Abstract
Background
Little is known about the trends in the incidence and outcomes of patients with end-stage renal disease (ESRD) attributed to human immunodeficiency virus-associated nephropathy (HIVAN). We sought to define relative incidence among ESRD patients, changes in mortality among patients with ESRD attributed to HIVAN, as well as changes in the excess mortality experienced by patients with ESRD attributed to HIVAN compared with otherwise similar ESRD patients with non-HIVAN causes.
Methods
We used the US Renal Data System to identify all individuals with reported HIVAN who initiated treatment for ESRD between 1989 and 2011. We plotted their counts and proportions among all incident ESRD patients and tabulated their characteristics across years. We then compared mortality within the HIVAN group across years using Cox regression. In addition, we studied the trends in relative mortality of HIVAN patients versus those with ESRD not reported as HIVAN.
Results
Overall, 14 719 individuals with HIVAN-ESRD were recorded, with significant reductions in recent years (893 in 2006; 525 in 2011). Compared with patients initiating dialysis between 1989 and 1992, mortality declined by 40% (HR = 0.60; 95% CI, 0.55–0.65) and 64% (HR = 0.36; 95% CI, 0.32–0.40) for patients initiating dialysis in 1999/2000 and 2009–11, respectively. The adjusted excess mortality of HIVAN-ESRD patients versus incident ESRD patients from other causes was >5-fold in 1989–92 (HR = 5.21; 95% CI, 4.84–5.60); this excess mortality has subsequently declined but remained at almost 3-fold in recent years (e.g. HR = 2.58; 95% CI, 2.37–2.80, 2009–11 incidence cohort).
Conclusions
Concurrent with the increasing availability of highly active antiretroviral therapy (HAART), both the incidence of ESRD due to HIVAN and the mortality of such patients have decreased substantially. However, HIVAN patients reaching ESRD continue to experience substantial excess mortality compared with other ESRD patients even in the current era of modern HAART.
Keywords: AIDS, dialysis, HAART, mortality, outcome
INTRODUCTION
Infection with the human immunodeficiency virus (HIV), often considered one of the most challenging diseases of modern times, has been transformed from a deadly and devastating disease to a relatively manageable chronic condition in most industrialized countries. Much of this progress can be attributed to the widespread use of increasingly potent and well-tolerated antiretroviral agents. Given that the survival of the HIV-infected population has dramatically improved, addressing HIV-associated chronic comorbidities is an increasingly significant concern to the medical community. Kidney disease is the fourth most common cause of non-AIDS-related mortality in the HIV-infected population (after cancer, cardiac and liver diseases). Concurrent with the advent and rise of highly active antiretroviral therapy (HAART), between 1985 and 1999, the proportion of dialysis centers in the USA treating HIV-positive patients with end-stage renal disease (ESRD) increased from 11 to 39% and the percentage of dialysis patients with HIV increased from 0.3 to 1.4% [1]. HIV-associated nephropathy (HIVAN) is the predominant histologic lesion seen in HIV patients with kidney disease. The number of patients with ESRD secondary to HIVAN has doubled from 1995 to 2000 [2], but appears to have leveled off, and, in fact, standardized incidence rates declined between 2001 and 2010 [3]. Despite these hopeful trends in incidence of ESRD, HIV patients on hemodialysis have been shown to have a worse prognosis than their HIV-negative hemodialysis counterparts [4].
We conducted the present study of patients who developed and were treated for ESRD between 1989 and 2011 to investigate whether recent advances in HIV treatment have impacted the incidence of ESRD due to HIVAN and trends in the survival of these patients once started on dialysis. Specifically, we examined the following three questions using data collected over 23 years: (i) whether the incidence of ESRD reportedly due to HIVAN (relative to ESRD from other causes) has changed over time; (ii) whether survival among patients with HIVAN initiating treatment for ESRD has improved; and (iii) whether the excess mortality of incident dialysis patients with reported HIVAN relative to patients with ESRD from other causes has decreased over time.
