Abstract
The analysis of receptor tyrosine kinases and their interacting ligands involved in vascular biology is often challenging due to the constitutive expression of families of related receptors, a broad range of related ligands and the difficulty of dealing with primary cultures of specialized endothelial cells. Here we describe a bioassay for the detection of ligands to the vascular endothelial growth factor receptor-2 (VEGFR-2), a key transducer of signals that promote angiogenesis and lymphangiogenesis. A cDNA encoding a fusion of the extracellular (ligand-binding) region of VEGFR-2 with the transmembrane and cytoplasmic regions of the erythropoietin receptor (EpoR) is expressed in the factor-dependent cell line Ba/F3. This cell line grows in the presence of interleukin-3 (IL-3) and withdrawal of this factor results in death of the cells within 24 hr. Expression of the VEGFR-2/EpoR receptor fusion provides an alternative mechanism to promote survival and potentially proliferation of stably transfected Ba/F3 cells in the presence of a ligand capable of binding and cross-linking the extracellular portion of the fusion protein (i.e., one that can cross-link the VEGFR-2 extracellular region). The assay can be performed in two ways: a semi-quantitative approach in which small volumes of ligand and cells permit a rapid result in 24 hr, and a quantitative approach involving surrogate markers of a viable cell number. The assay is relatively easy to perform, is highly responsive to known VEGFR-2 ligands and can accommodate extracellular inhibitors of VEGFR-2 signaling such as monoclonal antibodies to the receptor or ligands, and soluble ligand traps.
Keywords: Cellular Biology, Issue 109, Receptor, VEGF, ligand, inhibitor, signaling, soluble receptors, protein growth factor, bioassay, monoclonal antibody
Introduction
The vascular endothelial growth factor (VEGF) family of secreted protein growth factors and their cognate cell surface receptors is an important and diverse group of soluble ligands and membrane-embedded receptors, respectively that function in transducing signals across cellular membranes. They function mainly in endothelial cells but also in cells of epithelial origin and those of the immune system 1,2. Signaling pathways engaged by ligand-activated VEGF receptors (VEGFRs) are critical in major pathologies, such as age-related macular degeneration and cancer, and therapeutics targeting them are in frequent clinical use (e.g., the monoclonal antibody bevacizumab that targets VEGF-A) 3,4.
One of the complexities of the VEGF family is the diversity of soluble ligands present in nature (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF proteins encoded by the parapox virus family orf and snake venom VEGF, plus other inhibitory isoforms of VEGF-A)2.
These ligands interact with three members of the receptor tyrosine kinase family, namely VEGFR-1, VEGFR-2 and VEGFR-3. These receptors are variably expressed on different cell types but are often co-expressed on the surface of endothelial cells that line blood and lymphatic vessels of all sizes 5. VEGFR-2 can bind the mammalian ligands VEGF-A 6, VEGF-C 7 and VEGF-D 8,9 as well as orf virus VEGF10 and snake venom VEGF11. VEGFR-2 plays a major role in driving angiogenesis (the growth of new blood vessels from pre-existing vessels) in embryonic development, wound healing, cancer and eye diseases. In these contexts, ligands such as VEGF-A, -C and -D bind and activate the receptor on blood vascular endothelial cells12-15. On lymphatic endothelial cells, VEGFR-2 plays a role in lymphangiogenesis, the formation of new lymphatic vessels 16. VEGFR-2 can also promote dilation and expansion of major arteries and lymphatics in healthy tissues and disease 17. A complete understanding of VEGFR-2:ligand interactions is therefore important for the development of inhibitors for use in treating angiogenesis-dependent diseases18. While most isoforms of VEGF-A bind to VEGFR-2, proteolytic cleavage of VEGF-C and VEGF-D is required to release a fragment consisting of the VEGF-homology domain that exhibits high affinity binding to VEGFR-2 19,20.
We have developed a bioassay to monitor ligands of VEGFR-2 that is designed to circumvent the need for primary endothelial cells, which are technically difficult to passage, expensive to purchase and culture (requiring specialized medium) 21 and express multiple VEGFRs and associated co-receptors 22. Heterodimerization of VEGFR-2 with other VEGF receptors or co-receptors can cause unwanted complexity when aiming to study binary receptor-ligand interactions, evaluating activity attributable to a specific receptor, or assessing the effect of inhibitory reagents.23. The bioassay retains mobility of the relevant receptor in the cell membrane and allows evaluation of a ligand's ability to bind and cross-link the VEGFR-2 extracellular region.
