Abstract
Background
Implantable cardioverter-defibrillators and cardiac resynchronization therapy-defibrillators (ICD/CRT-Ds) are evidence-based preventative treatments for many patients with heart failure (HF), yet large numbers of eligible patients remain untreated. It is uncertain if localities with more frequent ICD/CRT-D use have had better rates of HF survival.
Objectives
To determine if U.S. Hospital Referral Regions (HRRs) with larger increases in the rate of ICD/CRT-D utilization during 2002–2007 also had commensurate increases in HF survival.
Research Design
Retrospective cohort.
Subjects
Medicare beneficiaries age 66–80 non-electively hospitalized for HF from 2002–2007.
Measures
Each HRR’s annual ICD/CRT-D rate was estimated from the cohort’s Medicare procedure claims. Survival duration was determined from Medicare mortality records. HRR-year-level panel regression models were estimated to assess whether an HRR’s ICD/CRT-D rate predicted HF survival, adjusting for baseline differences in survival across HRRs and secular trends.
Results
883,002 HF patients were propensity-score matched within HRR across 2002–2007. Across HRRs, growth in ICD/CRT-D use among such patients varied from 1–12 percentage points. Regression models indicated that a 1 percentage point increase in an HRR’s ICD/CRT-D utilization among hospitalized HF patients was associated with an increase in one-year survival of 0.12% (95% confidence interval[CI] 0.03% to 0.21%, p=0.009) and with a 0.26% increase in HF survival at two years (95% CI 0.14% to 0.37%, p<0.001).
Conclusions
Localities with greater increases in ICD/CRT-D utilization from 2002 to 2007 also had greater improvements in HF survival. Areas with persistently low ICD/CRT-D use may be good targets for programs designed to increase the evidence-based use of defibrillators.
Keywords: cardiovascular disease, congestive heart failure, health care technology, mortality, utilization
Implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds) have been demonstrated in randomized controlled trials to reduce mortality in patients with heart failure (HF) and reduced left ventricular ejection fraction.1, 2 Although clinical trials demonstrating the benefits of ICDs/CRT-Ds mostly enrolled patients younger than age 65,3 recent observational studies of ICD/CRT-D outcomes among clinically-eligible elderly HF patients have confirmed ICDs/CRT-Ds also improve survival among patients over age 65.4, 5 Guidelines have been jointly issued by the American Heart Association, American College of Cardiology, and Heart Rhythm Society recommending “primary prevention” ICD/CRT-D implantation in all clinically eligible HF patients as a Class IA indication.6 In January of 2005, the Centers for Medicare and Medicaid Services expanded coverage for ICDs to include device implantation for primary prevention, thereby reducing financial barriers to ICD/CRT-D receipt.7
Despite the published evidence, guidelines, and favorable coverage decisions supporting primary prevention ICDs, growth in ICD/CRT-D volume has not risen to a level commensurate with the estimated size of the ICD-eligible HF population,7, 8 suggesting a sizeable fraction of guidelines-eligible patients are not receiving devices.9 Quality-of-care initiatives for heart failure are increasingly focused on reducing the fraction of ICD-eligible HF patients who do not have an ICD.10 However, the public health impact of primary prevention ICDs/CRT-Ds among non-experimental HF patients nationwide is uncertain.11 If expanding ICD/CRT-D utilization among Medicare patients with HF during recent years has had relatively little impact on HF mortality, efforts to further increase the use of ICDs/CRT-Ds in this population would be questionable.
The primary goal of this research was to determine whether growth in the numbers of Medicare beneficiaries with HF receiving ICDs/CRT-Ds in 2002–2007 was associated with improvement in survival among these patients, and to estimate the future public health impact of increasing ICD/CRT-D utilization in localities with persistently low ICD/CRT-D use.
Methods
Overview
The study’s analytical approach involved the assembly of annual (i.e., 2002–2007) subcohorts of CHF patients within 306 small U.S. geographic areas. Patients initially were selected into subcohorts based on their being non-electively hospitalized for heart failure. These patients were then matched within areas across all 6 years by demographic and clinical characteristics. The local rates of ICD/CRT-D utilization and one-year survival in each annual HF subcohort were measured, and regression methods were used to estimate whether increasing ICD/CRT-D rates were associated with increasing survival.
