Abstract
Objective
Diamond Blackfan anemia (DBA) is a rare inherited bone marrow failure syndrome. The mainstays of treatment involve chronic red cell transfusions, long-term glucocorticoid therapy, and stem cell transplantation. Systematic data concerning endocrine function in DBA are limited. We studied patients in the DBA Registry (DBAR) of North America to assess the prevalence of various endocrinopathies.
Study design
In a pilot study, retrospective data were collected for 12 patients with DBA. Subsequently, patients with DBA aged 1–39 years were recruited prospectively. Combined, 57 patients were studied; 38 chronically transfused, 12 glucocorticoid-dependent and 7 in remission. Data were collected on anthropometric measurements, systematic screening tests of pituitary, thyroid, parathyroid, adrenal, pancreatic and gonadal function and ferritin levels. Descriptive statistics were tabulated and group differences assessed.
Results
Fifty-three percent of patients had ≥ 1 endocrine disorder, including adrenal insufficiency (32%), hypogonadism (29%), hypothyroidism (14%), growth hormone dysfunction (7%), diabetes mellitus (2%), and/or diabetes insipidus (2%). Ten of 33 patients with available heights had height SD <−2. Low 25(OH)D levels were present in 50%. A small proportion also had osteopenia, osteoporosis or hypercalciuria. Most with adrenal insufficiency were glucocorticoid-dependent; other endocrinopathies were more common in chronically transfused patients.
Conclusions
Endocrine dysfunction is common in DBA, as early as the teenage years. While prevalence is highest in transfused patients, patients taking glucocorticoids or in remission also have endocrine dysfunction. Longitudinal studies are needed to better understand the etiology and true prevalence of these disorders.
Keywords: Hypogonadism, Growth, Vitamin D deficiency, Osteoporosis, Adrenal insufficiency, Iron overload
INTRODUCTION
Diamond Blackfan anemia (DBA), a rare inherited bone marrow failure syndrome affecting 5–7 per million live births per year, is characterized by red cell aplasia, congenital anomalies and a predisposition to cancer. The most common mutations or deletions involve genes encoding ribosomal proteins[1–4].
The initial treatment is prednisone at a dose of 2 mg/kg/day commencing at 1 year of age, gradually weaned to ≤ 0.5 mg/kg/day. Although approximately 80% of patients respond to corticosteroids, only 40% have a sustained response without dose-limiting toxicity. Another 40% are transfusion dependent, requiring packed red cells every 3–4 weeks. Interestingly, about 20% of patients enter remission, requiring neither glucocorticoids nor transfusions for ≥6 months. Stem cell transplantation is considered in select patients[1].
Chronic glucocorticoid therapy predisposes patients to iatrogenic Cushing syndrome and adrenal insufficiency. Chronic blood transfusions place patients at risk for iron overload of liver, heart and endocrine organs. Growth failure, osteopenia, diabetes mellitus, and failure of the thyroid, parathyroids, adrenals, gonads and pituitary gland, may be related to therapy, and have been described in other hematologic diseases associated with iron overload and in aplastic anemia[5–8]. Endocrine dysfunction in DBA has only been described in case reports or small series[9–14]. Due to the rarity of DBA, there are no published systematic studies of endocrinopathies.
The DBA Registry (DBAR) of North America at the Feinstein Institute for Medical Research, North Shore-LIJ Health System (NSLIJHS) since 2000, provided an opportunity for a study of endocrine function in DBA[15]. Our aims were: (a) To study a cross-section of patients with DBA patients for glucose tolerance, pituitary, adrenal, thyroid, parathyroid and gonadal function; (b) To estimate the crude prevalence of hormone deficiencies in the DBA population; and (c) To compare the frequency of endocrine dysfunction in transfusion-dependent group to those treated with glucocorticoids or in remission.
SUBJECTS AND METHODS
Subjects
We first performed a retrospective chart review of patients enrolled in DBAR evaluated in the divisions of medical and pediatric endocrinology of NSLIJHS between 1/1/04 and 10/1/12. Twelve patients’ (aged 11–43) charts were reviewed. Subsequently subjects were recruited prospectively from 12/2012 to 4/2014. Inclusion criteria were: diagnosis of DBA according to DBAR criteria[1], aged 1–39 years, participating in DBAR, and a) receiving packed red cell transfusions every 3–4 weeks (at least 15 transfusions); or, b) in remission; or c) glucocorticoid dependent. Exclusion Criteria were: a) pregnancy or b) previous hematopoietic stem cell transplantation.
Study procedures
The protocol was approved by the NSLIJHS Institutional Review Board. Since the DBAR includes patients across North America, the principal investigator (PI) discussed consent via telephone, then sent consent and assent forms and the study questionnaire by mail. Patients visiting the DBAR at FIMR signed consent in person. Participants completed a short questionnaire about treatment details and symptoms. Data on clinical history, pubertal stage, anthropometrics and mid-parental heights were recorded. A broad age range was included due to the rarity of the disease. Serum ferritin and endocrine test results were collected and compared with age-appropriate reference standards (Table 1). If indicated, local endocrine evaluation was recommended.
Table I.