The bioassay relies on the creation of a chimeric receptor in which the extracellular region of a VEGF receptor (in this case VEGFR-2) is fused to the transmembrane and intracellular regions of the erythropoietin receptor (EpoR), a member of the cytokine receptor family 8,24. This fusion protein is then expressed in the factor-dependent pro-B cell line Ba/F3, upon which stimulation with a ligand capable of binding and cross-linking the extracellular domain of the receptor causes activation of the cytoplasmic effector region, which is capable of transducing a survival signal via Janus kinases (JAKs) to promote cell survival and/or proliferation. In contrast, expression of full-length VEGFR-2 in the same cell type, and stimulation with ligand, does not promote cell survival and proliferation, indicating that the proximal signaling effectors of the VEGFR-2 pathway are not available in this cell type.
We have used the assay in a variety of contexts to explore binding of novel VEGFR-2 ligands 10,19,20,24-29. In combination with a VEGFR-3-EpoR-Ba/F3 assay, we have compared the relative activities of the VEGF-C and VEGF-D growth factors for binding and cross-linking VEGFR-2 and VEGFR-3 30. The assay has been used to characterize the inhibitory activity of neutralizing monoclonal antibodies to VEGFR-2 or VEGF-D, soluble VEGFR-2 trap and peptidomimetics targeting the VEGF family31. The assay was also used to show the ability of VEGFs from different orf virus strains to bind and cross-link VEGFR-2 prior to testing in primary endothelial cells 10,26. The assay is particularly useful for the rapid screening of mutants of VEGFs which can be quickly assessed for activity before they are introduced to the more laborious endothelial cell assays 25 or when assessing protocols for purifying growth factors 27.
The assay we describe is easy to perform, and the semi-quantitative version allows for quick determinations that are sometimes required when monitoring the production or purification of growth factors, antibodies or soluble receptor domains for other experiments. The ease of use of the assay makes it an ideal complement to further and more complete studies performed with primary endothelial cells derived from blood or lymphatic vessels from specific tissues or organ systems.
Protocol
Source of IL-3 and Preparation of WEHI-3D-conditioned Medium
Note: The mouse granulocytic leukemia cell line WEHI-3D is cultured to generate a conditioned medium containing IL-3.
Culture WEHI-3D in Dulbecco's Modified Eagle's Medium (DMEM), 10% fetal bovine serum (FBS), 1% long-life glutamine supplement, 50 μg/ml gentamicin. Inoculate 5 × 106 cells in the log phase of growth into 50 ml of fresh culture medium in a T175 cm3 tissue culture flask and grow for about 7 days or until the cells have passed their log phase of growth by about 24 - 48 hr (avoid excessive cell death in the culture). Conditioned media is capable of being stored for multiple years at −20 °C, so batches of 200 − 500 ml can be produced at one time.
Decant fluid and cells from flasks and spin at 1,000 x g for 15 min to remove cells and cellular debris. Remove the top 90% of supernatant. Filter through a 0.22 μm filter unit.
Aliquot WEHI-3D conditioned medium (CM) into 1 ml, 50 ml and 200 ml volumes for storage at −20 °C or −70 °C. Smaller aliquots are useful for assay preparation, intermediate volumes for making culture medium for passage of factor-dependent cell lines, and larger volumes for long-term storage. IL-3 is relatively stable at 4 °C so thawed medium can be stored for a few weeks if used under sterile conditions.
Alternatively, use recombinant mouse IL-3 at levels of 50 ng/ml diluted into culture medium, after being filtered through a 0.22 µm filter unit.
2. Culture and Evaluation of VEGFR-2-EpoR-Ba/F3 and Control Ba/F3 Cell Lines
Culture control Ba/F3 cells in DMEM, 10% FBS, 50 µg/ml gentamicin or penicillin/streptomycin supplement, 1% stabilized L-glutamine and 10% WEHI-3D-CM. Passage cells at 1:15 dilutions about every three days from cells growing in log phase.