Data
Data for this study were fee-for-service inpatient and outpatient institutional Medicare claims from 2001 to 2007. Medicare Part A (inpatient) coverage is nearly universal for persons over age 65, and approximately 85% of Medicare beneficiaries are covered under fee-for-service,12, 13 thus these claims data are broadly representative of the elderly Medicare population nationwide. Although Part B (outpatient/physician) coverage is not universal, we determined that the vast majority (>98%) of hospitalized elderly patients with heart failure carried Part B coverage between 2001–2007, as would be expected in a population with a high level of anticipated health care utilization. Claims data for HF patients were linked to beneficiaries’ demographic information and ZIP codes in Medicare’s annual enrollment files.
Patients, Setting, and Exclusions
Patients in this study were Medicare beneficiaries ages 66–80 non-electively hospitalized with a diagnosis of heart failure between 2002 and 2007. Medicare claims do not include clinical indicators of ICD/CRT-D appropriateness such as left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) class, or QRS duration, so the study population was instead selected from acutely hospitalized HF patients, who have relatively high rates of ICD/CRT-D eligibility.9,14 We restricted the cohort to patients older than age 66 because universal Medicare coverage begins at age 65, and therefore patients 66 and older were likely to have at least one year of antecedent health care claims that could be used to identify prior HF admissions, prior ICD/CRT-D implantation, and comorbidities. Patients older than 80 were excluded as these patients have a high prevalence of heart failure15 but frequently have compelling contraindications (e.g., low life expectancy, dementia, etc.) to ICD/CRT-D implantation.16 To ensure that we were only evaluating patients with no prior device therapy, patients were also excluded if they had any prior Medicare claim indicating ICD/CRT-D implantation or device replacement from January 1, 2001 until their index HF hospitalization date. Patients were also excluded if they had been diagnosed with metastatic cancer at any point during the 12 months prior to their index HF hospitalization.
Identifying Patients with Heart Failure, ICD/CRT-D Recipients and Survival
To increase the probability that annual sub-cohorts of HF patients were reasonably uniform in their mortality risk across years, we excluded patients who had a HF hospitalization in the year prior to their index hospitalization, and we enrolled patients at their earliest hospital admission date of the calendar year, thus all patients were generally beginning a HF episode of care after a prolonged period without a HF hospitalization. To create unambiguous time boundaries between annual cohorts, we included only those patients with a HF hospital admission date in the first 10 months of each calendar year. The vital status of each patient at one year and two years (excepting 2007 patients, for whom two-year data were not available) after the admission date of the index hospitalization was determined from annual 2002–2008 Medicare Denominator files, which are linked to the Social Security Administration’s Death Master File and thus are reliable indicators of mortality.17 Among the selected HF patients, ICD/CRT-D implantations were identified based on the appearance of procedure codes 00.51, 00.54, 37.94, or 37.96 occurring during the index hospitalization claim or on a subsequent inpatient or outpatient (i.e., ambulatory surgical center) procedure claim, but no later than December 31 of the index hospitalization calendar year.
Geographic Unit of Analysis
The Dartmouth Atlas for Health Care’s Hospital Referral Region (HRR), defined as a contiguous locality within which most inter-facility cardiovascular referrals are contained, was used as the primary geographic unit of analysis.18
Propensity Score Matching of Annual HRR Heart Failure Cohorts
To further increase the likelihood that annual HF cohorts were stable over time in terms of demographic and clinical characteristics, each HRR’s HF patients selected via the methods described above were then matched across years using a propensity score logistic regression model.19, 20 This model’s independent variables included age, race, and gender as indicated by Medicare enrollment data, as well as comorbidity information coded on the index hospitalization claim and linked inpatient and outpatient claims from the 12 months prior to the index hospitalization. Specific comorbidities included in the model, identified using methods of Elixhauser et al.,21 were coronary artery disease, arrhythmias, valvular disease, diabetes, peripheral vascular disease, chronic liver disease, chronic kidney disease, chronic pulmonary disease, and non-metastatic cancer. Receipt of an ICD/CRT-D was the dependent variable. The propensity score regression model was estimated using HF patients in 2006–2007 only, as these years had the highest ICD/CRT-D rates. Coefficients derived from this model were then used to estimate a propensity score for each HF cohort patient from 2002 to 2007, and patients were subsequently matched within HRR across years by propensity score and minimal Mahalanobis distance between key covariates (i.e., age, coronary artery disease, renal disease, and non-metastatic cancer). Matching of patients across 2002–2007 was completed via a “closest match within calipers” strategy in groups of six, with one patient selected from each year. Unmatched patients were excluded from subsequent analysis. These methods ultimately yielded six annual subcohorts of HF patients that were identically distributed geographically and that were closely matched across factors predictive of ICD/CRT-D receipt.