MATERIALS AND METHODS
We conducted a retrospective cohort study using data from the United States Renal Data System (USRDS) on patients with incident ESRD between 1989 and 2011 [5]. Primary cause of kidney failure is documented by physicians in the ESRD Medical Evidence Report (form CMS-2728). All patients who were reported to have HIVAN through an International Classification of Diseases (9th Revision; ICD-9) code of 042.x constituted the group of interest. For comparative analyses, all incident ESRD patients with another reported cause (including unknown or missing) were considered. The exposure/effect modifier of interest was calendar year of incidence of ESRD. For descriptive analyses, patients were further categorized into three eras based on year of first dialysis service. HAART became available in the mid-1990s, so the temporal groups utilized aimed to capture pre-HAART (1989–1995) as well as early (1996–2001) and modern (2002–2011) HAART eras of the HIV/AIDS epidemic. Regression analyses used incidence years 1989–92 as the reference group to which each subsequent 2-year stratum was compared (the most recent stratum consisted of 3 years, 2009–11) when looking at mortality over time within HIVAN patients. When comparing mortality rates over time between HIVAN and non-HIVAN ESRD patients, the latter served as reference group.
From the PATIENTS file, we ascertained age at ESRD, sex, race, which was categorized as White, Black and other (including Asian, Native American, Pacific Islander and other race), as well as Hispanic ethnicity (from 1995 onwards). We also determined—at time of ESRD and for the years these data were available (from 1995 onwards)—employment status (Unemployed, Employed, Homemaker, Retired, Other) and categorized patients by their region of residence (West, Midwest, Northeast, South). We also ascertained type of insurance: Medicaid, Medicare, Department of Veterans Affairs, Employer Group Health Plan, Other Insurance, Uninsured. (Medicaid is a federally mandated, means-tested program that is jointly funded from federal and state funds. Each state Medicaid program is different in eligibility criteria and scope of covered services. Medicare is a federal health insurance program available to eligible persons aged 65 years or older; ESRD is the only disease that qualifies for Medicare regardless of age.) Patients were excluded if sex, race, or place of residence was missing, listed as unknown, or outside the 50 states or the District of Columbia.
Cohorts of patients, tabulated by era, were described by counts (%) for categorical and median (interquartile range; IQR) for continuous variables. The absolute counts of patients reaching ESRD due to HIVAN and their proportion of the overall population were plotted. Kaplan–Meier plots were used to graph actuarial survival by era and differences among curves were tested using the log-rank test. Survival analysis was carried out using Cox proportional hazards models where the endpoint was defined as either date of death or the end of the study period (1 January 2012). In cases where the patient died prior to the end of the study period, time to death was the difference between date of death and date of first dialysis service. The proportionality of hazards was assessed using interaction terms between each covariate and time. Given the large sample size, we further assessed this assumption by plotting scaled Schoenfeld residuals. If appropriate, and in the presence of violations of the proportional hazards assumption, final models were either stratified (for categorical variables) and/or included interaction terms with time (continuous variables). Effect modification by demographic characteristics (age, sex, race) and geographic region was examined using categorical multiplicative interaction terms and applying a global Wald test. Significance was determined by a 2-sided alpha of 0.05; interaction tests were considered significant at a more stringent alpha of 0.01. Statistical analysis was performed using SAS System for Windows version 9.3 (www.sas.com).
RESULTS
The study cohort included 14 719 patients who initiated dialysis between 1989 and 2011 with HIVAN listed as the primary cause of their ESRD. A flow chart of cohort assembly is shown in Supplementary Figure S1. Over 60% of the cohort was between the ages of 21 and 44 years, with an overall median age of 41 years at time of initiation of dialysis (interquartile range of 35–48). Table 1 shows the characteristics of the cohort, stratified by era: 70% were male and nearly 90% were African American. In regard to geographic distribution, 54% of patients resided in southern states, 31% in the Northeast, and 10 and 5% in the Midwest and West, respectively. ESRD due to HIVAN remained concentrated in the South across all time periods analyzed. The states with the highest counts of patients with ESRD secondary to HIVAN throughout the study period were New York and Florida, comprising 2629 (18%) and 1746 (12%) of all cases, respectively.
Table 1.