Culture VEGFR2-EpoR-Ba/F3 cells in DMEM, 10% FBS, 50 µg/ml gentamycin, 1% stabilized L-glutamine and 10% WEHI-3D-CM and 1 mg/ml G418. Passage cells at 1:15 dilutions from cells growing in log phase. See 24 for more details about the construction and expression of the chimeric receptor.
Harvest control Ba/F3 or VEGFR2-EpoR-Ba/F3 cells from mid log-phase cultures. Gently pipette to remove these non-adherent cells from the bottom of the flask. Wash three times in mouse tonicity phosphate-buffered saline, pH 7.3 (PBS), (10 ml, centrifuge at 750 x g for 5 min to recover cell pellet) to remove medium containing IL-3.
Wash cells once with DMEM and additives, without the WEHI-3D-CM or recombinant IL-3, and then resuspend in this medium at a concentration of 7.4 × 104 cells/ml (i.e., 1,000 cells per 13.5 µl; 10,000 cells per 135 µl as determined by counting using a hemocytometer) for use in the semi-quantitative or quantitative versions of the assay respectively.
Assess cells for viability by Trypan Blue exclusion (CAUTION). Mix Trypan Blue in PBS (0.4%) 1:1 with the cell population and count a minimum of 100 cells on a hemocytometer. Cells that take up the dye are considered dead or dying. Viability of greater than 98% is required to perform the assay.
3. Semi-quantitative Assay
Add washed cells (1,000 cells) contained in 13.5 μl of IL-3-deficient medium to the wells of a 72-well microwell plate at RT. Take care to mix the cell suspension during aliquotting to ensure cell settling by gravity does not bias cell concentration. Use a well-calibrated P20 automated pipette, and autoclaved tips.
Add test samples and controls to the wells at 10% volume (1.5 μl, making a final volume of 15 µl containing 1,000 cells per well) using a calibrated P20 pipette or, preferably, P2 pipette. Take care to ensure that samples are compatible with the culture conditions for Ba/F3 cells in terms of pH, salt and other potentially cytotoxic/cytostatic substances. Where possible, use a compatible medium or buffer (e.g., DMEM or PBS, respectively) or dilute in such medium or buffer. Trituration with the same tip will ensure mixing.
For control samples, include (i) Medium alone-containing no IL-3; (ii) WEHI-3BD-CM added to medium alone at 10% final volume; and/or (iii) VEGF-A diluted to 100 ng/ml in medium alone, containing no IL-3.
Fill any unused wells of the microwell plate with sterile water, PBS or medium and place the plate within a humidified container (with water soaked tissue paper) allowing gas exchange. Incubate cells within the containers in a humidified atmosphere of 10% CO2. This assay can be comfortably set up in 3 hr by one person if test samples and media components are available.
Assess plates typically after 16 hr of incubation at which time discrimination between positive and negative samples is evident. See Figure 3 for examples of how cells appear in culture. Analyze plates using a standard inverted phase-contrast microscope at 40 − 100× magnification. Note: Wells containing supportive growth factors such as IL-3 or VEGF-A will contain cells which appear round and translucent. Wells without supportive growth factors or with medium alone will have reduced numbers of round cells, as well as dead or dying cells and cellular debris (e.g., Figure 3C). Evaluating the assay at 24−48 hr post incubation is the optimum for sensitivity.
4. Quantitative Bioassay
Add washed cells (10,000 cells in 135 μl) in IL-3-deficient medium to the wells of a standard 96-well microtiter plate at RT. Take care to mix the cell suspension during aliquotting to ensure cell settling by gravity does not affect cell concentration.
Add test samples and controls to the wells at 10% volume (15 μl) using a calibrated pipette (P20). Take care to ensure that samples are compatible with the culture conditions for Ba/F3 cells in terms of pH, salt or other potentially cytotoxic/cytostatic substances. Where possible, use a compatible medium or buffer (e.g., DMEM or PBS) or dilute in such medium or buffer. Filter sterilize small volumes using a 0.22 µm pore cellulose acetate centrifuge tube filter unit.