Test results collected for evaluation of function of various endocrine glands
| Endocrine gland | Tests |
|---|---|
| Anterior pituitary gland* | insulin-like growth factor-1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3), prolactin |
| Posterior pituitary gland | serum and urine osmolality |
| Thyroid gland | thyroid stimulating hormone (TSH), total and free T4 |
| Parathyroid gland and vitamin D metabolism | calcium, intact parathyroid hormone (iPTH), 25-OH-vitamin D [25(OH)D] |
| Adrenal cortex: glucocorticoid synthesis | AM Cortisol |
| Adrenal cortex: mineralocorticoid synthesis | plasma renin activity (PRA), aldosterone |
| Adrenal cortex: sex steroid synthesis | androstenedione, dehydroepiandrosterone sulphate (DHEAS) |
| Pancreatic endocrine function/glucose metabolism** | fructosamine (or HbAlc in non-transfused patients) |
| Gonadal function for ages ≥ 8 years# | luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone and sex hormone binding globulin (SHBG) (in males); and estradiol (in females). |
Additional tests for evaluation of other pituitary hormones are mentioned along with the respective endocrine glands they regulate.
HbA1c value >6.5% as per ADA guidelines used to screen for diabetes mellitus in non-transfused patients, but not: as reliable in patients transfused frequently. Fasting and post-prandial glucose can provide a good measure of glycemic control in transfused patients, but was not feasible to obtain due to the design of study. Fructosamine used as a screen for short-term glycemic control and some studies show correlation between fasting glucose and fructosamine level. However no definitive fructosamine level is considered diagnostic for diabetes and patients with values outside laboratory reference range need further evaluation.
Patients on sex hormone replacement with a history of delayed puberty (defined as no breast development in girls >13 years and no testicular enlargement in boys >14 years) are considered to have hypogonadism. Additionally, patients with elevated FSH and/or LH with or without low sex hormones are considered to have primary hypogonadism. Secondary hypogonadism diagnosed in patients with low LH and/or FSH and sex hormones with evidence of other pituitary hormone deficiencies; those with history of hypogonadism and on treatment with human chorionic gonadotropins (HCG) or in males on long term androgen therapy for anemia.
Clinical and anthropometric data
Self-reported height and weight data were collected. Body surface area was calculated [16]. Height and weight SD scores were calculated based on Centers for Disease Control (CDC) 2000 standards. Short stature was defined as height SD below −2.0 for age and gender. Parental heights were used to calculate MPH. BMI was calculated to the nearest 0.1 kg/m2. For patients under 18 years, BMI was compared to age and gender specific CDC 2000 standards [17]. Adults were classified as normal BMI, overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥30 kg/m2).
Patients > 8 years were assessed for pubertal development using tanner staging based on physical examination within the past year, or based on parent-assessed pubertal development (breast development in girls; genital enlargement and body hair in boys). Adult patients were asked if they had received hormone therapy to induce growth or puberty. Puberty was considered delayed if boys had no signs of pubertal maturation by age 14, or girls had no breast development by age 13.
Biochemical testing
Laboratory testing was systematically performed between 0700–0800 h after an overnight fast, prior to a blood transfusion and over two days for subjects under 10 years or weighing <65 lbs. FSH and LH were measured by immunochromatographic membrane assay (ICMA), steroid hormones by liquid chromatography followed by tandem mass spectrometry (LC-MS/MS)[18,19].
Glucocorticoid-treated patients were presumed to have secondary adrenal insufficiency, unless adequate cortisol response to standard dose cosyntropin, (ACTH 1-24) was documented. Cosyntropin stimulation tests were performed in subjects with a screening 8 AM cortisol < 16 micrograms/dl[20]. Adrenal insufficiency was classified as symptomatic or subclinical. Symptomatic adrenal insufficiency was characterized by a subnormal 60 minute cortisol response following cosyntropin with symptoms of fatigue, hypoglycemia, hyponatremia, or hyperkalemia. Subclinical adrenal insufficiency was characterized by biochemical evidence of adrenal insufficiency without symptoms[21] (i.e. 8AM cortisol <5 micrograms/dL and/or inadequate cortisol rise to stimulation (peak <16 micrograms/dL)[21]. Mineralocorticoid production was assessed based on published standards for aldosterone: PRA ratio[22].
GH deficiency was defined by peak level <10 ng/mL in response to two drug stimuli (arginine + clonidine or glucagon) [23].
The standard calcium - iPTH nomogram was used to interpret parathyroid gland function[24]. Serum 25(OH)D levels were interpreted as sufficient (>30 ng/mL), insufficient (20–30 ng/mL), or deficient (<20 ng/mL)[25].
Statistical analysis
Results were expressed as median (range) or mean ± 1 SD for continuous variables and frequencies for categorical variables. ANOVA was used to test the difference between means of two (t-test) or more groups, and Wilcoxon-signed rank test was used to test the difference between non-parametric variables. P<0.05 was considered statistically significant. Analyses were performed using JMP IN 7 software (SAS Institute, Cary, NC).
RESULTS
Patient characteristics (Tables II and III)
Table II.
Patient Characteristics
| Parameter | Overall | On Chronic Transfusions | Glucocorticoid-dependent | Remission* |
|---|---|---|---|---|
| n | 57 | 38 | 12 | 7 |
| Age (in years) at endocrine evaluation€ | 14.4 (2–43) | 17.4 (2.2–43)§ | 10.5 (2–30.7) | 12.9 (3–33.2) |
| Males (%) | 56 | 58 | 50 | 57 |
| Height SDS#€ | −1.1 (−3.2 to 1.7) | −1.1 (−3.2 to 0.1) | −1.3 (−2.4 to 0.4) | −0.6 (−2.6 to 1.7) |
| Difference between subject’s height SDS and MPH height SDS€¥ | −1.2 (−4.6 to 0.6) | −1.2 (−4.6 to 0.3) | −0.6 (−2.3 to 0.6) | −1.2 (−1.6 to −1.0) |
Expressed as Median (range).
