Motor weakness is a common and important sequela of stroke, and motor recovery is mostly achieved within 3 months following stroke (Jorgensen et al., 1995; Fujii and Nakada, 2003), suggesting the importance of active rehabilitation during the early stage of stroke. Many studies have reported on neurological recovery during this period, however, little is known about pontine infarction (Jang et al., 2007; Kwon and Jang, 2012; Kwon et al., 2013; Yeo and Jang, 2013; Chang et al., 2014; Jang and Yeo, 2014; Seo and Jang, 2015). In this study, we attempted to demonstrate the recovery of an injured corticospinal tract (CST) using diffusion tensor tractography (DTT) and transcranial magnetic stimulation (TMS) during the early stage of rehabilitation following pontine infarction.
A 71-year-old woman presented with right hemiplegia that occurred at the onset of an infarct in the left pontine base (Figure 1A). She underwent conservative management for pontine infarction at the department of neurosurgery of a hospital. At 2 weeks after onset, she was transferred to the rehabilitation department to undergo rehabilitation. She had the severe weakness of the right upper and lower extremities at onset (the manual muscle test [MMT](Frese et al., 1987): upper, 0–1/lower, 2) and moderate weakness at the beginning of rehabilitation at 2 weeks after onset (the MMT: 3/3). During a period of 3 weeks from 2 to 5 weeks after onset, she underwent rehabilitative treatment, including administration of neurotrophic drugs (ropinirole, levodopa, and amantadine), movement therapy, and neuromuscular electrical stimulation of the right finger extensors and ankle dorsiflexor (Scheidtmann et al., 2001). Movement therapy for improving locomotion in the right upper and lower extremities was performed during the conventional therapy sessions five times per week (70 minutes/day). At 5 weeks after onset, her right weakness was improved, with 4/4 on the MMT; consequently, she was able to perform some fine motor activities using her right hand and walk independently. The patient provide signed, informed consent and our institutional review board approved the study protocol.
Figure 1.

T2-weighted brain magnetic resonance images and results of diffusion tensor tractography and transcranial magnetic stimulation in a 71-year-old female patient with an infarct in the left pontine base.
(A) T2-weighted MRI images (2 and 5 weeks after onset) showing an infarct in the left pontine base (red arrows). R: Right. (B) Diffusion tensor tractography (DTT) images of the corticospinal tract (CST). On both 2- and 5-week DTT images, the integrity of the left CST was preserved through the peri-infarct areas, however, the left CST was thickened on 5-week DTT images compared with that on 2-week DTT images (blank green arrow: thinning of the left CST on the 2-week DTT images, blue arrow: thickening of the left CST on the 5-week DTT image). R: Right; A: anterior. (C) Motor-evoked potentials (MEPs) elicited from the right abductor pollicis brevis (APB) muscle. In a 2-week transcranial magnetic stimulation (TMS) study, an MEP was elicited from the right APB muscle (latency: 28.5 ms; amplitude: 50 μV; excitatory threshold (ET): 100%). By contrast, the latency was shortened and the amplitude was increased in the 5-week TMS study (latency: 23.1 ms; amplitude: 350 μV; ET: 100 %). X-axis: Latency; Y-axis: amplitude.