Incubate the mixture of cells, growth factors and/or inhibitor agents in a humidified atmosphere of 10% CO2 for 48 hr. Perform the assay within a humidified container that has water-soaked tissue paper and allows gas-exchange. At the completion of the incubation period, evaluate the assay using one of the alternative methods listed below which are surrogate markers of the viable cell number in the well.
- Alternative#1: 3H-thymidine Incorporation Note: This quantification is designed for the 96-well plate format.
- After incubation of assay plates for 48 hr at 37 °C (150 µl volume per well), add 3H-thymidine in a volume of 50 µl of assay medium (Ba/F3 culture medium without IL-3/WEHI-3D CM) per well at a concentration of 20 µCi/ml, giving a final dose of 1 µCi per well.
- Incubate plates for a further 4 hr at 37 °C before harvesting cells using a cell harvester. Extract individual samples by placing the filters in scintillant and quantify with a Liquid Scintillation Counter.
- Alternative#2: Bioluminescence Detection of Cellular ATP Note: This approach uses an ATP monitoring reagent which when combined with cell lysate can generate a bioluminescence signal which reflects the viable cells in the population.
- Lyse cells to extract ATP and use an ATP Monitoring Reagent to generate a luminescent signal according to the manufacturer's instructions. Read luminescence using a luminometer compatible with a 96-well plate format.
- Alternative#3: Enzymatic Reduction of an Indicator Dye by Viable Cells Note: Resazurin based assays show good correlation to cell viability.
- Combine cultured cells with the resazurin based dye and quantify assays using a fluorescence plate reader according to the manufacturer's instructions.
Representative Results
In this section, we show the results of an experiment demonstrating the essential features of a VEGFR-2-EpoR-Ba/F3 bioassay (see Figure 1 for principles of the assay). Other published studies demonstrate broader applications of the assay for alternative VEGFR-2 ligands, mutant VEGF molecules and inhibitory monoclonal antibodies 8,10,19,24-30.
The data presented here represent an assay in which the VEGFR-2-EpoR-Ba/F3 bioassay is used to quantify the activity of the three recombinant human ligands (VEGF-A165, VEGF-C and VEGF-D) with specificity for VEGFR-2 in the presence of an inhibitory monoclonal antibody (bevacizumab) to VEGF-A165. Data have been normalized versus the response to VEGF-A165 alone (Figure 2). As expected, bevacizumab blocked the effect of VEGF-A165 in the assay, but had no effect on the activity of VEGF-C and VEGF-D.
In the semi-quantitative version of the assay or when observing the assay during its development, viewing the assay using a standard inverted phase microscope can provide valuable information about the progress of the assay. An analysis of an assay (Figure 3) shows the good viability of VEGFR-2-EpoR-Ba/F3 bioassay cells when incubated with medium containing IL-3, or ligand for VEGFR-2, e.g., VEGF-A. These cells are large and translucent, and there is no or minimal evidence of granularity in the cellular cytoplasm or cell debris in the culture. In the well with no additional growth factors there is already evidence of reduced cell numbers and few healthy cells. Cells present have significant granularity in their cytoplasm and cell debris is a consistent feature of the culture. After 48 hr there is no sign of viable cells.