HRR-level ICD/CRT-D Rates, Survival Rates, and Regression Analysis
Using matched HF patients only, for each HRR and year, the percentage of ICD/CRT-D recipients was calculated as well as the percentage of patients alive at 1 and 2 years after index admission. The study’s final analytical dataset thus comprised 6 consecutive annual observations of ICD/CRT-D rate as well as one-year and two-year HF survival rates for each of 306 HRRs, totaling 1,836 HRR-year-level observations. An ordinary least squares panel regression model weighted by each HRR’s matched HF population size was then estimated using this HRR-year-level data. The regression had one-year survival as the dependent variable and ICD/CRT-D rate as the key independent variable, as well as HRR fixed effects to control for any non-time-dependent differences across HRRs and year fixed effects (i.e., year dummy variables) to control for secular trends.22
Sensitivity Analysis
The assumption that any significant association at the HRR level between changes in ICD/CRT-D rate and changes in HF survival solely resulted from improved survival among ICD/CRT-D recipients was tested via a sensitivity analysis in which all ICD/CRT-D recipients and their matches were removed from the HF cohorts. Survival percentages at the HRR-year-level were re-calculated in these “reduced cohorts,” and the regression models were re-estimated using the reduced cohort survival percentages as dependent variables and the full cohort ICD/CRT-D rates as independent variables. This analysis revealed whether there was any residual association between ICD/CRT-D rates and the survival rates of non-ICD/CRT-D recipients, which would suggest the presence of residual confounding.
Projection of Improved Survival
To estimate the potential public health benefit of increasing ICD/CRT-D utilization in localities with low use, HRRs were divided into quintiles based on their ICD/CRT-D rates in 2006–2007, and the results of the regression model and the observed 2006 sub-cohort two-year survival rates were used to project the number of premature deaths that might have been averted had ICD/CRT-D use been higher in HRRs with low ICD/CRT-D rates. In the first counterfactual projection, ICD/CRT-D utilization in the lowest three HRR quintiles in 2006–2007 was raised to the national average ICD/CRT-D rate. In the second counterfactual projection, ICD/CRT-D utilization in the lowest four quintiles of HRRs in 2006–2007 was raised to that of the top quintile.
Institutional Oversight and Statistical Software
The University of Pennsylvania’s Institutional Review Board approved the study protocol. Statistical analyses were performed using SAS version 9.2 (Cary, NC) or STATA version 11.1 (College Station, TX).
Results
Study population
We initially identified 919,323 patients with HF who met study entry criteria. After propensity score matching, there were 147,167 patients included in each annual subcohort, totaling 883,002 patients across 6 years in the full study cohort. These six matched subcohorts were well balanced in terms of demographics, comorbidities, and propensity scores (Table 1). Seventy-six percent of these patients had at least one prior outpatient HF diagnosis, with a median duration of diagnosed HF prior to the index hospital admission date equal to 24 months. The mean ICD/CRT-D rate in this cohort increased from 2.6% in 2002 to 7.0% in 2007 (p<0.001).
Table 1.
Characteristics of Propensity-Score Matched Heart Failure Cohorts, 2002–2007*
| Characteristic | 2002 (n=147,167) | 2003 (n=147,167) | 2004 (n=147,167) | 2005 (n=147,167) | 2006 (n=147,167) | 2007 (n=147,167) |
|---|---|---|---|---|---|---|
| Age, years (std) | 74 (4) | 74 (4) | 74 (4) | 74 (4) | 74 (4) | 74 (4) |
| Female | 79,923 (54) | 80,575 (55) | 80,396 (55) | 80,510 (55) | 81,494 (55) | 80,739 (55) |
| Non-white | 26,840 (18) | 26,514 (18) | 26,457 (18) | 26,956 (18) | 26,648 (18) | 26,052 (18) |
| U.S. Census Region† | ||||||
| Northeast | 29,544 (20) | 29,571 (20) | 29,590 (20) | 29,577 (20) | 29,377 (20) | 29,524 (20) |
| Midwest | 37,983 (26) | 38,007 (26) | 37,965 (26) | 37,963 (26) | 38,169 (26) | 38,112 (26) |
