Abstract
Background
The effect of intensive blood pressure (BP) lowering on kidney function among persons with established cerebrovascular disease and preserved estimated glomerular filtration rate (eGFR) is not established.
Methods and Results
Among 2610 participants randomized to lower SBP target (<130 mmHg) vs. higher (130-149 mmHg) with repeated measures of serum creatinine, we evaluated differences by study arm in annualized eGFR decline and rapid decline (eGFR decline >30%) using linear mixed models and logistic regression, respectively. We assessed associations of both treatment and kidney function decline with stroke, major vascular events (MVE) and the composite stroke, death, MVE or myocardial infarction, using multivariable Cox regression, separately and jointly including test for interaction. Analyses were conducted by treatment arm. Mean age was 63±11; 949 (36%) were diabetic, mean eGFR was 80±19 ml/min/1.73m2. At 9 months, achieved SBP was 137±15 in higher vs. 127±14 mmHg in lower BP group, and differences were maintained throughout follow-up (mean 3.2 years). Compared with higher, lower BP target had -0.50 (95% CI - 0.79 to -0.21) ml/min/1.73m2/year faster eGFR decline. Differences were most pronounced during the first year (-2.1 ml/min/1.73m2 (95% CI -0.97 to -3.2)), whereas rates of eGFR decline did not differ after year 1 (-0.095, -0.47 to 0.23). A total of 313 (24%) persons in the lower BP group had rapid kidney function decline, compared with 247 (19%) in higher (OR 1.4 (95% CI 1.1 to 1.6)). Differences in rapid decline by treatment arm were apparent in the first year (OR 1.4, 1.1-1.8), but were not significant after year 1 (OR 1.0, 0.73-1.4). Rapid decline was associated with higher risk for stroke, MVE and composite after full adjustment among persons randomized to the higher BP target (Stroke HR 1.93 (1.15 to 3.21), but not the lower BP arm, stroke HR 0.93 (0.50 to 1.75) (all p interaction <0.06).
Conclusions
In persons with prior lacunar stroke and relatively preserved kidney function, intensive BP lowering was associated with greater likelihood of rapid kidney function decline. Differences were primarily observed during the first year of anti-hypertensive treatment. Rapid kidney function decline was not associated with increased risk for clinical events among those undergoing intensive BP lowering.
Clinical Trial Registration Information
ClinicalTrials.gov. Identifier: NCT00059306.
Keywords: Cerebrovascular disease/stroke, hypertension, kidney
Introduction
Management of hypertension remains the mainstay of treatment in chronic kidney disease (CKD), defined as an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2 or presence of proteinuria.1 However, the optimal blood pressure (BP) level to attenuate CKD progression remains an issue of active debate. Observational data show a graded association of higher blood pressure (BP) levels with progression to end stage renal disease (ESRD), but the BP threshold for the observed higher risk is variable. 2-4 Data from randomized trials among persons with established CKD have not consistently shown benefit from achieving BP to targets lower than 140/90 mmHg, particularly among persons without proteinuria.5-9
Less is known about the effect of higher vs. more intensive BP lowering on changes in kidney function among persons with preserved eGFR (>60 ml/min/1.73m2). Some studies among diabetic persons suggest that intensive BP lowering may reduce the level of proteinuria, but it may not attenuate progression of disease.10-13 On the other hand, it is also possible that intensive BP lowering may accelerate kidney function decline, particularly in persons with established vascular disease and little or no proteinuria.14 The Systolic Blood Pressure Intervention Trial (SPRINT) showed that, while more intensive BP lowering resulted in lower rates of cardiovascular events and death compared with usual target, aggressive BP treatment led to higher rates of kidney function decline among participants without CKD.15 Understanding potential renal effects of intensive BP lowering is an especially pressing question among individuals at high cardiovascular risk (i.e. atherosclerotic disease, prior stroke, elderly), because the optimal systolic BP goal for cardiovascular protection in these persons remains an issue of active debate.16-18 Given longer life expectancy rates, understanding the effects of intensive vs. usual BP lowering on kidney function change is important in addressing the increasingly high morbidity from renal disease in elders and persons at high cardiovascular risk.