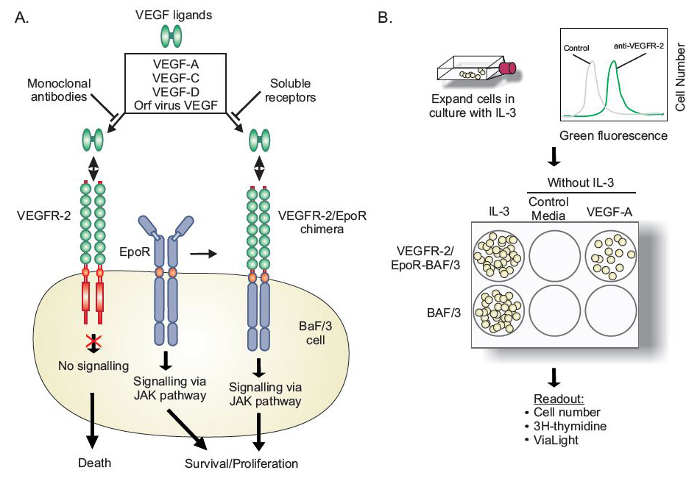
Figure 1. Schematic Diagram Describing the Principles of the VEGFR-2-EpoR-Ba/F3 Bioassay. (A) Ba/F3 cells are factor-dependent and require an exogenous growth factor such as IL-3 to stimulate cell growth/viability via endogenous IL-3 receptors and the JAK signaling pathway. If EpoR is expressed in these cells rescue can also occur via the JAK pathway. Expression of a full-length receptor tyrosine kinase such as VEGFR-2 results in minimal signaling as downstream targets of VEGFR-2 activation do not engage the JAK pathway. A chimeric receptor with the extracellular region of VEGFR-2 fused to the transmembrane and cytoplasmic regions of EpoR, when transfected into Ba/F3 cells and stimulated with specific VEGFR-2 ligands, is capable of activating the JAK pathway and driving cell survival and proliferation. (B) VEGFR-2-EpoR-Ba/F3 cells express significant levels of the chimeric VEGFR-2-EpoR receptor which can be quantified using flow cytometry. Once washed out of IL-3 containing medium, cells are placed in a variety of culture conditions which drive either survival/proliferation of cells. Untransfected Ba/F3 cells or VEGFR-2-EpoR-Ba/F3 cells without specific growth factors fail to grow/survive. Please click here to view a larger version of this figure.
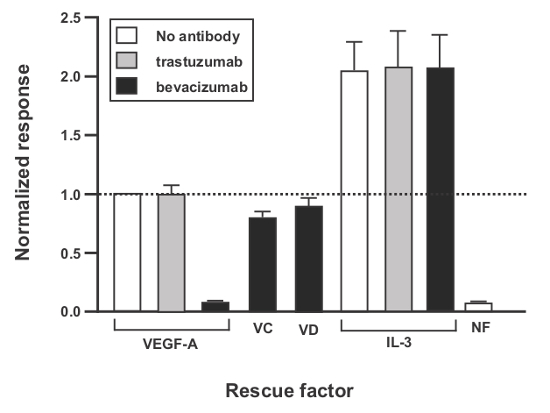
Figure 2. Evaluation of VEGFR-2 Ligands in the VEGFR-2-EpoR-Ba/F3 Bioassay. IL-3-dependent Ba/F3 cells expressing a mouse VEGFR-2-EpoR chimera were seeded in medium without IL-3 (except for the +IL-3 subset, as indicated) plus human VEGF-A165, VEGF-CΔNΔC (VC) (this is a form of VEGF-C which consists of the central region and excludes the N and C-terminal propeptides), or VEGF-DΔNΔC (this is a form of VEGF-D which includes the central region and excludes the N and C-terminal propeptides (VD)) at 100 ng/ml and humanized neutralizing monoclonal antibody bevacizumab (which binds and inhibits VEGF-A, but not VEGF-C or VEGF-D), or control non-neutralising antibody trastuzumab, as indicated at 0.75 μM. NF = medium containing no additional growth factors. Forty-eight hours later, relative live cell number was quantified using bioluminescence detection of cellular ATP. Vertical bars represent means (normalized versus the response to VEGF-A165 alone, first bar) and standard error of the mean of three independent experiments. Please click here to view a larger version of this figure.
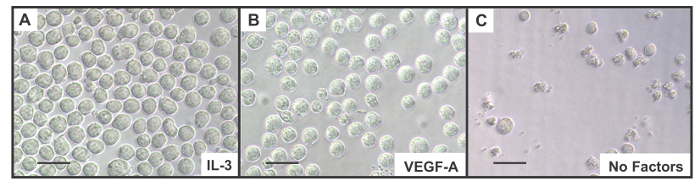
Figure 3. VEGFR-2-EpoR-Ba/F3 Cells are Dependent on External Growth Factors for Survival. Examples of VEGFR-2-EpoR-Ba/F3 bioassay in which IL-3-dependent Ba/F3 cells expressing a mouse VEGFR-2-EpoR chimera were seeded in medium (A) containing 10% WEHI-3D conditioned medium that provides IL-3, (B) 100 ng/ml human VEGF-A165 (no IL-3), or (C) medium with no IL-3 or additional growth factors. Culture is depicted 24 hr into the assay showing highly viable cells in the presence of IL-3 (A) or VEGF-A165 (B), whereas in the culture with no IL-3 or additional growth factors (C) cell death and reduced viability are clearly evident. Scales bars are 100 µm. Please click here to view a larger version of this figure.