< 0.05 for DBA transfused group versus glucocorticoid-dependent group.
Duration of remission >2 years (range 2–28 years) in all subjects except for 1 patient (6 months).
Height SDS data available for 33 DBA patients (n=19, 10 and 4 respectively in chronic transfusion, glucocorticoid-dependent and remission groups).
Both patient and mid parental height SDS data were available for 28 DBA patients (n=19, 5 and 4 respectively in chronic transfusion, glucocorticoid-dependent and remission groups).
Table III.
Table showing frequencies of various endocrinopathies in patients with Diamond Blackfan anemia
| Overall* | On chronic Transfusions* | Glucocorticoid-dependent* | Remission* | |
|---|---|---|---|---|
| Patients with endocrinopathies a) None b) One only c) Two only d) Three only e) Greater than three f) Indeterminate screening labs |
a) 30% (17/57) b) 37% (21/57) c) 10% (6/57) d) 4% (2/57) e) 2% (1/57) f) 17% (10/57) |
a) 34% (13/38) b) 26% (10/38) c) 11% (4/38) d) 5% (2/38) e) 3% (l/38) f) 21% (8/38) |
a) 0/12 b) 83% (10/12) c) 17% (2/12) d) 0/12 e) 0/12 f) 0/12 |
a) 57% (4/7) b) 14% (l/7) c) 0/7 d) 0/7 e) 0/7 f) 29%(2/7) |
| Adrenal insufficiency (for glucocorticoids) a) Symptomatic b) Subclinical |
a) 4% (2/50) b) 28% (14/50) |
a) 6% (2/33) b) 3% (1/33) |
a) 0/12 b) 100% (12/12) |
a) 0/5 b) 20% (1/5) |
| Mineralocorticoid insufficiency | 0/30 | 0/19 | 0/8 | 0/3 |
| Adrenal Sex steroid insufficiency | 12% (4/32) | 12% (3/24) | 0/5 | 33% (1/3) |
| Growth hormone dysfunction | 7% (3/45) | 10% (3/30) | 0/10 | 0/5 |
| Diabetes insipidus | 2% (1/39) | 4% (1/27) | 0/9 | 0/3 |
| Hypothyroidism | 14% (8/55) | 22% (8/36) | 0/12 | 0/7 |
| Diabetes mellitus | 2% (1/40) | 4% (1/28) | 0/9 | 0/3 |
| Ca-iPTH abnormalities a) Probable Hypoparathyroid ism b) Hypercalcemia with normal iPTH |
a) 4% (2/46) b) 2% (1/46) |
a) 3% (1/31) b) 0/31 |
a) 10% (1/10) b) 10% (1/10) |
a) 0/5 b) 0/5 |
| Vitamin D a) insufficiency b) deficiency |
a) 33% (17/51) b) 24% (12/51) |
a) 35% (12/34) b) 26% (9/34) |
a) 27% (3/11) b) 9% (1/11) |
a) 33% (2/6) b) 33% (2/6) |
| Hypogonadism (among children >8 years and adults) | 29% (10/34) | 40% (10/25) | 0/5 | 0/4 |
| Iron overload | 80% (37/46) | 97% (32/33) | 40% (4/10) | 33% (1/3) |
Data expressed as: Percentage of patients with the disorder (number of patients with the disorder/number of patients for whom data was available)
Fifty-seven patients were included, 38 of whom were transfusion-dependent whereas 19 were non-transfusion dependent (seven in remission; 12 glucocorticoid-dependent. (Table II). Of the total 679 patients in the DBA Registry, 450 patients were eligible. Ninety-two patients were randomly selected and contacted for recruitment in the prospective study. Of these 45 patients consented and completed study participation (10% of those eligible). An attempt was made to obtain complete hormonal profile for all recruited patients; however for a number of patients for whom data was available in each sub-group was variable for different endocrine functions (see Table III). All percentages are expressed as a fraction of the patients with available data and refer to the entire cohort unless otherwise indicated.
Endocrine testing
Adrenal cortical function
Adrenal insufficiency as defined by the above criteria was present in 32% of patients (symptomatic: n=2, both transfusion dependent; sub-clinical: n=14, one patient receiving glucocorticoids and transfusions, one in remission and 12 receiving glucocorticoids). Median prednisone dose for the glucocorticoid-dependent patients was 3.8 mg/m2/day (range 1.4–15.2). The median hydrocortisone equivalent dose (calculated using a 1:5 conversion factor) was 18.8 mg/m2/day (range 7–75.8). An additional 40% (n=20) had low cortisol levels suggestive of adrenal insufficiency, however confirmatory ACTH stimulation tests were not performed. Of these, 16 patients had relatively low AM cortisol levels (range: 6.2–12.6 micrograms/dL). Four patients, all transfusion dependent, had an absolutely low morning cortisol level (<5 micrograms/dL)].