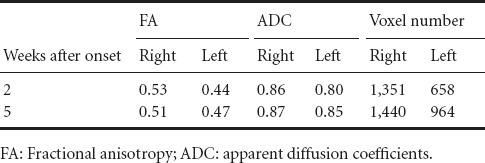
A 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Best, Netherlands) with 32 gradients was applied for acquisition of diffusion tensor imaging data (twice: 2 and 5 weeks after onset respectively). Seventy contiguous slices were acquired. Parameters of imaging were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192, field of view = 240 × 240 mm2, repetition time = 10,398 ms, echo time = 72 ms, parallel imaging reduction factor = 2, echo-planar imaging factor = 59 and b = 1,000 s/mm2, number of excitations = 1, and a slice thickness of 2.5 mm. Philips Extended MR Work Space 2.6.3 based on deterministic fiber tracking was used for estimation of the CST with two regions of interest (the posterior limb of the internal capsule and pyramid in the upper medulla on the axial images) (Kwon et al., 2011). The set of fiber tracking used default values (fractional anisotropy (FA) of > 0.15 and a direction threshold of < 27°) and FA, apparent diffusion coefficients, and voxel number was measured. On both 2- and 5-week DTT images, the integrity of the left CST was preserved between the primary sensorimotor cortex and pyramid in the medulla through the peri-infarct areas (Figure 1B), however, compared with 2-week DTT images, the left CST was thickened on 5-week DTT images; on 5-week DTT image, the FA and voxel number of the left CST was increased to 0.47 and 964 voxels compared with those (0.44 and 658 voxels) on 2-week DTT images (Table 1).
Table 1.
Diffusion tensor image parameters of the corticospinal tract in a patient with pontine infarction
TMS was also performed at the same time with DTTs using a Magstim Novametrix 200 magnetic stimulator (Novametrix Inc., Wallingford, CT, USA) equipped with a circular coil (mean diameter: 9 cm). The counterclockwise current was used to stimulate the left hemisphere. Motor-evoked potentials (MEPs) were elicited from abductor pollicis brevis (APB) muscles. Excitatory threshold (ET) was set as the minimum stimulus needed to elicit an MEP with a peak-to-peak amplitude of 50 μV or greater in two out of four attempts. Each site was stimulated three times and the average peak to peak amplitudes were adopted. On the 2-week TMS, an MEP was elicited from the right APB (latency: 28.5 ms; amplitude: 50 μV; excitatory threshold (ET): 100%) (Figure 1C). By contrast, on 5-week TMS, the latency was shortened and the amplitude was increased (latency: 23.1 ms; amplitude: 350 μV; ET: 100 %) (Figure 1C).
In this study, we found that the injured left CST was thickened on 5-week DTT image compared with 2-week DTT image; this thickening was consistent with the increment of FA and voxel numbers, which demonstrated that the number of neural fibers changed from 658 voxels on 2-week DTT image to 964 voxels on 5-week DTT image (Schaechter et al., 2008; Kwak et al., 2010; Jang et al., 2013, 2014). The change of the left CST observed on DTT also coincided with the change of the amplitude of MEP, which indicates the amount of CST fibers (50 μV on 2-week TMS was increased to 350 μV on 5-week DTT) and with the clinical motor recovery of the right extremities (3/3 on the MMT at 3 weeks after onset to 4/4 at 5 weeks after onset) (Rossini et al., 1994). Previous studies have reported that the initial recovery within 2 weeks after stroke was attributed to the resolution of local factors such as peri-lesional edema or inflammation (Furlan et al., 1996; Witte, 1998). Therefore, we believe that the changes observed on DTT and TMS images during a 3-week period from 2 weeks to 5 weeks after onset were mainly attributed to brain plasticity, but not to resolution of local factors such as edema (Furlan et al., 1996; Witte, 1998).
In summary, we report on a patient who had recovery of an injured CST during the early stage of rehabilitation following a pontine infarct. Many previous studies have reported on recovery of an injured CST during the early stage of stroke, however, most of these studies focused on supratentorial intracerebral hemorrhage (Jang et al., 2007; Kwon and Jang, 2012; Kwon et al., 2013; Yeo and Jang, 2013; Chang et al., 2014; Jang and Yeo, 2014; Seo and Jang, 2015). To the best of our knowledge, this is the first study to demonstrate the recovery of an injured CST during the early stage of rehabilitation in a patient with a pontine infarct. However, this study is limited to a case report. Further studies involving large numbers of cases are needed. Future studies will also focus on neurological recovery during the early stage of rehabilitation in patients with an injury in other brain regions or with other pathologies.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1A2A2A01004073).
