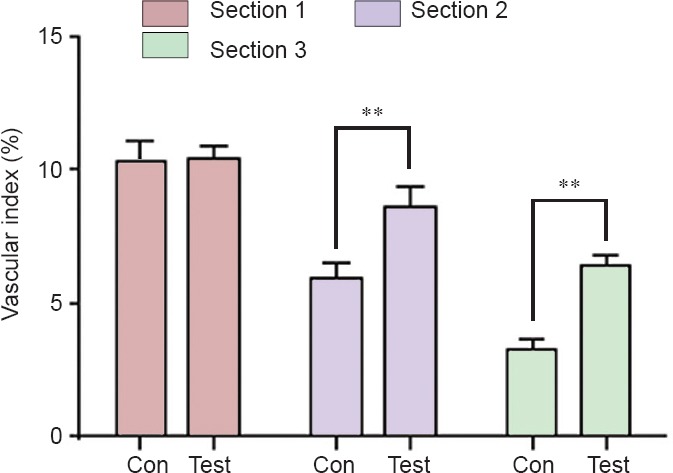
Vascular index in the control (Con) and test groups at 14 days postoperatively.
In section 1, the vascular index was not statistically different between the Con group and the test group. In sections 2 and 3, the vascular index in the Con group was less than that in the test group. Vascular index = the total area of the vascular development region/cross-sectional area of the nerves × 100%. Data are expressed as the mean ± SD (n = 6). One-way analysis of variance followed by the least significant difference post hoc tests was used. **P < 0.01, vs. Con group.
Discussion
Although our understanding of the cell biology of nerve regeneration is improving (Hobson et al., 1997), it remains unclear whether there is a direct correlation between vascularization and the efficacy of nerve regeneration within a nerve conduit (Best and Mackinnon, 1994; Chang et al., 2007; Fukuda et al., 2014). Hobson (2002) pointed out that there is a close relationship between the neural orientation and vascular elements within a silicone nerve regeneration chamber. In that study, Schwann cells and axonal orientation were longitudinal and located behind a more random regeneration front, which was reflected in the vascular staining pattern. Interestingly, Hobson et al. (2000) also found that vascular endothelial growth factor significantly increased Schwann cell migration and axonal regeneration in acellular nerves at 30 days postoperatively and that the number of myelinated axons was increased by 78% at 180 days postoperatively. Kosaka (1990) established an artery within a silicone tube and demonstrated its benefits for nerve regeneration. He suggested that the advantages of his scaffold resulted from an increased supply of nutrients for regenerating nerve fibers and Schwann cells and an increased supply of oxygen from the highly oxygenated arterial blood. A prospective, multicenter controlled clinical trial showed that human acellular nerve grafting is a safe and effective alternative for the repair of nerve defects of 1–5 cm in size (He et al., 2015). In one meta-analysis review of, papers on upper limb peripheral nerve injuries published in the last 20 years (He et al., 2014), the one-way analysis of variance demonstrated that a 1 cm increase in the defect length resulted in a 9% decrease in the odds ratio of good to excellent recovery, indicating a worse recovery from longer defects (Louissaint et al., 2002; Carmeliet, 2003). In addition, recent genetic findings have shown that blood vessels and nerves have much more in common than originally anticipated. They rely on similar signals and principles to differentiate, grow, and migrate towards their targets (Dickson, 2002; Lawson and Weinstein, 2002; Gerhardt et al., 2003). The results of the present study demonstrated both epineurial neovascularization and endoneurial neovascularization, and moreover, that the endoneurial neovascularization occurred earlier than the epineurial neovascularization. These findings are consistent with a previous study that found that axonal regeneration and neovascularization depend on similar finger-like growth cones, which are sensory structures present at the leading tip of extending axons and blood vessels (Carmeliet and Storkebaum, 2002; Louissaint et al., 2002; Rivera and Bergers, 2014).