Discussion
The assay described here relies on using cells of high viability, which are dependent on growth factors. Cells therefore need to be carefully cultured to ensure they are factor-dependent, and retain expression of the chimeric receptor. Ensuring that the medium is freshly made and not stored for an excessively long period and that WEHI-3D CM is highly active is important. Cells need to be thoroughly washed from IL-3 containing medium into the assay medium to ensure that no residual IL-3 contaminates the assay when exposing the cells to the rescuing ligands. As ligands can come in a number of different forms, care needs to be taken when preparing these for assaying. Preservatives, anti-bacterial agents, salt or buffers of different pH can affect the assay, and this is why parallel testing of untransfected Ba/F3 cells can inform about buffer-related issues.
We use both the semi-quantitative and quantitative protocols. The semi-quantitative assay allows for rapid assessment of the activity of samples prior to committing to a fully quantitative assay which requires more time and sample. The quantitative method is used exclusively when generating data for publication. The alternative methods for detecting the viability of the culture are relatively straightforward. The quantitative assay has been adapted to a number of different formats for quantification of proliferation, and we have used (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide)MTT 30, bioluminescence detection of cellular ATP (unpublished), resazurin-based dye (Figure 2) and 3H-thymidine31 with success. In recent years we have evolved away from the use of radiation to adapt bioluminescence approaches and have used the resazurin-based dye due to its cost-effectiveness. All of the approaches give appropriate results and the choice may depend on the availability of reagents and the associated specialized plate readers in a given laboratory/institute or familiarity with particular approaches.
Troubleshooting revolves around the activity of three main components of the assay, i) IL-3 CM, ii) VEGFR-2-EpoR-Ba/F3 cells and iii) stimulating ligands. Poor levels of IL-3 in CM due to inadequate WEHI 3D cells in the initial culture, poor storage conditions, excessive freeze-thawing cycles or using at too high a dilution can create poorly growing cultures that will hamper the assay. This is usually detected in control wells as cell populations growing slowly, losing their normal bright and round appearance. The VEGFR-2-EpoR-Ba/F3 assay cells can lose cell surface expression of the chimeric construct over time if kept in culture for extended periods. Maintaining good numbers of liquid nitrogen stocks allows fresh cultures of cells highly expressing the chimeric receptor to be available at all times. If required a high expressing population can be achieved by growing cells in VEGFR-2 ligands and selecting the population expressing high levels of the chimeric receptor by flow cytometry sorting. These cells are then further expanded in culture for freezing.
While the technique is a convenient and simplified alternative for analyzing interactions with the VEGFR-2 extracellular domain it cannot be seen to replace more sophisticated experiments where the native receptor on primary endothelial cells is probed in the presence of other VEGFRs, co-receptors such as neuropilins. The cells on which this assay is based (B cells) are distinct from endothelial cells and should therefore not be used to study the cytoplasmic signaling pathways and transcription factors that respond to VEGFR-2 activation in endothelial cells. Nevertheless, the method provides a useful approach to analyze receptor-ligand interaction that lies between a simple binding assay that examines interaction of a ligand with an immobilized receptor construct (such as a soluble receptor extracellular region or a receptor extracellular region fused to immunoglobulin), and the analysis of receptor binding and downstream effects seen in binding assays to primary endothelial cells. It provides a system to allow binding and cross-linking of receptors, the first two stages by which many receptor-type tyrosine kinases commonly initiate their signal transduction cascade. Its application in a number of academic and commercial settings has shown it is accepted as a useful assay for examining binding characteristics, especially when evaluating variant ligands or inhibitors of the receptor-ligand interaction.
The assay is also useful for analysis of new inhibitors of the receptor extracellular domain or of its ligands. The assay could be further used to quickly screen variants of the ligands or receptor extracellular regions seen in the context of genomic mutations (both inherited and somatic) found in human genes in diseases such as cancer 32,33 to determine their functional consequence. Hence the applications of this VEGFR-2 assay are likely to expand in the future. Further, the general approach underlying this assay can be utilized more broadly for the cell surface receptor tyrosine kinases, which constitute a large and important family of signaling molecules in health and disease.