References
- Chang MC, Jung YJ, Jang SH. Motor recovery via transcallosal and transpontine fibers in a patient with intracerebral hemorrhage. Am J Phys Med Rehabil. 2014;93:708–713. doi: 10.1097/PHM.0000000000000076. [DOI] [PubMed] [Google Scholar]
- Frese E, Brown M, Norton BJ. Clinical reliability of manual muscle testing. Middle trapezius and gluteus medius muscles. Phys Ther. 1987;67:1072–1076. doi: 10.1093/ptj/67.7.1072. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Nakada T. Cortical reorganization in patients with subcortical hemiparesis: neural mechanisms of functional recovery and prognostic implication. J Neurosurg. 2003;98:64–73. doi: 10.3171/jns.2003.98.1.0064. [DOI] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- Jang SH, Yeo SS. Recovery of an injured corticoreticular pathway via transcallosal fibers in a patient with intracerebral hemorrhage. BMC Neurol. 2014;14:108. doi: 10.1186/1471-2377-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SH, Kim SH, Cho SH, Choi BY, Cho YW. Demonstration of motor recovery process in a patient with intracerebral hemorrhage. NeuroRehabilitation. 2007;22:141–145. [PubMed] [Google Scholar]
- Jang SH, Chang CH, Lee J, Kim CS, Seo JP, Yeo SS. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke. 2013;44:1099–1104. doi: 10.1161/STROKEAHA.111.000269. [DOI] [PubMed] [Google Scholar]
- Jang SH, Kim K, Kim SH, Son SM, Jang WH, Kwon HG. The relation between motor function of stroke patients and diffusion tensor imaging findings for the corticospinal tract. Neurosci Lett. 2014;572:1–6. doi: 10.1016/j.neulet.2014.04.044. [DOI] [PubMed] [Google Scholar]
- Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:406–412. doi: 10.1016/s0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- Kwak SY, Yeo SS, Choi BY, Chang CH, Jang SH. Corticospinal tract change in the unaffected hemisphere at the early stage of intracerebral hemorrhage: a diffusion tensor tractography study. Eur Neurol. 2010;63:149–153. doi: 10.1159/000281108. [DOI] [PubMed] [Google Scholar]
- Kwon HG, Jang SH. Significance of rehabilitative management during the critical period for motor recovery in intracerebral hemorrhage: a case report. J Rehabil Med. 2012;44:280–284. doi: 10.2340/16501977-0931. [DOI] [PubMed] [Google Scholar]
- Kwon HG, Son SM, Chang MC, Kim S, Kwon YH, Jang SH. Characteristics of the aberrant pyramidal tract in comparison with the pyramidal tract in the human brain. BMC Neurosci. 2011;12:108. doi: 10.1186/1471-2202-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HG, Choi BY, Chang CH, Kim SH, Jung YJ, Jang SH. Recovery of an injured corticospinal tract during a critical period in a patient with intracerebral hemorrhage. NeuroRehabilitation. 2013;32:27–32. doi: 10.3233/NRE-130820. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39:1370–1382. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K, Fries W, Muller F, Koenig E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet. 2001;358:787–790. doi: 10.1016/S0140-6736(01)05966-9. [DOI] [PubMed] [Google Scholar]
- Seo YS, Jang SH. Recovery of a degenerated corticospinal tract after injury in a patient with intracerebral hemorrhage: confirmed by diffusion tensor tractography imaging. Neural Regen Res. 2015;10:829–831. doi: 10.4103/1673-5374.156990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte OW. Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol. 1998;11:655–662. doi: 10.1097/00019052-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Yeo SS, Jang SH. Recovery of an injured corticospinal tract and an injured corticoreticular pathway in a patient with intracerebral hemorrhage. NeuroRehabilitation. 2013;32:305–309. doi: 10.3233/NRE-130848. [DOI] [PubMed] [Google Scholar]