Characteristics of patients with end-stage renal disease from HIV-associated nephropathy
| Overall | Pre-HAART 1989–1995 |
Early HAART 1996–2001 |
Modern HAART 2002–2011 |
|
|---|---|---|---|---|
| N = 14 719 (100%) | N = 2371 (16.1%) | N = 4721 (32.1%) | N = 7627 (51.8%) | |
| Patient age (year; median; interquartile range) | 41 (35–48) | 37 (32–43) | 40 (34–46) | 44 (37–51) |
| Female sex (%) | 30.0 | 20.8 | 29.1 | 33.4 |
| Race (%) | ||||
| White | 9.7 | 8.9 | 7.7 | 11.2 |
| Black | 88.7 | 89.5 | 90.2 | 87.5 |
| Other | 1.6 | 1.6 | 2.1 | 1.3 |
| Hispanic ethnicity (%) | 5.6 | N.A. | 4.2 | 6.0 |
| Insurance status (%) | ||||
| Medicaid | 52.4 | N.A | 52.8 | 52.2 |
| Medicare | 20.7 | 19.2 | 22.0 | |
| Department of Veterans Affairs | 1.8 | 1.9 | 1.7 | |
| Employer-based group health plan | 14.8 | 15.2 | 14.1 | |
| Uninsured | 20.6 | 23.0 | 19.2 | |
| Other | 7.9 | 8.3 | 7.6 | |
| Employment status (%) | N.A | |||
| Employed | 10.3 | 10.1 | 10.4 | |
| Homemaker | 0.5 | 0.5 | 0.4 | |
| Unemployed | 54.7 | 55.9 | 53.9 | |
| Retired | 25.4 | 21.8 | 27.6 | |
| Other | 9.1 | 11.6 | 7.7 | |
| Geographic region (%) | ||||
| Northeast | 31.0 | 39.0 | 29.7 | 28.6 |
| Midwest | 10.0 | 8.0 | 10.6 | 10.6 |
| South | 54.0 | 45.0 | 55.9 | 56.1 |
| West | 5.0 | 8.0 | 3.8 | 4.7 |
Insurance status and employment status were not reported until the 1995 version of the Medical Evidence Report.
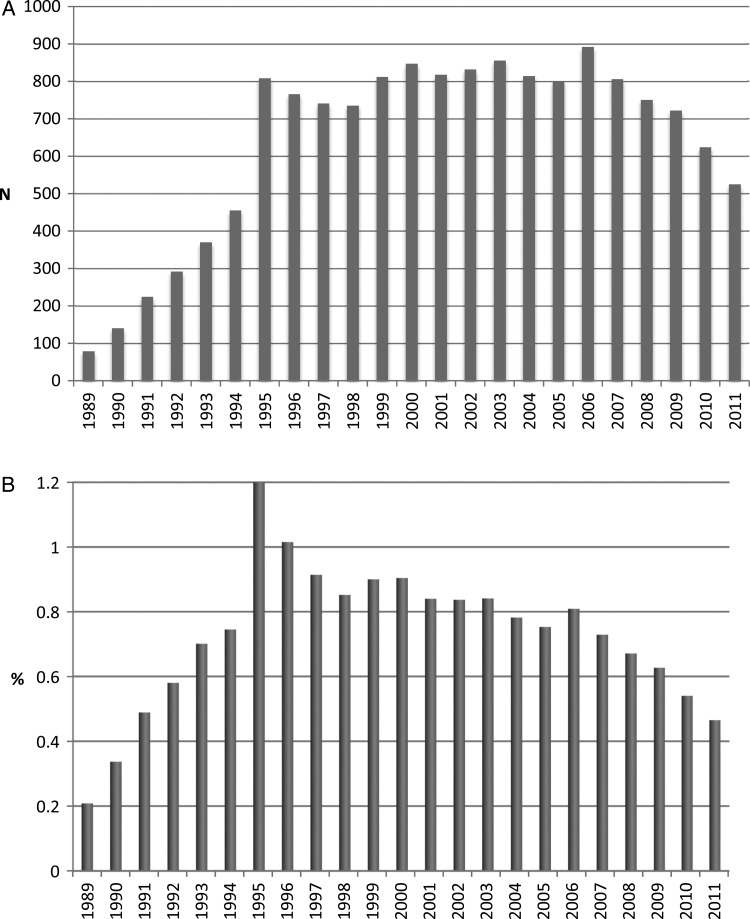
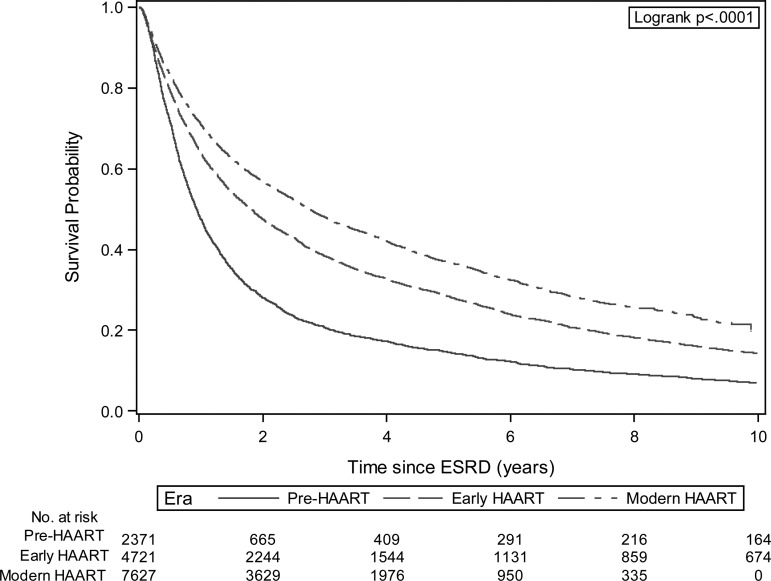
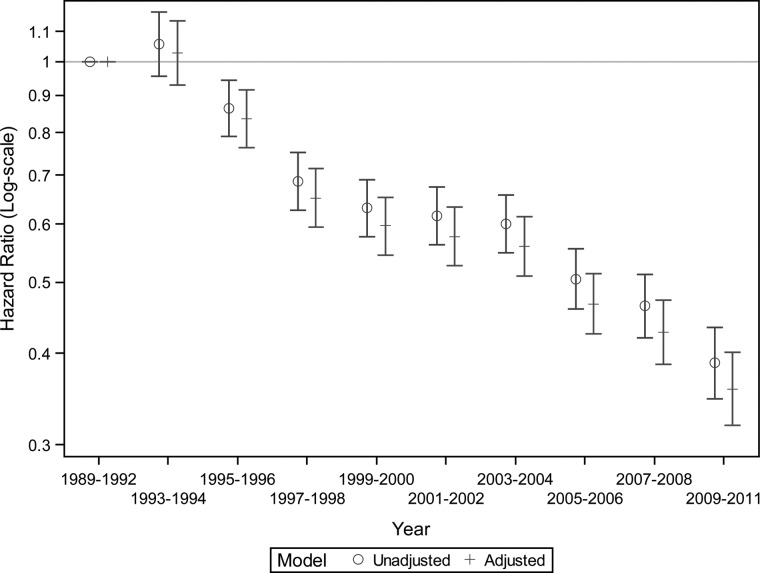