| South | 62,678 (43) | 62,621 (43) | 62,598 (43) | 62,613 (43) | 62,629 (43) | 62,504 (42) |
| West | 16,962 (12) | 16,968 (12) | 17,014 (12) | 17,014 (12) | 16,992 (12) | 17,027 (12) |
| Coronary artery disease | 77,758 (53) | 77,764 (53) | 77,758 (53) | 77,763 (53) | 77,752 (53) | 77,765 (53) |
| Arrhythmia | 53,846 (37) | 53,885 (37) | 55,657 (38) | 57,287 (39) | 58,857 (40) | 60,237 (41) |
| Chronic pulmonary disease | 52,624 (36) | 53,294 (36) | 55,090 (37) | 56,197 (38) | 57,058 (39) | 57,166 (39) |
| Diabetes, uncomplicated | 49,243 (33) | 49,564 (34) | 50,220 (34) | 50,745 (34) | 51,352 (35) | 50,531 (34) |
| Chronic kidney disease | 12,821 (9) | 12,829 (9) | 12,823 (9) | 12,826 (9) | 12,819 (9) | 12,816 (9) |
| Peripheral vascular disease | 12,710 (9) | 11,906 (8) | 11,667 (8) | 11,291 (8) | 11,119 (8) | 10,437 (7) |
| Diabetes with complications | 9,973 (7) | 9,335 (6) | 9,630 (7) | 9,392 (6) | 8,103 (6) | 7,879 (5) |
| Valvular disease | 1,590 (1.1) | 1,654 (1.1) | 817 (0.6) | 699 (0.5) | 552 (0.4) | 3,502 (2.4) |
| Chronic liver disease | 1,542 (1.0) | 1,503 (1.0) | 1,487 (1.0) | 1,554 (1.1) | 1,535 (1.0) | 1,683 (1.1) |
| Cancer (no metastases) | 1,342 (0.9) | 1,340 (0.9) | 1,340 (0.9) | 1,338 (0.9) | 1,351 (0.9) | 1,340 (0.9) |
| DxCG risk score‡, mean (std) | 1.2 (1.1) | 1.2 (1.1) | 1.2 (1.1) | 1.2 (1.1) | 1.2 (1.1) | 1.2 (1.1) |
| Propensity score§, mean (std) | −2.8 (0.7) | −2.8 (0.7) | −2.8 (0.7) | −2.8 (0.7) | −2.8 (0.7) | −2.8 (0.7) |
| ICD recipients | 3,870 (2.6) | 5,416 (3.7) | 7,785 (5.3) | 10,979 (7.5) | 10,419 (7.1) | 10,228 (7.0) |
Data are n (percentage) unless otherwise specified. Percentages may not add to 100 due to rounding
Although patients were matched across years within HRRs and thus the number of patients in each HRR was the same for each year, there was slight variation in the number of patients living in the 4 U.S. Census regions across years, as some HRRs straddle Census region boundaries
The DxCG Risk Score is a well-validated, claims based predictor of future health care cost accrual
Propensity scores presented as logits (i.e., log (probability/(1-probability))
Geographic Variation in ICD/CRT-D Utilization
There was substantial variation in the 2002–2007 change in ICD/CRT-D rates across HRRs, ranging from 0 to 12 percentage points. Comparisons of HRR characteristics across quintiles of HRRs ranked by the 2006–2007 ICD/CRT-D rate indicated HRRs with the lowest rates of ICD/CRT-D use had significantly lower population density, lower per capita income, fewer academic hospitals per capita, and fewer cardiologists per capita than HRRs with the highest ICD/CRT-D rates (Table 2). Low-ICD-utilizing HRRs were relatively evenly distributed across the United States (Figure).
Table 2.
Characteristics of HRRs, by Quintiles of 2006–2007 ICD Use
| Characteristic | Low Quintile (n=12,368)* | Quintile 2, (n=29,945) | Quintile 3, (n=38,502) | Quintile 4 (n=32,118) | High Quintile (n=34,234) | P-value |
|---|---|---|---|---|---|---|
| ICD use, mean† | 4.1 | 5.0 | 6.3 | 7.6 | 10.5 | 0.01 |
| HRR population, mean‡ | 530,669 | 911,884 | 1,253,472 | 996,217 | 1,174,092 | <0.001 |
| HRR population density, per square mile‡ | 630 | 481 | 324 | 554 | 1,157 | <0.001 |
| Black population‡ | 7 | 11 | 11 | 13 | 10 | 0.007 |
| Per capita income, $‡ | 19,400 | 19,100 | 20,420 | 21,230 | 21,034 | 0.004 |
| Acute care hospital beds, n per million population§ | 2,520 | 2,620 | 2,480 | 2,470 | 2,490 | 0.87 |
| Academic hospitals, n per million population§ | 0.5 | 0.7 | 0.9 | 0.9 | 0.8 | 0.007 |
| ICD/CRT-D hospitals, n per million population‖ | 0.7 | 0.6 | 0.6 | 0.6 | 0.6 | 0.62 |
| Cardiologists, n per million population¶ | 50 | 57 | 58 | 64 | 61 | <0.001 |
Annual number of heart failure study cohort patients in quintile
Average annual ICD percentage use in the 2006 and 2007 heart failure study subcohorts
Source: 2000 U.S. Census
Source: Medicare Hospital Cost Report Information System
Source: Hospitals that submitted claims to Medicare for ICD/CRT-D implantation in 2006–2007
Source: Area Resource File
Abbreviations: HRR—hospital referral region; ICD—implantable cardioverter-defibrillator; CRT-D—cardiac resynchronization therapy defibrillator.