Disclosures
Steven A. Stacker and Marc M. Achen are shareholders in Circadian Technologies Ltd., a company developing therapeutics by targeting the VEGF family of growth factors.
Acknowledgments
SAS and MGA are supported by Project Grants, a Program Grant and Research Fellowships from the National Health and Medical Research Council of Australia (NHMRC), and by funds from the Operational Infrastructure Support Program provided by the Victorian Government, Australia. MMH has support from a Peter MacCallum Foundation Grant.
References
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Achen MG, Stacker SA. Vascular endothelial growth factor-D:signalling mechanisms, biology and clinical relevance. Growth Factors. 2012;5:283–296. doi: 10.3109/08977194.2012.704917. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007;58:491–504. doi: 10.1146/annurev.med.58.061705.145635. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- Korpelainen EI, Alitalo K. Signaling angiogenesis and lymphangiogenesis. Curr Opin Cell Biol. 1998;10:159–164. doi: 10.1016/s0955-0674(98)80137-3. [DOI] [PubMed] [Google Scholar]
- Senger DR, et al. Tumour cells secrete a vascular permeability factor that promotes accumulation of ascities fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Joukov V, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt-4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- Achen MG, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci USA. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen VM, et al. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood. 2011;117:1507–1515. doi: 10.1182/blood-2010-08-301549. [DOI] [PubMed] [Google Scholar]
- Wise LM, et al. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc Natl Acad Sci USA. 1999;96:3071–3076. doi: 10.1073/pnas.96.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Takani K, Atoda H, Morita T. Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF receptor 2) J Biol Chem. 2003;278:51985–51988. doi: 10.1074/jbc.C300454200. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- Stacker SA, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- Skobe M, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- Karnezis T, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181–195. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery. Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Stacker SA, et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–32136. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- McColl BK, et al. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med. 2003;198:863–868. doi: 10.1084/jem.20030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Pacifici RE, Thomason AR. Hybrid tyrosine kinase/cytokine receptors transmit mitogenic signals in response to ligand. J Biol Chem. 1994;269:1571–1574. [PubMed] [Google Scholar]
- Stacker SA, et al. A mutant form of vascular endothelial growth factor (VEGF) that lacks VEGF receptor-2 activation retains the ability to induce vascular permeability. J Biol Chem. 1999;274:34884–34892. doi: 10.1074/jbc.274.49.34884. [DOI] [PubMed] [Google Scholar]
- Davydova N, Roufail S, Streltsov VA, Stacker SA, Achen MG. The VD1 neutralizing antibody to vascular endothelial growth factor-D: binding epitope and relationship to receptor binding. J Mol Biol. 2011;407:581–593. doi: 10.1016/j.jmb.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Wise LM, et al. Viral vascular endothelial growth factors vary extensively in amino acid sequence, receptor-binding specificities, and the ability to induce vascular permeability yet are uniformly active mitogens. J Biol Chem. 2003;278:38004–38014. doi: 10.1074/jbc.M301194200. [DOI] [PubMed] [Google Scholar]
- Davydova N, et al. Preparation of human vascular endothelial growth factor-D for structural and preclinical therapeutic studies. Protein Expr. Purif. 2012;82:232–239. doi: 10.1016/j.pep.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Baldwin ME, et al. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J. Biol. Chem. 2001;276:44307–44314. doi: 10.1074/jbc.M106188200. [DOI] [PubMed] [Google Scholar]
- Baldwin ME, et al. The specificity of receptor binding by vascular endothelial growth factor-D is different in mouse and man. J. Biol. Chem. 2001;276:19166–19171. doi: 10.1074/jbc.M100097200. [DOI] [PubMed] [Google Scholar]
- Makinen T, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBOJ. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen MG, et al. Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur J Biochem. 2000;267:2505–2515. doi: 10.1046/j.1432-1327.2000.01257.x. [DOI] [PubMed] [Google Scholar]
- Bamford S, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasance ED, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]