The annual number of patients with incident ESRD secondary to HIVAN increased steadily from 1989 to 1995 and then remained stable until 2006. However, we observed a strong and persistent decline in the patient counts and the proportion of incident ESRD cases attributed to HIVAN between 2006 and 2011 (P < 0.001; Figure 1). Among patients with incident ESRD secondary to HIVAN, overall survival improved during the 23-year study period as shown in Kaplan–Meier plots (P < 0.001; Figure 2). These findings remained robust after stratification or adjustment for age, sex, race and geographic region (Figure 3). Compared with patients initiating dialysis between 1989 and 1992, mortality declined by 40% (HR = 0.60; 95% CI, 0.55–0.65) for patients initiating dialysis in 1999 or 2000, remained similar and then declined again starting with the 2005–2006 ESRD cohort. Patients with HIVAN initiating treatment for ESRD in 2009–11 had approximately one third of the mortality experienced by patients starting dialysis in 1989–92 (HR = 0.36; 95% CI, 0.32–0.40). Only few patients received a kidney transplant during follow-up (N = 315; 2.1%). Tests for effect modification were nominally significant for age, race, and region and stratified analyses are shown in Supplementary Figure S2. It appears that the mortality of children and seniors aged 65 or older did not improve as much as in the two middle-aged groups although the numbers were smaller and the confidence limits wide. Survival analysis by geographic region indicated that although survival improved in every region, less dramatic improvements were noted in the Northeastern and Southern United States. Regarding the reported causes of death, infectious causes—while still dominant—have declined considerably over time and causes of death more typically seen in the general population such as cardiovascular or cancer have increased (Supplementary Figure S3).