The Secondary Prevention of Small Subcortical Strokes (SPS3) study compared the effectiveness of a lower systolic blood pressure (<130 mmHg) vs. a higher target (130-149 mmHg) to reduce recurrent stroke among persons with a history of lacunar stroke. The lower BP target did not significantly reduce the risk of stroke or the composite outcome of stroke, myocardial infarction or vascular death.19 In this report, we examined the effects of intensive vs. usual BP lowering on renal outcomes among these persons with largely preserved kidney function.
Methods
Participants
SPS3 was a randomized, multi-center clinical trial designed to evaluate the effectiveness of two antiplatelet treatments (aspirin vs. aspirin plus clopidogrel) and two target levels of systolic blood pressure (SBP) in preventing strokes among persons with previous lacunar stroke. Details of the study design have been previously published.20 Briefly, persons in North America, Latin America and Spain, ages 30 years or older and with a recent symptomatic lacunar stroke were randomized, in a 2-by-2 factorial design, to the anti-platelet intervention (double-blind) and to lower SBP target of <130 mmHg or higher target (130-149 mmHg), at least two weeks after the qualifying stroke. The SBP intervention used the Prospective Randomized Open, Blinded End-point (PROBE) design. Persons were excluded if they had a disabling stroke, hemorrhagic or cortical ischemic stroke. In addition, persons were excluded if they had advanced kidney disease, defined as an eGFR <40 ml/min/1.73m2. For these analyses, we included all SPS3 participants who had at least two measures of serum creatinine during the study period. A total of 3020 SPS3 participants entered SPS3. We excluded 410 participants who had only one measure of serum creatinine, for a final sample size of 2610 persons. All participants signed informed consent and the trial was approved by the appropriate institutional review board.
Blood Pressure Targets
Participants were randomly assigned to a higher SBP target (130-149 mmHg) vs. lower (<130 mmHg). Relevant to this study, there was no washout period of anti-hypertension medication. As previously described, patients were seen monthly until BP target was achieved, then quarterly for BP measures and medication adjustment. If participants randomized to the higher target were below target, antihypertensive medications were discontinued or reduced, unless their use was indicated for other reasons. BP was measured using the Colin 8800C automated device, and management was overseen by a physician at each study site. The physician prescribed anti-hypertensive medication from the available study formulary, which included at least one drug of the major classes, and medications were provided to participants. Classes or doses of medications were not managed per protocol. All participants were followed to a common end-study date. For these analyses, we consider year 5 the end of this study due to the very low number of participants with follow-up renal measures > 5 years.
Kidney Function Measures
Kidney function was measured by serum creatinine among all participants at yearly intervals until end of study. Serum creatinine was measured at each study site. The eGFR was estimated using the CKD Epi equation.21 For these analyses, there were two primary kidney outcomes: (1) annualized eGFR change and (2) rapid kidney function decline. Rapid decline was defined as a reduction in eGFR ≥30% from baseline. This definition is recommended as a valid surrogate outcome for kidney disease trials, and it is a strong predictor of adverse cardiovascular events, death and ESRD.22-24 In sensitivity analyses, we defined rapid decline as ≥40%, as this outcome has been shown to have even stronger associations with ESRD, 22, 23 and we also examined rates of incident CKD during the entire study period, defined as eGFR <60 ml/min/1.73m2 plus a decline ≥30% among persons with eGFR >60 at baseline, consistent with SPRINT.15
Clinical Outcomes
Trial outcomes were adjudicated by a committee blinded to treatment arm, as has been previously described in detail.20 For these analyses, we define three clinical outcomes: (1) the SPS3 primary outcome of any stroke (ischemic or hemorrhagic confirmed by neuroimaging); (2) the secondary endpoint of need for hospitalization due to a major vascular event (MVE): and (3) a composite outcome of stroke, death, MVE or myocardial infarction (MI). MI was defined based on clinical history, electrocardiographic changes and cardiac enzymes.