Although 25% of the patients reported salt craving and/or dizziness, none were found to have mineralocorticoid deficiency. Adrenal androgens (DHEAS and/or androstenedione) were low for age and pubertal stage in 12% of patients (n=4). This included one patient in remission; the remainder were transfusion dependent. Of the transfused patients, two were pubertal (age 15.7 years, tanner IV and age 20 years, tanner V) and had normal AM cortisol level, while the third had no signs of puberty (age 15 years) and a low AM cortisol.
Anterior pituitary function
Seven percent of patients (n=3) had received recombinant human growth hormone (rhGH); all were male and transfusion-dependent. Two of these patients were treated for GH deficiency (final height −1.3 SD and −2.3 SD; MPH −0.2 SD for both). The third patient had normal GH level, but low IGF-1 (<−3 SD for age), short stature (height −2.7 SD) and poor growth velocity; he was treated for presumed GH resistance. His final height was −3 SD (poor treatment adherence; MPH −1.3 SD). Additionally, 16% of patients (n=7) had low IGF-1 or IGFBP-3 levels.
Height SD was available for 33 patients, of whom 10 had a height <−2 SD (6 transfusion-dependent, 2 glucocorticoid-dependent, 2 in remission). Both patient and mid-parental height SD values were available for 28 patients. Of these, 64% had a height SD of −1 below their MPH.
BMI could be calculated for 31 patients. Of these, 10% were underweight (n=3), 29% overweight (n=9) and 19% obese (n=6). The relative proportion of overweight and obese individuals was highest in the transfusion-dependent patients.
Posterior pituitary function
Diabetes insipidus (DI) was diagnosed in a single 16.5-year-old transfusion-dependent patient.
Glycemic control
The diagnosis of diabetes mellitus was made in one 14.5-year-old transfusion-dependent patient who presented with symptoms of hyperglycemia. Screening fructosamine levels, when available, were normal in all other patients.
Thyroid function
Hypothyroidism was reported in 14% of the patients (n=8, all transfusion-dependent). Four additional patients had mildly elevated TSH (range: 4.98–9.36 μIu/ml) and normal T4 ; and one asymptomatic glucocorticoid-dependent patient had a low free T4 with a low-normal TSH. Calcium metabolism, parathyroid function and Vitamin D.
Two patients had probable hypoparathyroidism (low calcium with inappropriately normal iPTH level), though none reported symptoms of hypocalcemia. One patient taking calcium supplements was hypercalcemic with a normal iPTH level. Approximately 28% of patients were taking supplemental vitamin D. Overall, 33% of patients (n=17) had insufficient 25(OH)D levels; 24% (n=12) were vitamin D deficient including some on supplemental vitamin D. Though data was not prospectively collected for urine calcium excretion, hypercalciuria (elevated spot or 24 hour urine calcium: creatinine ratio) and/or kidney stones were reported by 7% of the patients (n=4, ages 11.6–30.3 years, while three of these were transfusion-dependent and receiving chelation with deferasirox, the fourth patient was receiving both glucocorticoids and transfusions and chelation with deferoxamine). Vitamin 25(OH)D levels on these patients were in the low-normal range; three had a family history of kidney stones.
Data for bone density was not prospectively collected, however osteoporosis was reported in 4 patients: three adults (aged 27–34.9; 2 males, 1 female) and one male child (age 11.6), all transfusion-dependent. [26] All had BMD ≤ −2 SD. The child also had multiple vertebral compression fractures (children diagnosed based on most recent densitometry guidelines [26]). Three additional patients had adverse skeletal outcomes that developed during glucocorticoid therapy (ages 10.4–20, including a hairline leg fracture and avascular necrosis of the femoral neck). Osteopenia was reported in another 2 patients: a 19-year-old glucocorticoid-dependent female and 21-year-old male treated with glucocorticoids and transfusions.
Gonadal function
Among children >8 years and adults, hypogonadism was present in 29% of patients (n=10), three of whom were not previously diagnosed. All were diagnosed at ≥ 14.4 years of age and were transfusion-dependent. The male to female ratio was 8:2 (diagnosed at or after 14.4 years). Considering age cut-offs for delayed puberty (Table 1), 47% of males >14 years had hypogonadism, while only 15% of females >13 years had hypogonadism. None of the patients attempted pregnancy during the study.
Iron overload
Ferritin levels were available for 46 patients, 80% of which (n=37) were above respective reference ranges [median 1141 ng/mL (range 214 – 5993)]. Of these 37 patients, most were chronically transfused, four were glucocorticoid-dependent and one was in remission. The ferritin level for the glucocorticoid dependent patients ranged from 330 – 689 ng/mL (last transfused 11 months to 7 years prior to this study). The ferritin level for the patient in remission was 1993 ng/mL (last transfusion 30 years earlier). On further investigation this patient was found to be homozygous for the C282Y mutation in the HFE gene, indicating primary hemochromatosis. There were insufficient liver iron concentration data by MRI to quantitate iron burden. Therefore, statistical analysis of the relationship between iron burden and endocrine abnormalities could not be performed.
DISCUSSION
Our report evaluates endocrine function in patients with DBA treated with transfusions or glucocorticoids and those in remission. We found a considerably higher incidence of endocrinopathies than previously reported. In our cohort, 53% of patients had one or more endocrinopathies (not including vitamin D deficiency/insufficiency). In order of frequency, these included adrenal insufficiency, hypogonadism, hypothyroidism, growth hormone deficiency/resistance, diabetes mellitus and diabetes insipidus (Figure 1).