Angiogenesis and the formation of patent vasculature are vital for successful nerve regeneration (Kannan et al., 2005; Laschke et al., 2006), but only a few studies have examined angiogenic outcomes while examining nerve regeneration (Gora-Kupilas and Josko, 2005; Chang et al., 2009; Fukuda et al., 2014). The aims of the present study were to characterize the patterns of neovascularization in acellular nerve grafts and to examine the effects of a variant of Ang-1, COMP-Ang-1, on the neovascularization. Ang-1 exerts anti-apoptotic and neurotrophic effects on neurons in both the central and peripheral nervous system (Kosacka et al., 2005; Kingham et al., 2014). COMP-Ang-1, a newly developed soluble and stable form of Ang-1, has been shown to improve the neovascularization of the sciatic nerve in rats with diabetic neuropathy (Kosacka et al., 2012). In the present study, COMP-Ang-1 improved the velocity of vascularization during the first 7 postoperative days. The Ang-1/Tie-2 signaling pathway likely participates in the angiogenic advantages induced by COMP-Ang-1 (Cho et al., 2004, 2005). The Ang-1/Tie-2 signaling pathway participates in endothelial cell migration, pericyte recruitment, and the formation, remodeling, and maturation of blood vessels; it also promotes angiogenesis (Fukuhara et al., 2010; Ibberson et al., 2013; Rivera and Bergers, 2014). Essential insights into the normal roles played by the Ang-1/Tie-2 signaling system have been made by analyzing knockout mice that lack these gene products (Yancopoulos et al., 2000). The vasculature of embryos lacking these genes fails to undergo normal remodeling. Although Ang-1 does not actually direct specific vascular remodeling events, it does play a permissive role by optimizing the integration of endothelial cells with supporting cells, allowing them to receive other critical signals from their environment (Carmeliet and Storkebaum, 2002; Louissaint et al., 2002; Carmeliet, 2003; Gerhardt et al., 2003; Nowicki et al., 2009). In the present study, the vascular density and vascular area were increased in the test group compared with the control group. Those results were similar to the findings by Kosacka et al (2012), which suggested that stronger phosphorylation of Tie-2 leads to activation of Akt, as well as the p38 MAPK signaling pathways, in pericytes and perineurial cells, improving the efficacy of nerve regeneration.
Although it is better to include an autograft group as a positive control for comparison, it was not feasible to do so in this study, which is one limitation. In addition, the univariate linear analysis for calculating velocity in the present study is not entirely accurate. However, little has been reported on how to calculate the velocity of neovascularization, which remains a vague concept. Based on the observed coordination of neovascularization and nerve regeneration (Bagnard et al., 2001; Ollikainen et al., 2014) and the increase in nerve regeneration with time (Wang et al., 2005; Wang et al., 2008; Chen et al., 2010; Rui et al., 2012), we simplified the relationship between the velocity of neovascularization and time to a linear relationship. This was done as a way to calculate the neovascularization velocity of acellular nerves. In future studies, it would be appropriate to investigate the effects and time intervals for the velocity of neovascularization in more detail.
In conclusion, the present study characterized the patterns of vascularization in acellular nerve grafts, showing that neovascularization began from both anastomotic ends at postoperative day 7 and gradually covered the entire graft at day 21. Neovascularization was enhanced by COMP-Ang-1 administration. In addition, epineurial neovascularization was accompanied by endoneurial neovascularization, and moreover, the endoneurial neovascularization occurred earlier than the epineurial neovascularization in the acellular nerve. In the present study, we used a two-dimensional radiography method to observe neovascularization in acellular nerve grafts, which should make it possible to directly observe the relationship between nerve regeneration and vascularization in future studies.