Analyses
We first compared baseline characteristics of 2610 SPS3 participants by study arm and by baseline eGFR. We then evaluated BP levels and use of each major medication class (ACE/ARB, diuretic, calcium channel blocker and beta-blocker) in the higher and lower BP study arms over the study period. We then compared annualized eGFR decline in the higher vs. lower BP groups using linear mixed models with random intercepts. We used smoothing splines to pictorially depict eGFR decline over the study period by treatment arm. We compared differences up to 5 years of follow up. We also stratified our results by study period (baseline to year 1 vs. year 1 to end of study) to determine if any observed differences were seen in short and long term renal function changes.25 We further examined whether any differences in eGFR decline by study arm differed by age (> or <65), diabetic status or presence of CKD (eGFR <60 ml/min/1.73m2). In sensitivity analyses, we stratified at eGFR < 90 or ≥90 ml/min/1.73m2 to ensure consistency of our findings across baseline eGFR levels. Then, we estimated the proportion of persons with rapid decline by study arm. We compared odds of rapid decline by usual vs. intensive BP treatment using logistic regression. In these analyses, we present comparisons for the entire study, and by study period as above. These analyses followed intention-to-treat principles.
Next, we were interested in understanding the clinical relevance of rapid decline. To do so, we first evaluated whether or not kidney function loss could explain the trial null findings. Specifically, based on the intention to treat principles, we used Cox regression models to compare time to event for the clinical outcomes by treatment arm. We then adjusted for eGFR slope or rapid decline in year 1, separately, and determined the importance on the treatment effect. In a second step, we used an observational design. We estimated the association of baseline characteristics and achieved SBP (defined as mean SBP at 6 and 9 months) with rapid kidney function decline, using multivariable logistic regression. Our final objective was to compare the associations of rapid kidney function decline in the first year with the clinical outcomes of stroke, major vascular event, and the composite outcome (death, MVE, MI, or stroke). We used Cox proportional hazards regression models adjusted for treatment arm, and then additionally adjusted for age, gender, ethnicity, smoking, diabetes, hypertension, use of ACE/ARB, baseline SBP, and baseline eGFR. We included tests of the interaction between rapid decline and treatment arm in order to determine whether the effects of kidney decline on the clinical outcomes differed by intensive versus usual BP treatment, and we repeated the analyses stratified by treatment arm.
All analyses were performed in SAS version 9.2 and 9.4
Results
Among 2610 participants included in these analyses, a total of 2041 (78%) had ≥3 creatinine measures, over a mean follow up time of 3.2 years (range 1-5). At baseline, the mean age was 63.4 ± 10.7 years, 384 (15%) were Black, 854 (33%) were Hispanic, 949 (36%) were diabetic, and 2339 (90%) had hypertension. The mean eGFR was 80 ± 18.5 ml/min/1.73m2, and 410 (16%) had an eGFR <60 ml/min/1.73m2 at time of randomization. There were no significant differences in baseline characteristics by study arm, except for a higher proportion of males in the higher target group. (Table 1) Persons with lower eGFR at baseline were more likely to be older, hypertensive, and a current user of an angiotensin converting enzyme inhibitor (ACE), angiotensin receptor blocker (ARB) or a diuretic. (Supplemental Table 1)
Table 1. Characteristics of SPS3 Participants by treatment group at baseline.