Figure.
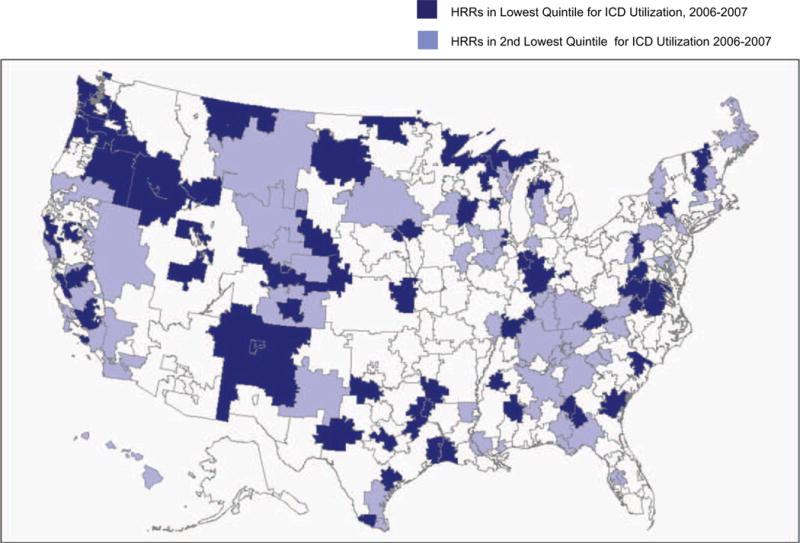
Location of low-use hospital referral regions, 2006–2007. The dark blue areas indicate HRRs in the lowest quintile (i.e., bottom 20% of HRRs) of ICD/CRT-D utilization among study cohort heart failure patients during 2006–2007. The light blue areas indicate HRRs in the second lowest quintile of ICD/CRT-D utilization among study cohort heart failure patients during 2006–2007. Abbreviations: ICD/CRT-D—implantable cardioverter defibrillator/cardiac resynchronization therapy defibrillator; HRR—hospital referral region.
Changes in ICD/CRT-D Utilization and Changes in CHF Mortality
The ordinary least squares regression model assessing the relationship between HRR ICD/CRT-D rates and HF mortality indicated a 1% increase in ICD/CRT-D rate was associated with a 0.12% increase in HF survival at one year (95% confidence interval[CI] 0.03% to 0.21%, p=0.009). When 2007 data were removed (two-year survival was unavailable for 2007 patients) and the model was re-estimated with two-year survival as the dependent variable, a 1% increase in an HRR’s ICD/CRT-D rate was associated with a 0.26% increase in two-year survival (95% CI 0.14% to 0.37%, p<0.001).
When the one-year and two-year survival regression models were re-estimated with survival rates recalculated after ICD/CRT-D patients and their matches had been removed from the cohort, ICD/CRT-D use was no longer significantly associated with improved survival—a 1% increase in ICD/CRT-D use was associated with a one-year survival increase of −0.05% (95% CI −0.15% to 0.05%, p=0.31) and a two-year survival increase of 0.05% (95% CI −0.08% to 0.18%, p=0.41). These findings implied no residual association between the HRR ICD/CRT-D utilization rate and mortality in the remaining members of the cohort.
Because a small fraction (5%) of patients appeared more than once in the cohort and thus could have influenced the results, we reanalyzed the data by requiring all patients appear no more than once (i.e., patients appearing in 2002 were ineligible for 2003–2007, etc.). The results were essentially unchanged.
Opportunities for Improving HF Outcomes
If the levels of ICD/CRT-D utilization in HRRs in the lower 3 quintiles of ICD/CRT-D use in 2006–2007 had been raised to the national average ICD/CRT-D rate, the regression model predicted that 405 premature deaths at two years would have been averted (Table 3) among the 147,167 patients in the 2006 subcohort. Further increasing ICD/CRT-D use in the lower 4 quintiles to the average ICD/CRT-D rate in the top quintile would have prevented an additional 940 premature deaths at two years.
Table 3.