FIGURE 1:
Trends in the incidence of end-stage renal disease from HIV-associated nephropathy (1989–2011). (A) Counts. (B) Proportion of all ESRD Cases.
FIGURE 2:
Kaplan–Meier plot of actuarial survival among patients with end-stage renal disease from HIV-associated nephropathy (1989–2011); by Era.
FIGURE 3:
Trends in the survival among incident patients with end-stage renal disease from HIV-associated nephropathy. All hazard ratios are compared to patients with incident ESRD due to HIVAN in 1989–92. Adjusted model included age, sex, race (black, white, other), and geographic location (Northeast, Midwest, South, West). P-value for trend using year as a continuous variable, <0.001.
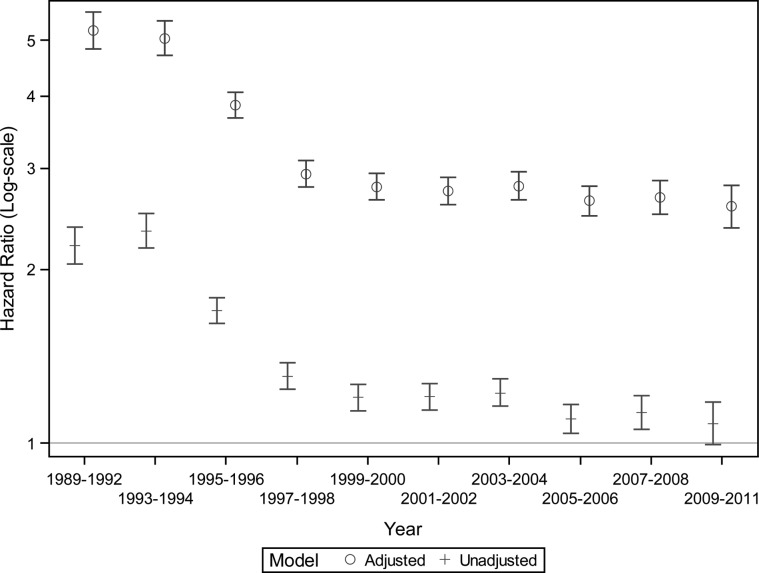
Next we compared patients with HIVAN to those with ESRD secondary to other causes; their characteristics, by era, are shown in Supplementary Table S1. Patients with HIVAN were younger, more likely to be male, black, unemployed, uninsured or on Medicaid, and residing in the South or Northeast. Compared to patients with ESRD secondary to causes other than HIVAN, the mortality of HIVAN ESRD patients was 5-fold for those initiating treatment for ESRD between 1989 and 1995 (Figure 4). Thereafter, the excess mortality declined substantially (P for interaction, <0.001), but has remained at ∼2.5- to 3-times the mortality of patients with ESRD from non-HIVAN causes. Effect modification by region was present and stratified analyses showed more pronounced improvements in relative survival in the West, but perhaps less so in the South (Supplementary Figure S4).
FIGURE 4:
Trends in the relative survival of incident patients with end-stage renal disease from HIV-associated nephropathy versus those with end-stage renal disease from other causes. Adjusted model included age, sex, race (black, white, other), and geographic location (Northeast, Midwest, South, West).
DISCUSSION
Contrary to predictions made a decade ago asserting that increased patient survival in the HAART era would translate into more ESRD cases secondary to HIVAN [6], our results indicate a persistent decline in the counts of incident ESRD cases attributed to HIVAN since 2006. This is likely related, in part, to increased efficacy and safety of combined antiretroviral therapy. Since HIV replicates in kidney epithelial cells [7], viral load reduction would be expected to minimize the direct effect of the virus on disturbing kidney function. In addition, it appears to us that in recent years fewer patients with HIV and kidney disease have presented with the classic features of HIVAN, but increasingly with other phenotypes like focal-segmental glomerulosclerosis alone, or interstitial nephritis, or other pathologies [8]. Whether this is the case and to what extent cannot be determined using the data we have available.