| Overall | Higher BP | Lower BP | ||
|---|---|---|---|---|
| “Usual” | “Intensive” | |||
|
| ||||
| n | 2610 | 1309 | 1301 | P-value |
| Age (years) | 63 (11) | 64 (11) | 63 (11) | 0.34 |
| Male | 1655 (63%) | 862 (66%) | 793 (61%) | 0.01 |
| Race | ||||
| Non-Hispanic White | 1300 (50%) | 641 (49%) | 659 (51%) | 0.75 |
| Black | 413 (16%) | 205 (16%) | 208 (16%) | |
| Hispanic | 834 (32%) | 430 (33%) | 404 (31%) | |
| Other/multiple | 63 (2%) | 33 (3%) | 30 (2%) | |
| Region | ||||
| North America | 1672 (64%) | 835 (64%) | 837 (64%) | 0.87 |
| Latin America | 647 (25%) | 330 (25%) | 317 (24%) | |
| Spain | 291 (11%) | 144 (11%) | 147 (11%) | |
| Smoking | ||||
| Current | 506 (19%) | 255 (19%) | 251 (19%) | |
| Past | 1069 (41%) | 525 (40%) | 544 (42%) | |
| Never | 1035 (40%) | 529 (40%) | 506 (39%) | 0.65 |
| BMI (kg/m2) | 29 (7) | 29 (8) | 29 (6) | 0.27 |
| Diabetes | 950 (36%) | 469 (36%) | 481 (37%) | 0.54 |
| Hypertension | 2337 (90%) | 1176 (90%) | 1161 (89%) | 0.62 |
| SBP (baseline) | 143 (19) | 144 (19) | 142 (18) | 0.10 |
| SBP (3 mo) | 133 (16) | 137 (15) | 130 (15) | <.0001 |
| SBP (9 mo) | 132 (15) | 137 (15) | 127 (14) | <.0001 |
| eGFR (ml/min/1.73m2) | 80 (19) | 80 (18) | 80 (19) | 0.59 |
| eGFR category at baseline: | ||||
| <60 | 410 (16%) | 195 (15%) | 215 (17%) | 0.25 |
| >60 | 2198 (84%) | 1113 (85%) | 1085 (83%) | |
Data are presented as Mean (SD) or numbers (percent).
SBP=systolic blood pressure in mmHg
eGFR= glomerular filtration rate estimated by CKD Epi equation in ml/min/1.73m2
2 persons are missing creatinine data at baseline, but have at least two values at follow-up.
From the initial 3020 SPS3 participants, 410 were excluded for these analyses due to loss to follow up or an event (and thus no follow up creatinine measures), and the proportion excluded did not differ by treatment arm (11.7 vs. 11.2%). When comparing those excluded to participants in these analyses, there were no significant differences in baseline age, gender, BMI, diabetes, hypertension, history of TIA, eGFR, SBP, use of ACE/ARB. Excluded participants were more likely to come from Spain (18% vs. 11%), and they were less likely to be Hispanic (21% vs. 33%) or use thiazide diuretic at baseline (30% vs 51%). There were no differences in the characteristics of persons who discontinued the study by treatment arm (all p >0.1).
Blood Pressure and Anti-hypertensive Treatment in SPS3
BP levels were reduced in both treatment arms over the study period, compared to baseline. At 9 months, the achieved SBP was 137 (±15) mmHg and achieved DBP was 76 (±10) mmHg among participants randomized to the higher BP target. The achieved SBP was 127 (±14) mmHg and DBP was 70 (±9) mmHg among those randomized to the lower BP group. Approximately 72% of persons in the intensive arm had achieved the SBP target at 9 months. As previously reported, differences were maintained to the end of the study.19 Relative to the higher BP arm, use of ACE or ARB, diuretic and CCB were all similarly increased by approximately 20% among persons in the lower BP arm. (Figure 1)
Figure 1.
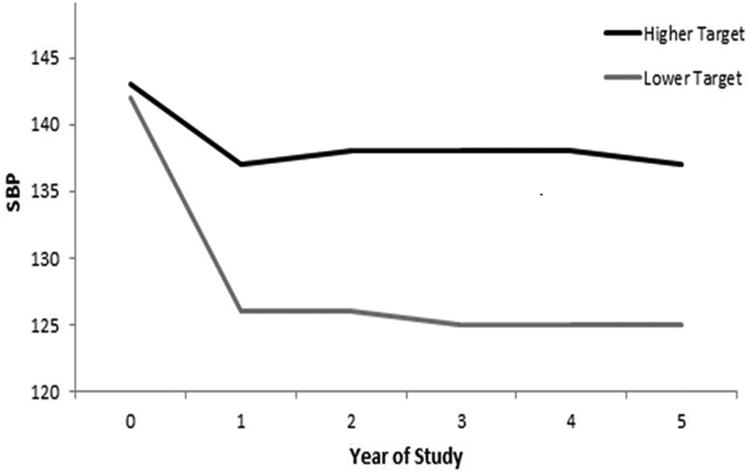
Blood Pressure Level and Use of Each Anti-Hypertensive Class in SPS3 (Systolic Blood Pressure vs. Year of Study).