Footnotes
Funding: This study was supported by the Specialized Research Fund for Science and Technology Plan of Guangdong Province in China, No. 201313060300007; the National High-Technology Research and Development Program of China (863 Program), No. 2012AA020507; the National Basic Research Program of China (973 Program), No. 2014CB542201; the Doctoral Program of Higher Education of China, No. 20120171120075; and Doctoral Start-up Project of the Natural Science Foundation of Guangdong Province in China, No. S201204006336 and 1045100890100590.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by McCarty W, Stow A, Wang J, Wang L, Li CH, Song LP, Zhao M
References
- Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Puschel AW, Belin MF, Bolz J, Thomasset N. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Weddell AG. A descriptive study of the blood vessels of the sciatic nerve in the rat, man and other mammals. Brain. 1984;107:871–898. doi: 10.1093/brain/107.3.871. [DOI] [PubMed] [Google Scholar]
- Bellamkonda RV. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–3518. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Best TJ, Mackinnon SE. Peripheral nerve revascularization: a current literature review. J Reconstr Microsurg. 1994;10:193–204. doi: 10.1055/s-2007-1006587. [DOI] [PubMed] [Google Scholar]
- Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242:S19–21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Storkebaum E. Vascular and neuronal effects of VEGF in the nervous system: implications for neurological disorders. Semin Cell Dev Biol. 2002;13:39–53. doi: 10.1006/scdb.2001.0290. [DOI] [PubMed] [Google Scholar]
- Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Hsu SH, Yen HJ, Chang H, Hsu SK. Effects of unidirectional permeability in asymmetric poly(DL-lactic acid-co-glycolic acid conduits on peripheral nerve regeneration: an in vitro and in vivo study. J Biomed Mater Res B Appl Biomater. 2007;83:206–215. doi: 10.1002/jbm.b.30785. [DOI] [PubMed] [Google Scholar]
- Chang JY, Ho TY, Lee HC, Lai YL, Lu MC, Yao CH, Chen YS. Highly permeable genipin-cross-linked gelatin conduits enhance peripheral nerve regeneration. Artif Organs. 2009;33:1075–1085. doi: 10.1111/j.1525-1594.2009.00818.x. [DOI] [PubMed] [Google Scholar]
- Chen YS, Chung SS, Chung SK. Aldose reductase deficiency improves Wallerian degeneration and nerve regeneration in diabetic thy1-YFP mice. J Neuropathol Exp Neurol. 2010;69:294–305. doi: 10.1097/NEN.0b013e3181d26487. [DOI] [PubMed] [Google Scholar]
- Cho CH, Kim KE, Byun J, Jang HS, Kim DK, Baluk P, Baffert F, Lee GM, Mochizuki N, Kim J, Jeon BH, McDonald DM, Koh GY. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res. 2005;97:86–94. doi: 10.1161/01.RES.0000174093.64855.a6. [DOI] [PubMed] [Google Scholar]
- Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Wang W, Ichinose S, Katakura H, Mukai T, Takakuda K. Laser perforated accordion nerve conduit of poly (lactide-co-glycolide-co-varepsilon-caprolactone) J Biomed Mater Res B Appl Biomater. 2014;102:674–680. doi: 10.1002/jbm.b.33046. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol Histopathol. 2010;25:387–396. doi: 10.14670/HH-25.387. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gora-Kupilas K, Josko J. The neuroprotective function of vascular endothelial growth factor (VEGF) Folia Neuropathol. 2005;43:31–39. [PubMed] [Google Scholar]
- He B, Zhu Z, Zhu Q, Zhou X, Zheng C, Li P, Zhu S, Liu X, Zhu J. Factors predicting sensory and motor recovery after the repair of upper limb peripheral nerve injuries. Neural Regen Res. 2014;9:661–672. doi: 10.4103/1673-5374.130094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Zhu Q, Chai Y, Ding X, Tang J, Gu L, Xiang J, Yang Y, Zhu J, Liu X. Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: a prospective, multicentre controlled clinical trial. J Tissue Eng Regen Med. 2015;9:286–295. doi: 10.1002/term.1707. [DOI] [PubMed] [Google Scholar]
- Hobson MI. Increased vascularisation enhances axonal regeneration within an acellular nerve conduit. Ann R Coll Surg Engl. 2002;84:47–53. [PMC free article] [PubMed] [Google Scholar]
- Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat 197 Pt. 2000;4:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson MI, Brown R, Green CJ, Terenghi G. Inter-relationships between angiogenesis and nerve regeneration: a histochemical study. Br J Plast Surg. 1997;50:125–131. doi: 10.1016/s0007-1226(97)91325-4. [DOI] [PubMed] [Google Scholar]