Following the introduction of HAART in 1995, improvements were noted in the survival of HIV-infected dialysis patients, but previous studies were restricted to the early HAART era, the decade following the initial widespread distribution of antiretrovirals [1, 9]. Our current investigation demonstrates further improvements in survival throughout the two decades following the introduction of HAART up through the current era of combined antiretrovirals. Indeed, compared with 1989–92, mortality declined by ∼40% one decade later, but by almost 70% two decades later. During the same time, the survival of the overall ESRD population has not improved as markedly, which leads to the observed persistent narrowing in the excess mortality of HIVAN ESRD cases compared to ESRD secondary to all other causes in more recent years. As previously observed by Ross et al., patients with incident ESRD secondary to HIVAN in 1996 had an almost 5-fold increased risk of mortality compared to other ESRD patients [6]. Our finding of a significantly diminishing excess mortality, 2.5- to 3-fold in the most recent years, lends support to previous projections that the survival of patients with ESRD due to HIVAN may eventually reach levels similar to the rest of the ESRD population [10]. However, one of the big issues that remain unresolved is the apparent acceleration of chronic disease progression in HIV-positive patients. Whether there is some low level of chronic inflammatory state or the effect of drugs, patients who are HIV positive even under treatment seem to have more rapid progression of diabetes, cardiac disease, liver disease, and other chronic conditions, which is also reflected in the trends of causes of death shown in our study [11, 12]. The persistence of this excess mortality through 2011 data, despite a significant narrowing of the gap, clearly indicates that morbidity and mortality of HIVAN patients remain high and that much work remains to be done to further improve the survival in this specific patient group.
Our results also confirm the previously reported racial predilection of HIVAN for African Americans. While African Americans make up a disproportionately high proportion of the HIV-infected population in the USA and also have a greater incidence of ESRD than any other race, the fact that they constitute almost 90% of patients with ESRD due to HIVAN is striking. Prior data suggest that the racial differences in ESRD rates may be explained in part by genetic factors (e.g. apolipoprotein L1 genotype) causing a more aggressive natural disease history in African Americans [13]. While it has been shown that African Americans with HIV experience faster decline in kidney function than whites [14], the putative interaction between apolipoprotein L1 genotype and the HIV on progression of kidney disease has not specifically been studied. In addition, it has been shown that African Americans are less likely to receive HAART [14], which may add to the accelerated decline in kidney function in this demographic group.
In regard to geographic distribution of ESRD secondary to HIVAN, over 60% of cases arose in areas designated as ‘high impact’ HIV/AIDS regions by the CDC [15]. These areas are defined as places with a particular heavy burden of HIV disease and include California, District of Columbia, Florida, Georgia, Illinois, Maryland, New York, Pennsylvania and Texas. There is a concentration in certain urban areas of the East and West Coast and there is a rapid increase in HIV incidence in the South East—particularly within the African American population. Our analysis confirms that ESRD secondary to HIVAN is more prevalent in the Southern states across all time periods studied which likely reflects the disproportionate impact of the epidemic within African American communities in the South East. Although the majority of HIVAN cases leading to ESRD are amongst African Americans (nearly 90%), the Northeast has a disproportionately higher burden of cases relative to the density of African Americans in this region. This suggests that factors other than race may be contributing to the progression to HIVAN among HIV infected patients. Access to medical care and HIV medications are proposed modifiable risk factors that may be impacting HIVAN disease progression.
As Southern states have disproportionately high rates of both incident and prevalent HIV diagnoses, HIV-related complications such as HIVAN are thus likely to remain concentrated in these areas in the near future. The CDC initiated a new 5-year HIV prevention plan in 2012 that aims to better align funding to the current geographic burden of the national HIV epidemic [16]. Future investigations may address if such strategies have contributed to narrowing regional gaps in survival and reducing the burden of HIV and HIV related renal disease in current highly impacted areas.