Projections of Avoidable Premature Heart Failure Deaths in Study Cohort Resulting from Increasing ICD Use in Low-Utilization HRRs
| Quintile* | N | Actual ICD rate† (%) | Actual 2-year survival | Projection 1: Increasing ICD rate in lower 3 quintiles to national average ICD rate | Projection 2: Increasing ICD rate in lower 4 quintiles to highest quintile ICD rate | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hypothetical ICD rate | Projected 2-year survival‡ | Projected premature deaths averted in cohort | Hypothetical ICD rate | Projected 2-year survival‡ | Projected premature deaths averted in cohort | ||||
| High | 34,234 | 10.6 | 66.0 | 10.6 | 66.0 | – | 10.6 | 66.0 | – |
| 4 | 32,118 | 8.1 | 64.6 | 8.1 | 64.6 | – | 10.6 | 65.2 | 202 |
| 3 | 38,502 | 6.1 | 64.8 | 7.0 | 65.0 | 92 | 10.6 | 65.9 | 443 |
| 2 | 29,945 | 4.7 | 64.3 | 7.0 | 64.9 | 179 | 10.6 | 65.8 | 452 |
| Low | 12,368 | 2.8 | 63.1 | 7.0 | 64.2 | 134 | 10.6 | 65.1 | 247 |
| TOTAL | 147,167 | 7.0 | 64.8 | 8.0 | 65.1 | 405 | 10.6 | 65.7 | 1,345 |
HRRs divided into quintiles by the percentage of ICD use in 2006–2007 among study cohort
Average ICD rate, 2006–2007
Projected survival based on results of regression model
Abbreviations: ICD—implantable cardioverter-defibrillator; HRR—hospital referral region
Discussion
Localities with greater increases in ICD/CRT-D utilization among HF patients from 2002–2007 had greater reductions in HF mortality rates. The ICD/CRT-D effect on mortality we observed in our cohort was equivalent to a 0.65 (95% CI 0.51–0.88) relative risk of death for patients receiving ICDs/CRT-Ds (calculations in Appendix). This is comparable to outcomes reported by the primary prevention ICD/CRT-D clinical trials—the MADIT-II trial’s ICD/CRT-D relative risk was 0.69 (95% CI 0.51–0.93), while the SCD-HeFT trial’s relative risk was 0.77 (95% CI 0.62–0.96). Our similar results indicate that ICD/CRT-D use was associated with improved HF survival in a broadly defined, nationally representative non-experimental HF population. Furthermore, sensitivity analysis demonstrated no relationship between the rate of ICD/CRT-D utilization and HF survival after removal of ICD/CRT-D recipients and their matched controls from the cohort, suggesting there were no unobservable confounders between high-ICD-use and low-ICD-use HRRs that could have otherwise explained the association between ICD/CRT-D rates and HF mortality rates.
We observed wide geographic variation in ICD/CRT-D utilization across HRRs, suggesting an important opportunity for improvement in HF outcomes, as demonstrated by our hypothetical projections. While the projected number of lives saved by increasing ICD/CRT-D rates in our cohort was modest, this study’s selection criteria necessarily formed a cohort that comprised only a small fraction of all ICD-eligible HF patients nationwide. To the extent that ICD/CRT-D utilization in our cohort was generalizable to all ICD-eligible HF patients, the potential number of premature deaths averted by increasing ICD/CRT-D rates in low-use localities may number in the several thousands.
Our findings suggest that ICDs/CRT-Ds were underused in many localities even several years after the publication of clinical trials, issuance of guidelines, and establishment of insurance coverage supporting the use of ICDs. Other investigators have reached similar conclusions: Bradfield et al. determined that less than 50% of eligible patients from 2002–2007 in an academically-affiliated Veterans Affairs hospital received ICDs,23 and Hernandez et al. determined that only 40% of eligible HF patients received ICDs/CRT-Ds between 2005–2007 at hospitals participating in the American Heart Association’s heart failure guidelines dissemination program.9 Our research extends these findings by demonstrating an association between lower rates of ICD/CRT-D utilization and lower heart failure survival at the population level.
Geographic variation in technology utilization can be caused by overuse of unnecessary therapy as well as underuse of necessary therapy.24 Nevertheless, our analysis found little evidence that high-use localities were, on net, overusing ICDs, as this phenomenon would have reduced the observed survival benefit of ICDs/CRT-Ds in the present study below the levels estimated by the ICD/CRT-D clinical trials. Overuse of ICDs/CRT-Ds was potentially inhibited by Medicare’s requirements that ICD/CRT-D implantation be accompanied by data entry into a national registry that includes detailed clinical indications for device therapy. While a recent analysis of national ICD registry data reported the possibility of inappropriate ICD use in some circumstances, prevalence estimates of low-ejection-fraction HF greatly exceed estimates of ICD utilization rates,9, 25, 26 suggesting that in most localities it is likely that ICDs are under-utilized rather than over-utilized.