Figure 1. Endocrine dysfunction in patients with Diamond Blackfan Anemia.
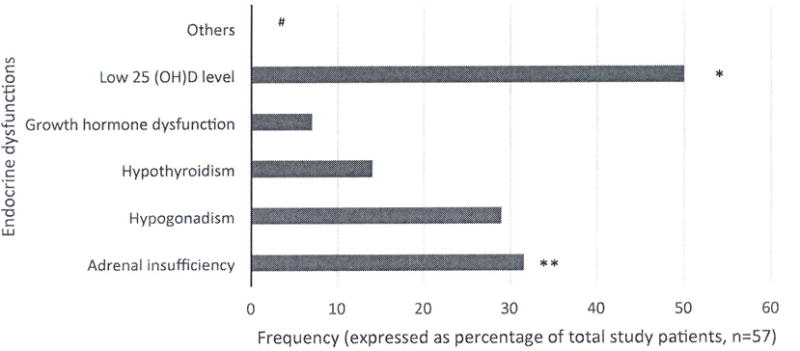
Bar diagram showing frequencies of various endocrine dysfunction in our patients with Diamond Blackfan anemia
* This includes all patients with 25(OH)D levels <20 ng/mL and 20–30 ng/mL
** Most of these patients were receiving chronic glucocorticoid therapy and had presumed secondary adrenal insufficiency.
*** Other endocrinopathies including diabetes mellitus, diabetes insipidus, osteoporosis, osteopenia and hypercalciuria were present in a minority of patients
#Total number of patients evaluated for each endocrinopathy somewhat varied as detailed in Table III.
Osteoporosis/osteopenia and hypercalciuria were also reported by history, although primary data were not uniformly available. Published reports from European DBA registries have focused primarily on chronically transfused patients[9–13]. In the Italian cohort, 23% of patients (7 of 31, median age 9.2 years, range 1.8–29.6) had one or more iron-related endocrine or other organ complications. In the French cohort, ~14% of patients (13 of 95, mean age 13.5 ± 10.4 years) had endocrinopathies; few iron overloaded transfused patients were tested. In both reports, hypothyroidism was most frequent, followed by hypogonadism[12,13]. Amongst our chronically transfused patients, 45% (17 of 38) had one or more endocrinopathies, likely due to their older age (median age 17.4 years, range 2.3–43), and because screening was done irrespective of symptoms or degree of iron overload.
In our study, patients underwent their first endocrine evaluation anywhere from 1.5 to 30.6 years after DBA diagnosis, with glucocorticoid-dependent patients among the earliest evaluated. The median interval from diagnosis to first endocrine evaluation was 3.5 years for those patients who were corticosteroid-dependent, 4.5 years for those in remission, and 8.3 years for transfusion-dependent patients. This is noteworthy, as high transfusion burden was most often associated with endocrinopathies. Approximately 33% of all patients indicated that they had never undergone a prior endocrine evaluation. While the age at diagnosis of pre-existing endocrinopathies was not uniformly captured in our study, for the few patients that this information was available, the age at diagnosis of a first endocrinopathy ranged from 13.5–19 years. This does not include glucocorticoid-dependent patients with presumed iatrogenic adrenal insufficiency.
Adrenal insufficiency was the most common endocrinopathy in our cohort: 32%, including all glucocorticoid-dependent patients who were presumed to have secondary adrenal insufficiency by the virtue of treatment with prolonged or supraphysiologic doses. Although less common, sub-normal cortisol response was also seen in patients receiving transfusions (9%) or in remission (20%). The etiology of adrenal insufficiency in the latter groups was unclear; we postulate that this may be related to prior transfusions. In comparison, subclinical adrenal insufficiency has been reported in 13–46% of beta-thalassemia patients[27,28]. Sources of variability include the test methodology and cortisol cut-off used.
Hypogonadism in patients >8 years age was the second most common endocrinopathy in our cohort (29%; M:F ratio =3:1), all from the transfusion-dependent group. Clinical presentations included failure to initiate puberty, arrest of puberty, and loss of libido or sexual function after puberty. Prior studies in patients with DBA have reported a hypogonadism prevalence of 8–13%, somewhat lower than our study[12,13]. The reported prevalence in beta-thalassemia patients ranges from 32–55%, increasing with age[5,29,30]. Pituitary iron overload in transfusion dependent patients with thalassemia and DBA can begin in the first decade of life, but clinically significant pituitary volume loss occursin the second decade[31]. At least half the patients with hypogonadism in our cohort had central hypogonadism, suggesting pituitary or hypothalamic iron overload.
Hypothyroidism has been previously reported in 3% and 16 % of patients with DBA in the Italian and French cohorts, respectively[12,13]. This is similar to our findings of 14% in our whole cohort and 22% of transfused patients. In comparison, prevalence of hypothyroidism in the beta-thalassemia population was found to be 8.7% (range 0–18%)[5], depending on the number and age of subjects.