In regard to further funding, AIDS Drug Assistance Programs (ADAPs) were put into place in all 50 states through the federal Ryan White Comprehensive AIDS Resources Emergency (CARE) Act of 1990 [17]. Prior reports have indicated that Southern states receive less Ryan White funding per AIDS case than other states, most notably in the Deep South (AL, GA, LA, MS, NC, SC) [18]. Although this regional gap in Ryan White drug assistance funding has somewhat narrowed based on 2010 data, Southern states have also been shown to provide Medicaid coverage for a lower proportion of HIV patients and less funding per HIV-positive beneficiary in comparison to national averages [18]. Our data suggest that perhaps enhancing geographic targeting of HIV/AIDS resources could also help address the pockets of heavy HIVAN disease burden which follow a similar pattern of distribution. As HIVAN tends to be a late manifestation of HIV infection [19], upstream efforts at prevention, early screening, and access to HAART may improve renal outcomes such as the progression of HIVAN in the HIV-infected population. As the use of antiretroviral therapy has been associated with a slower progression to renal replacement therapy among patients with HIVAN [20, 21], and our data suggest decreasing incidence of ESRD secondary to HIVAN in the late HAART era, existing or new kidney dysfunction may compel clinicians to introduce HAART irrespective of CD4 count.
There are certain limitations of our study that require discussion. First, we ascertained the presence of HIVAN as the cause of ESRD from the Medical Evidence Report submitted to the Centers for Medicare and Medicaid Services. A previous study has validated the reporting of glomerular diseases using a biopsy registry and found limited sensitivity, but excellent positive predictive values for most glomerular diseases [22]. The accuracy of reported HIVAN was not specifically studied since only a single patient had this diagnosis in the study database. As renal biopsy is the only reliable test to establish or exclude the presence of HIVAN [6], the lack of biopsy-proven diagnoses for the entire USRDS patient population may translate into underestimates of the true incidence of ESRD secondary to HIVAN (or other renal phenotypes secondary to HIV or its treatment) in the USA. It appears, however, that any inferences on trends in (relative) mortality among HIVAN patients as well as when comparing HIVAN patients to those without HIVAN would remain unbiased if the positive predictive value of HIVAN reporting as a cause of ESRD was also high. Another limitation of our study is the absence of patient-level data on CD4 T-cell counts, HIV viral load and antiretroviral therapy. Trends in the presence of low CD4 counts and/or high HIV viral loads could serve as confounders, but more likely as mediators of our findings. Our cohort is stratified by time periods within our 23-year study period, which we correlate with the introduction and distribution of HAART. We are assuming that HIV patients in the mid- and late-HAART eras are utilizing these medications but this was not confirmed by medication prescription data. The eras we have chosen correspond roughly to the introduction of certain medications; their impact on (renal) outcomes may be subject to time lag, which is unaccounted for in our study. Thus, any differences in prescribing practices, access, and compliance to antiretroviral drugs are not captured and cannot inform our observations of geographic heterogeneity in trends. While this current study focuses on those patients with HIVAN who have ultimately progressed to ESRD, many patients have chronic kidney disease secondary to HIVAN who are not yet on dialysis and are thus not captured in the USRDS database. This will be a crucial population to study in future investigations in regard to exploring factors that may prevent or slow progression to ESRD in the HIVAN population. As HIV continues to evolve into a relatively chronic condition that is clinically vastly different than it was just two decades ago, the associated comorbidities will undoubtedly continue to evolve as well and will require continued re-evaluation in regards to their impact on overall survival in this patient population.
Our study indicates favorable trends in the incidence and outcomes of ESRD secondary to HIVAN over the past two decades. While the ultimate goal has to be eradication of HIVAN as a cause of advanced kidney disease, for now it appears similarly important to identify strategies that lead to further reduction of the excess mortality experienced by patients with HIVAN who do reach ESRD and require long-term dialysis treatment.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
A.R.Z. is an employee of ViiV Healthcare. P.E.K. serves on the Scientific Advisory Board of Gilead. W.C.W. acknowledges salary and research support through the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine. None of the remaining authors have any disclosures.
Supplementary Material
ACKNOWLEDGEMENTS
The results presented in this paper have not been published previously in whole or part, except in abstract format. Data reported herein were supplied by the United States Renal Data System (USRDS). This work was conducted under a Data Use Agreement between Dr Winkelmayer and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK). The manuscript was reviewed and approved for publication by an officer of the NIDDK. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. This work was supported by National Institutes of Health grants P01 DK056492 and T32 DK007357.