There are many plausible reasons for under-utilization of ICDs. Implantation of an ICD/CRT-D remains a momentous decision for patients, particularly for those with no history of sudden cardiac death.11 This is in part due to concerns regarding device safety and difficulties in quantifying the risks and benefits associated with primary prevention ICDs. Patients have significant concerns about the adverse effects of ICDs, particularly in regard to driving, sexual activity, and physical exertion.27 In addition, clinicians caring for heart failure patients may be reluctant to screen for low ejection fraction and/or to refer otherwise appropriate patients for ICD/CRT-D implantation because of concerns about the potential for adverse clinical consequences from ICDs.28
Geographic comparisons revealed that HRRs with low ICD/CRT-D use had significantly fewer cardiologists and fewer academic hospitals per capita. It is therefore possible that lower rates of ICD/CRT-D use resulted in part from some localities having fewer hospitals offering ICD services, or fewer physicians who implanted the devices. Although there is little geographic variation across HRRs in the number of hospitals providing ICD/CRT-D implantation, it is also possible that academic centers with larger electrophysiology services were more likely to proactively identify appropriate candidates for primary prevention device therapy. Both of these possibilities are speculative, however, as our data could not establish a causal link between the availability of physician and hospital services and the likelihood of ICD implantation.
Limitations
Due to the absence of the key clinical indicators for ICD/CRT-D eligibility in claims data, our study could not be restricted solely to patients who were clinically eligible for ICDs. Undoubtedly many patients in our cohorts were ineligible for ICDs; for example, 24% of patients in our cohort had no antecedent diagnosis of HF prior to hospitalization, and thus might have appropriately undergone a trial of HF pharmacotherapy prior to consideration for ICD/CRT-D implantation. While the combination of our patient selection criteria and our propensity score match was designed to produce highly similar annual subcohorts of HF patients within each HRR, and thus the proportion of ICD-eligible patients would likely be constant across years, it remains possible that this proportion changed with time. If the proportion of ICD-eligible patients grew more quickly in some HRRs than others, this potentially would explain higher ICD/CRT-D use in these areas. However, it is less clear how an HRR with a rising proportion of ICD-eligible patients among its HF population would simultaneously have improving HF survival, as clinical eligibility for ICDs/CRT-Ds and cardiac resynchronization therapy is generally associated with more advanced heart failure and worse long-term survival.
It is also possible that HRRs with higher rates of ICD/CRT-D utilization had simultaneous improvements in the health of their HF populations, either due to medical therapies correlated with higher ICD/CRT-D use (e.g., increasing use of beta-blockers) or other factors that could have affected HF population health (e.g., declining smoking rates). If so, rising ICD/CRT-D utilization in an HRR may have been a proxy for improvements in the general quality of HF care. However, there are few published data suggesting that utilization of evidence-based HF therapies other than devices increased dramatically from 2002 to 2007, thus there is no strong empirical reason to believe that other aspects of health care produced the observed results.
Our analysis could have potentially been specified as a patient-level survival analysis as opposed to a HRR-year-level analysis. However, the time-varying effect of the changing ICD rate on each patient complicates interpretation of these models. Furthermore, it is unlikely that the HRR-level ICD frequency was uniformly influential on each patient’s likelihood of receiving an ICD. Our selected approach avoided both of these analytical difficulties.
Finally, although we observed that increasing ICD/CRT-D utilization was associated with reduction in HF mortality among large populations of Medicare beneficiaries, it is unlikely that this relationship would continue to hold as the percentage of HF patients receiving ICDs/CRT-Ds increasingly included patients who were clinically ineligible. Were ICDs/CRT-Ds to be increasingly used in such patients, who have reduced potential for clinical benefit from therapy, HF mortality rates associated with ICD/CRT-D use would decline more slowly, remain stable, or potentially increase.
Summary
Localities with higher growth of ICD/CRT-D utilization between 2002 and 2007 had more pronounced declines in HF mortality rates during this period than areas with lower ICD/CRT-D growth rates. These findings were not explained by time-invariant differences across localities, baseline differences in the health of HF patients, or temporal changes in mortality risk. This finding may signal an important opportunity to improve survival in heart failure patients living in areas with low rates of ICD/CRT-D utilization.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the administrative and design contributions of Janell Olah, MFA, and Mollie Epstein, MA in the production of the Tables and Figures for this manuscript.