Of our patients with available height measurements, 30% had short stature, similar to that reported from the French DBA registry[12] and to the 25–31% for patients with beta-thalassemia[5,29]. Of our patients, 60% had heights > 1 SD below MPH. Short stature in DBA patients may be associated with skeletal malformations, type of treatment and iron overload status[12,32]. Data on skeletal malformations were not collected in our study. Three patients in our study received rhGH therapy. There have been reports of positive outcomes using growth hormone for both growth hormone deficiency and resistance in patients with DBA [11,12,33]. A recently published retrospective report on rhGH therapy in patients with DBA showed significant increases in height velocity up to two years and height Z-scores up to four years after starting GH therapy. Adult heights were not obtained. Of note, in this study, the baseline peak stimulated GH level for the treated patients was 11.5±10.6 ng/mL indicating that only some were GH deficient based on the most liberal cut-off of 10 ng/mL[34]. The association of DBA and various malignancies, especially osteosarcoma[15,35], raises theoretical concerns about GH safety that must be explained to the patient and family. GH treatment should be monitored by maintaining serum IGF1 in the mid-normal range for age.
Vitamin D insufficiency and deficiency were common in our cohort, with 57% having a 25(OH)D level below 30 ng/mL (75 nmol/L), despite some taking supplements. This proportion is somewhat lower than estimates in the general population and beta-thalassemia patients[5,36]. These findings suggest that either supplemental doses of vitamin D were inadequate or that patients were not adherent to the prescribed regimen. Chronic transfusions and associated co-morbidities are known risk factors for fractures, reported in up to 36% of patients with thalassemia[37]. Osteopenia, osteoporosis and/or fragility fractures were reported in a minority of our patients (n=9), though the information was based on self-report only and bone density data were not uniformly available. Hypercalciuria, nephrocalcinosis and/or kidney stones were present in 4 patients. Among the risk factors for nephrolithiasis were treatment with deferasirox[38] and a family history of kidney stones. Although hypercalciuria has been described in up to 21% of patients with beta-thalassemia, perhaps due to deferasirox [5,38], this was a new finding in patients with DBA.
While diabetes mellitus developed in only one transfusion-dependent patient at age 14.5, it has been described in both transfusion-dependent and glucocorticoid-dependent patients[13,14]. It appears to be less frequent in DBA than in beta-thalassemia (14.1%)[5]. The best screening test for diabetes in transfusion-dependent patients is controversial. One recent study in thalassemia patients found continuous glucose monitoring promising[39].
One patient with panhypopituitarism due to iron overload developed diabetes insipidus, another finding not previously reported in DBA. Symptomatic hypoparathyroidism was not diagnosed, although it was suspected in two patients and has been described[12,13].
Ferritin is not a reliable marker of iron burden or the chronicity of iron overload. Hence, statistical correlation between iron burden and the presence of endocrinopathies was not performed.
Intrinsic limitations of our study include the small population due to the rarity of DBA, the cross-sectional nature of data collection, and geographic distance from the study center for the majority of DBAR patients. Other limitations were the relative young median age of our patients, self-reported parental heights, growth measurements and pubertal stage for several patients, assays done in different laboratories and the lack of data on hepatic and cardiac MRIs as measures of iron burden. Due to the small number of patients in remission, the study was not adequately powered to exclude a contributing effect of DBA itself on any of the endocrine diagnoses. Despite these limitations, this study is unique in that it evaluated endocrine dysfunction in a large DBA cohort, both children and adults, regardless of mode of treatment, and has thus improved our knowledge in this field.
In summary, over half of the studied DBA patients (53%) had one or more endocrinopathies, several beginning in childhood. While most of these were present in transfused patients, glucocorticoid-dependent patients and those in remission also showed endocrine dysfunction. Based on our experience, we make the following suggestions regarding endocrine screening of DBA patients:
Graphing height and weight should be done in children and adolescents every 6–12 months to timely diagnose growth failure. BMI should also be monitored.
Screening for gonadal insufficiency should be considered in all transfused patients with delayed puberty, cessation of pubertal progression, or loss of libido or sexual function in adults.
Hypothyroidism screening should be considered in all DBA patients irrespective of treatment type or remission state after age 14.
Periodic screening by early morning serum cortisol measurement is suggested in iron overloaded patients, and cosyntropin testing in patients after discontinuation of glucocorticoids to ensure normal adrenal function.
Vitamin D supplementation should be given to maintain 25(OH)D levels above 30 ng/mL (75 nmol/L). Consider screening urine calcium:creatinine ratio annually for hypercalciuria. Periodic bone density measurements should begin in late childhood.
Annual screening for diabetes mellitus should begin by age 14. HbA1c may be used to screen non-transfused patients, with fructosamine or oral glucose tolerance test in transfused patients. Screening for diabetes insipidus should also be considered in symptomatic patients.
Since prevalence of endocrinopathies tends to increase with age, normal screening labs at any age should not preclude lifetime monitoring of these patients. Timely diagnosis and treatment may serve to avoid further morbidities.
Acknowledgments
The authors express their gratitude to the patients with DBA, their families and their physicians for their support of our research and their contribution of data to the DBA Registry. We acknowledge our many colleagues who work tirelessly to understand DBA as well as our collaborators in the Intramural Research Programs of the National Cancer Institute and the National Human Genome Research Institute. We also acknowledge the contribution of Dr Martin L. Lesser, PhD and Dr Patricia M. Vuguin, MD in statistical analysis, respectively and DBAR Nurse Ellen Muir, RN, MSN, for help with recruiting patients.