REFERENCES
- 1.Ahuja TS, O'Brien WA. Special issues in the management of patients with ESRD and HIV infection. Am J Kidney Dis 2003; 41: 279–291 [DOI] [PubMed] [Google Scholar]
- 2.Choi AI, Rodriguez RA, Bacchetti P, et al. Low rates of antiretroviral therapy among HIV-infected patients with chronic kidney disease. Clin Infect Dis 2007; 45: 1633–1639 [DOI] [PubMed] [Google Scholar]
- 3.Sexton DJ, Reule S, Solid C, et al. End-stage renal disease from human immunodeficiency virus-associated nephropathy in the United States, 2001 through 2010. JAMA Intern Med 2014; 174: 809–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tourret J, Tostivint I, du Montcel ST, et al. Outcome and prognosis factors in HIV-infected hemodialysis patients. Clin J Am Soc Nephrol 2006; 1: 1241–1247 [DOI] [PubMed] [Google Scholar]
- 5.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis 2013; 61 (1 Suppl 1): A7, e1–476 [DOI] [PubMed] [Google Scholar]
- 6.Ross MJ, Klotman PE. HIV-associated nephropathy. AIDS 2004; 18: 1089–1099 [DOI] [PubMed] [Google Scholar]
- 7.Bruggeman LA, Ross MD, Tanji N, et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol 2000; 11: 2079–2087 [DOI] [PubMed] [Google Scholar]
- 8.Ahmed S, Truong L, Eknoyan G, et al. Evolving spectrum of HIV-associated nephropathy. Nephron Clin Pract 2012; 121: c131–c135 [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez RA, Mendelson M, O'Hare AM, et al. Determinants of survival among HIV-infected chronic dialysis patients. J Am Soc Nephrol 2003; 14: 1307–1313 [DOI] [PubMed] [Google Scholar]
- 10.Abbott KC, Trespalacios FC, Agodoa LY, et al. HIVAN and medication use in chronic dialysis patients in the United States: analysis of the USRDS DMMS Wave 2 study. BMC Nephrol 2003; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160: 458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 2009; 50: 499–505 [DOI] [PubMed] [Google Scholar]
- 13.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 2013; 369: 2183–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alves TP, Hulgan T, Wu P, et al. Race, kidney disease progression, and mortality risk in HIV-infected persons. Clin J Am Soc Nephrol 2010; 5: 2269–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Funding Opportunity Announcement (FOA) PS12-1201: Comprehensive Human Immunodeficiency Virus (HIV) Prevention Programs for Health Departments. http://www.cdc.gov/hiv/policies/funding/announcements/ps12-1201/index.html .
- 16. High-Impact HIV Prevention: CDC's Approach to Reducing HIV Infections in the United States. http://www.cdc.gov/hiv/policies/hip.html .
- 17. Part B: AIDS Drug Assistance Program. http://hab.hrsa.gov/abouthab/partbdrug.html . [PubMed]
- 18.Reif S, Whetten K. SASI Update: The Continuing HIV Crisis in the US South Duke Center for Health Policy and Inequalities Research, Duke University, Durham, NC: December 2012. http://southernaids.files.wordpress.com/2011/10/sasi-update-the-continuing-hiv-crisis-in-the-us-south.pdf (Accessed date 4/8/2014) [Google Scholar]
- 19.Ganesan A, Krantz EM, Huppler Hullsiek K, et al. Determinants of incident chronic kidney disease and progression in a cohort of HIV-infected persons with unrestricted access to health care. HIV Med 2013; 14: 65–76 [DOI] [PubMed] [Google Scholar]
- 20.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int 2004; 66: 1145–1152 [DOI] [PubMed] [Google Scholar]
- 21.Ahuja TS, Borucki M, Grady J. Highly active antiretroviral therapy improves survival of HIV-infected hemodialysis patients. Am J Kidney Dis 2000; 36: 574–580 [DOI] [PubMed] [Google Scholar]
- 22.Layton JB, Hogan SL, Jennette CE, et al. Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 2010; 5: 2046–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.