Research support: This research was supported by the National Heart, Lung, and Blood Institute (1R01HL086919) and by the Agency for Healthcare Research and Quality (1R01HS018403). Dr. Groeneveld was additionally supported by a Career Development Transition Award from the Department of Veterans Affairs’ Health Services Research and Development Service, Washington, DC. This project was also funded, in part, under a grant from the Pennsylvania Department of Health, which specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
None of the authors had any personal or financial conflicts of interest in regard to this study.
References
- 1.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Yarnoz MJ, Curtis AB. Why cardioverter-defibrillator implantation might not be the best idea for your elderly patient. Am J Geriatr Cardiol. 2006;15(6):367–371. doi: 10.1111/j.1076-7460.2006.05935.x. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez AF, Fonarow GC, Hammill BG, et al. Clinical effectiveness of implantable cardioverter-defibrillators among medicare beneficiaries with heart failure. Circ Heart Fail. 2010;3(1):7–13. doi: 10.1161/CIRCHEARTFAILURE.109.884395. [DOI] [PubMed] [Google Scholar]
- 5.Chan PS, Nallamothu BK, Spertus JA, et al. Impact of age and medical comorbidity on the effectiveness of implantable cardioverter-defibrillators for primary prevention. Circ Cardiovasc Qual Outcomes. 2009;2(1):16–24. doi: 10.1161/CIRCOUTCOMES.108.807123. [DOI] [PubMed] [Google Scholar]
- 6.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. Circulation. 2006;114(10):e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 7.McClellan MB, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005;352(3):222–224. doi: 10.1056/NEJMp048354. [DOI] [PubMed] [Google Scholar]
- 8.Assessment of appropriate implantable cardioverter defibrillator (ICD) implantation for primary prevention of sudden cardiac death. Clinicaltrial.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT00926159. Accessed November 3, 2009.
- 9.Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298(13):1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 10.Get with the Guidelines-Heart Failure Fact Sheet. American Heart Association; Available at: http://www.americanheart.org/downloadable/heart/1251477226167factsheet-HF-02.pdf. Accessed Nov 10, 2009. [Google Scholar]
- 11.Al-Khatib SM, Sanders GD, Carlson M, et al. Preventing tomorrow’s sudden cardiac death today: dissemination of effective therapies for sudden cardiac death prevention. Am Heart J. 2008;156(4):613–622. doi: 10.1016/j.ahj.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 12.United States House of Representatives Committee on Ways and Means. Medicare and Health Care Chartbook. Washington: U.S. Government Printing Office; 1999. [Google Scholar]
- 13.Health and Human Services Budget in Brief:FY 2008. United States Department of Health and Human Services; Available at: http://www.hhs.gov/budget/08budget/2008BudgetInBrief.pdf. Accessed August 12, 2008. [Google Scholar]
- 14.McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297(22):2502–2514. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 15.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AE, Kay GN, Plumb VJ, et al. Implantable cardioverter-defibrillator prescription in the elderly. Heart Rhythm. 2009;6(8):1136–1143. doi: 10.1016/j.hrthm.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology. 2001;12(2):259–261. doi: 10.1097/00001648-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Center for the Evaluative Clinical Sciences - Dartmouth Medical School. The Dartmouth Atlas of Health Care; Available at: www.dartmouthatlas.org. Accessed January 4, 2010. [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Allison PD. Survival Analysis Using SAS: A Practical Guide. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 23.Bradfield J, Warner A, Bersohn MM. Low referral rate for prophylactic implantation of cardioverter-defibrillators in a tertiary care medical center. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S194–197. doi: 10.1111/j.1540-8159.2008.02281.x. [DOI] [PubMed] [Google Scholar]
- 24.Wennberg J. Which rate is right? N Engl J Med. 1986;314(5):310–311. doi: 10.1056/NEJM198601303140509. [DOI] [PubMed] [Google Scholar]
- 25.Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115(12):1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 26.Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm. 2009;6(3):325–331. doi: 10.1016/j.hrthm.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Groeneveld PW, Matta MA, Suh JJ, Yang F, Shea JA. Quality of life among implantable cardioverter-defibrillator recipients in the primary prevention therapeutic era. Pacing Clin Electrophysiol. 2007;30(4):463–471. doi: 10.1111/j.1540-8159.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 28.Schoenfeld MH. Contemporary pacemaker and defibrillator device therapy: challenges confronting the general cardiologist. Circulation. 2007;115(5):638–653. doi: 10.1161/CIRCULATIONAHA.106.618587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