- Hu JZ, Wu TD, Zhang T, Zhao YF, Pang J, Lu HB. Three-dimensional alteration of microvasculature in a rat model of traumatic spinal cord injury. J Neurosci Methods. 2012;204:150–158. doi: 10.1016/j.jneumeth.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Ibberson M, Bron S, Guex N, Faes-van’t Hull E, Ifticene-Treboux A, Henry L, Lehr HA, Delaloye JF, Coukos G, Xenarios I, Doucey MA. TIE-2 and VEGFR kinase activities drive immunosuppressive function of TIE-2-expressing monocytes in human breast tumors. Clin Cancer Res. 2013;19:3439–3449. doi: 10.1158/1078-0432.CCR-12-3181. [DOI] [PubMed] [Google Scholar]
- Isaacs J, Browne T. Overcoming short gaps in peripheral nerve repair: conduits and human acellular nerve allograft. Hand (N Y) 2014;9:131–137. doi: 10.1007/s11552-014-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong BC, Kim HJ, Bae IH, Lee KN, Lee KY, Oh WM, Kim SH, Kang IC, Lee SE, Koh GY, Kim KK, Koh JT. COMP-Ang1, a chimeric form of Angiopoietin 1, enhances BMP2-induced osteoblast differentiation and bone formation. Bone. 2010;46:479–486. doi: 10.1016/j.bone.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Charchanti A, Soucacos PN. Nerve repair: experimental and clinical evaluation of neurotrophic factors in peripheral nerve regeneration. Injury. 2008;39(Suppl 3):S37–42. doi: 10.1016/j.injury.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26:1857–1875. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kim SO, Lee HS, Ahn K, Park K. COMP-angiopoietin-1 promotes cavernous angiogenesis in a type 2 diabetic rat model. J Korean Med Sci. 2013;28:725–730. doi: 10.3346/jkms.2013.28.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014;23:741–754. doi: 10.1089/scd.2013.0396. [DOI] [PubMed] [Google Scholar]
- Kosacka J, Figiel M, Engele J, Hilbig H, Majewski M, Spanel-Borowski K. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 2005;320:11–19. doi: 10.1007/s00441-004-1068-2. [DOI] [PubMed] [Google Scholar]
- Kosacka J, Nowicki M, Kloting N, Kern M, Stumvoll M, Bechmann I, Serke H, Bluher M. COMP-angiopoietin-1 recovers molecular biomarkers of neuropathy and improves vascularisation in sciatic nerve of ob/ob mice. PLoS One. 2012;7:e32881. doi: 10.1371/journal.pone.0032881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka M. Enhancement of rat peripheral nerve regeneration through artery-including silicone tubing. Exp Neurol. 1990;107:69–77. doi: 10.1016/0014-4886(90)90064-y. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rucker M, Junker D, Haufel JM, Carvalho C, Heberer M, Germann G, Vollmar B, Menger MD. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12:2093–2104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Mompeo B, Engele J, Spanel-Borowski K. Endothelial cell influence on dorsal root ganglion cell formation. J Neurocytol. 2003;32:123–129. doi: 10.1023/b:neur.0000005597.28053.2a. [DOI] [PubMed] [Google Scholar]
- Nowicki M, Kosacka J, Spanel-Borowski K, Borlak J. Deferoxamine-induced neurite outgrowth and synapse formation in postnatal rat dorsal root ganglion (DRG) cell cultures. Eur J Cell Biol. 2009;88:551–562. doi: 10.1016/j.ejcb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Ollikainen E, Tulamo R, Frosen J, Lehti S, Honkanen P, Hernesniemi J, Niemela M, Kovanen PT. Mast cells, neovascularization, and microhemorrhages are associated with saccular intracranial artery aneurysm wall remodeling. J Neuropathol Exp Neurol. 2014;73:855–864. doi: 10.1097/NEN.0000000000000105. [DOI] [PubMed] [Google Scholar]
- Rivera LB, Bergers G. Angiogenesis. Targeting vascular sprouts. Science. 2014;344:1449–1450. doi: 10.1126/science.1257071. [DOI] [PubMed] [Google Scholar]
- Rui J, Dadsetan M, Runge MB, Spinner RJ, Yaszemski MJ, Windebank AJ, Wang H. Controlled release of vascular endothelial growth factor using poly-lactic-co-glycolic acid microspheres: in vitro characterization and application in polycaprolactone fumarate nerve conduits. Acta Biomater. 2012;8:511–518. doi: 10.1016/j.actbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- Skold MK, Svensson M, Tsao J, Hultgren T, Landegren T, Carlstedt T, Cullheim S. Karolinska institutet 200-year anniversary. Symposium on traumatic injuries in the nervous system: injuries to the spinal cord and peripheral nervous system - injuries and repair, pain problems, lesions to brachial plexus. Front Neurol. 2011;2:29. doi: 10.3389/fneur.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Thurston G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61–68. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, Yang LM, Wang HG, Yi JH. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008;1188:44–53. doi: 10.1016/j.brainres.2007.09.098. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu W, Cao Y, Yao J, Wu J, Gu X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Zhu Z, He B, Mao Y, Zhao L, Zhou X, Zhu Q, Zhu J, Tang M, Liu X. Comparative study of 3D reconstruction of rat sciatic nerve microvessels using Evans blue and lead oxide. Neurol Res. 2014;36:992–1000. doi: 10.1179/1743132814Y.0000000394. [DOI] [PubMed] [Google Scholar]