Grant support: This work (JML, AV) is supported by grants from the National Heart Lung and Blood Institute (R01HL079571), the Pediatric Cancer Foundation, the Diamond Blackfan Anemia Foundation and the Daniella Maria Arturi Foundation.
Abbreviations
- DBA
Diamond Blackfan Anemia
- DBAR
Diamond Blackfan Anemia Registry
- SD
Standard deviation
- IGF-1
Insulin-like growth factor 1
- IGFBP-3
Insulin-like growth factor binding protein 3
- TSH
Thyroid stimulating hormone
- T4
Thyroxine
- iPTH
Intact parathyroid hormone
- 25(OH)D
25-hydroxy vitamin D
- ACTH
Adrenocorticotrophic hormone
- PRA
Plasma renin activity
- DHEAS
Dehydroepiandrosterone sulphate
- LH
Luteinizing hormone
- FSH
Follicular stimulating hormone
- SHBG
Sex hormone binding globulin
- HbA1c
Hemoglobin A1c
- GH
Growth hormone
- MPH
Midparental height
- rhGH
Recombinant human growth hormone
- DI
Diabetes insipidus
Footnotes
CONFLICT OF INTEREST STATEMENT
None of the authors have a conflict of interest real or perceived.
Disclosure statement: The Authors have nothing to disclose.
References
- 1.Vlachos A, Ball S, Dahl N, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. British journal of haematology. 2008;142(6):859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlachos A, Blanc L, Lipton JM. Diamond Blackfan anemia: a model for the translational approach to understanding human disease. Expert review of hematology. 2014 doi: 10.1586/17474086.2014.897923. [DOI] [PubMed] [Google Scholar]
- 3.Vlachos A, Dahl N, Dianzani I, et al. Clinical utility gene card for: Diamond-Blackfan anemia–update 2013. European journal of human genetics: EJHG. 2013;21(10) doi: 10.1038/ejhg.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazda HT, Preti M, Sheen MR, et al. Frameshift mutation in p53 regulator RPL26 is associated with multiple physical abnormalities and a specific pre-ribosomal RNA processing defect in diamond-blackfan anemia. Human mutation. 2012;33(7):1037–1044. doi: 10.1002/humu.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogiatzi MG, Macklin EA, Trachtenberg FL, et al. Differences in the prevalence of growth, endocrine and vitamin D abnormalities among the various thalassaemia syndromes in North America. British journal of haematology. 2009;146(5):546–556. doi: 10.1111/j.1365-2141.2009.07793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fix OK, Kowdley KV. Hereditary hemochromatosis. Minerva medica. 2008;99(6):605–617. [PubMed] [Google Scholar]
- 7.Matthews AL, Grimes SJ, Wiesner GL, et al. Clinical consult: iron overload–hereditary hemochromatosis. Primary care. 2004;31(3):767–770. xii–xiii. doi: 10.1016/j.pop.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Giri N, Batista DL, Alter BP, et al. Endocrine abnormalities in patients with Fanconi anemia. The Journal of clinical endocrinology and metabolism. 2007;92(7):2624–2631. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- 9.Beck W, Stubbe P, Tillmann W. Endocrine studies in Blackfan-Diamond anemia: evidence for hypothalamic-pituitary dysfunction under frequent transfusion therapy. European journal of pediatrics. 1980;135(1):103–105. doi: 10.1007/BF00445904. [DOI] [PubMed] [Google Scholar]
- 10.Berdel D, Romahn A, Burmeister W. Pluriglandular insufficiency due to transfusion haemosiderosis in Blackfan-Diamond anaemia (author’s transl) Klinische Padiatrie. 1980;192(1):91–94. doi: 10.1055/s-2008-1033864. [DOI] [PubMed] [Google Scholar]
- 11.Lanes R, Muller A, Palacios A. Multiple endocrine abnormalities in a child with Blackfan-Diamond anemia and hemochromatosis. Significant improvement of growth velocity and predicted adult height following growth hormone treatment despite liver damage. Journal of pediatric endocrinology & metabolism: JPEM. 2000;13(3):325–328. doi: 10.1515/jpem.2000.13.3.325. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Warszawski J, Bader-Meunier B, et al. Diamond-blackfan anemia and growth status: the French registry. The Journal of pediatrics. 2005;147(5):669–673. doi: 10.1016/j.jpeds.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Roggero S, Quarello P, Vinciguerra T, et al. Severe iron overload in Blackfan-Diamond anemia: a case-control study. American journal of hematology. 2009;84(11):729–732. doi: 10.1002/ajh.21541. [DOI] [PubMed] [Google Scholar]
- 14.Willig TN, Niemeyer CM, Leblanc T, et al. Identification of new prognosis factors from the clinical and epidemiologic analysis of a registry of 229 Diamond-Blackfan anemia patients. DBA group of Societe d’Hematologie et d’Immunologie Pediatrique (SHIP), Gesellshaft fur Padiatrische Onkologie und Hamatologie (GPOH), and the European Society for Pediatric Hematology and Immunology (ESPHI) Pediatric research. 1999;46(5):553–561. doi: 10.1203/00006450-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Vlachos A, Rosenberg PS, Atsidaftos E, et al. Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood. 2012;119(16):3815–3819. doi: 10.1182/blood-2011-08-375972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosteller RD. Simplified calculation of body-surface area. The New England journal of medicine. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity National health statistics reports. Hyattsville, MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 18.Rosner W, Auchus RJ, Azziz R, et al. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. The Journal of clinical endocrinology and metabolism. 2007;92(2):405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 19.Rosner W, Hankinson SE, Sluss PM, et al. Challenges to the measurement of estradiol: an endocrine society position statement. The Journal of clinical endocrinology and metabolism. 2013;98(4):1376–1387. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dullaart RP, Pasterkamp SH, Beentjes JA, et al. Evaluation of adrenal function in patients with hypothalamic and pituitary disorders: comparison of serum cortisol, urinary free cortisol and the human-corticotrophin releasing hormone test with the insulin tolerance test. Clinical endocrinology. 1999;50(4):465–471. doi: 10.1046/j.1365-2265.1999.00679.x. [DOI] [PubMed] [Google Scholar]
- 21.Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. The Journal of clinical endocrinology and metabolism. 2008;93(11):4245–4253. doi: 10.1210/jc.2008-0710. [DOI] [PubMed] [Google Scholar]
- 22.McKenna TJ, Sequeira SJ, Heffernan A, et al. Diagnosis under random conditions of all disorders of the renin-angiotensin-aldosterone axis, including primary hyperaldosteronism. The Journal of clinical endocrinology and metabolism. 1991;73(5):952–957. doi: 10.1210/jcem-73-5-952. [DOI] [PubMed] [Google Scholar]
- 23.Growth Hormone Research S. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. The Journal of clinical endocrinology and metabolism. 2000;85(11):3990–3993. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 24.Fuleihan GERC, Mulder JE, Brown EM. In: Parathyroid hormone secretion and action. Post TW, editor. UpToDate; Waltham, MA: 2014. [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Gordon CM, Leonard MB, Zemel BS, et al. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17(2):219–224. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Gulati R, Bhatia V, Agarwal SS. Early onset of endocrine abnormalities in beta-thalassemia major in a developing country. Journal of pediatric endocrinology & metabolism: JPEM. 2000;13(6):651–656. doi: 10.1515/jpem.2000.13.6.651. [DOI] [PubMed] [Google Scholar]
- 28.Jaruratanasirikul S, Tanchotikul S, Wongcharnchailert M, et al. A low dose adrenocorticotropin test (1 microg ACTH) for the evaluation of adrenal function in children with beta-thalassemia receiving hypertransfusion with suboptimal iron-chelating therapy. Journal of pediatric endocrinology & metabolism: JPEM. 2007;20(11):1183–1188. doi: 10.1515/jpem.2007.20.11.1183. [DOI] [PubMed] [Google Scholar]
- 29.Toumba M, Sergis A, Kanaris C, et al. Endocrine complications in patients with Thalassaemia Major. Pediatric endocrinology reviews: PER. 2007;5(2):642–648. [PubMed] [Google Scholar]
- 30.De Sanctis V, Eleftheriou A, Malaventura C, et al. Prevalence of endocrine complications and short stature in patients with thalassaemia major: a multicenter study by the Thalassaemia International Federation (TIF) Pediatric endocrinology reviews: PER. 2004;2(Suppl 2):249–255. [PubMed] [Google Scholar]
- 31.Noetzli LJ, Panigrahy A, Mittelman SD, et al. Pituitary iron and volume predict hypogonadism in transfusional iron overload. American journal of hematology. 2012;87(2):167–171. doi: 10.1002/ajh.22247. [DOI] [PubMed] [Google Scholar]
- 32.Ball SE, McGuckin CP, Jenkins G, et al. Diamond-Blackfan anaemia in the U.K.: analysis of 80 cases from a 20-year birth cohort. British journal of haematology. 1996;94(4):645–653. doi: 10.1046/j.1365-2141.1996.d01-1839.x. [DOI] [PubMed] [Google Scholar]
- 33.Scott EG, Haider A, Hord J. Growth hormone therapy for short stature in Diamond Blackfan anemia. Pediatric blood & cancer. 2004;43(5):542–544. doi: 10.1002/pbc.20075. [DOI] [PubMed] [Google Scholar]
- 34.Howell JC, Joshi SA, Hornung L, et al. Growth hormone improves short stature in children with diamond-blackfan anemia. Pediatric blood & cancer. 2015;62(3):402–408. doi: 10.1002/pbc.25341. [DOI] [PubMed] [Google Scholar]
- 35.Lipton JM, Federman N, Khabbaze Y, et al. Osteogenic sarcoma associated with Diamond-Blackfan anemia: a report from the Diamond-Blackfan Anemia Registry. Journal of pediatric hematology/oncology. 2001;23(1):39–44. doi: 10.1097/00043426-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Archives of internal medicine. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogiatzi MG, Macklin EA, Fung EB, et al. Bone disease in thalassemia: a frequent and still unresolved problem. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2009;24(3):543–557. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efthimia V, Neokleous N, Agapidou A, et al. Nephrolithiasis in beta thalassemia major patients treated with deferasirox: an advent or an adverse event? A single Greek center experience. Annals of hematology. 2013;92(2):263–265. doi: 10.1007/s00277-012-1558-3. [DOI] [PubMed] [Google Scholar]
- 39.Choudhary A, Giardina P, Antal Z, et al. Unreliable oral glucose tolerance test and haemoglobin A1C in beta thalassaemia major–a case for continuous glucose monitoring? British journal of haematology. 2013;162(1):132–135. doi: 10.1111/bjh.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]