Intensive vs. Usual BP Target and Kidney Function Decline
Among all participants, the mean eGFR decline was -3.2 ± 8.6 ml/min/1.73m2 per year during follow up. Overall, there was a pronounced eGFR decline during the first year of the study in both groups, followed by a steady decline in eGFR from year 1 to the end of the study. (Figure 2) Compared with usual, persons in the intensive BP target had -0.50 (- 0.79 to -0.21) ml/min/1.73m2 per year faster eGFR decline overall. In analyses stratified by study period, in the first year, persons in the lower BP group declined by 2.1 ml/min/1.73m2 (95% CI 0.97 to 3.2) faster, compared with higher target (p=0.0002). The differences in eGFR decline between BP treatment arms were not statistically significant when only considering changes from year 1 to end of study (Figure 2). Findings were not materially different by age or diabetic status. However, differences in eGFR decline by treatment arm were not observed among persons with eGFR <60 ml/min/1.73m2 at baseline, p for interaction 0.04. (Supplemental Figure 1)
Figure 2.
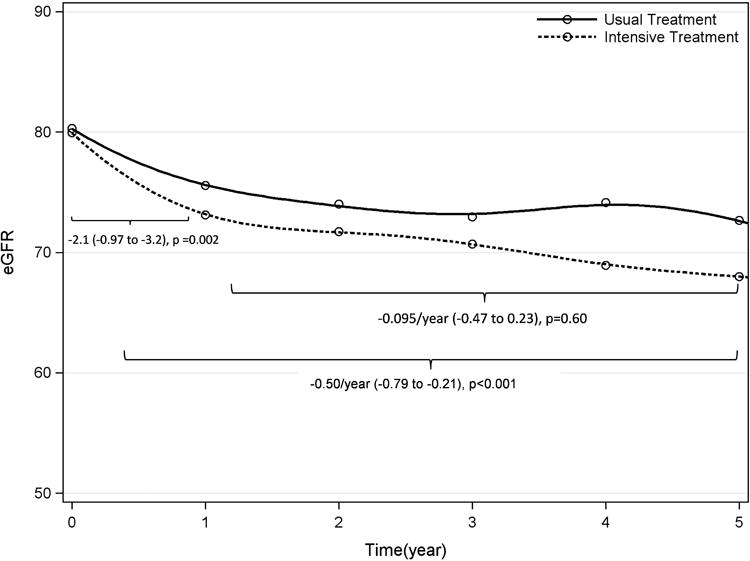
The Effect of Usual vs. Intensive BP Lowering on Kidney Function Change (ml/min/1.73m2 per year) in the SPS3 Study (eGFR vs. Time (year)).
A total of 313 (24%) persons in the lower BP group had rapid kidney function decline, compared with 247 (19%) in the usual BP arm, OR 1.4 (95% CI 1.1 to 1.6). There were no differences in these estimates when we stratified by baseline GFR ≥90 or < 90 ml/min/1.73m2. For example, among persons with eGFR ≥90, the OR comparing the lower to the higher BP target group was 1.4 (1.0 to 2.0), and it was 1.3 (1.1 to 1.7) among persons with eGFR <90 ml/min/1.73m2. We found that differences in rapid decline by treatment arm were apparent in the first year, but were not statistically significant after year 1. (Table 2) When we defined rapid decline as ≥40%, OR for rapid decline was 1.3 (95% CI: 1.01, 1.7) during follow up. Among persons with preserved eGFR at randomization, 14% had incident CKD in the intensive BP arm, compared with 11% in the usual, OR 1.41 (1.09, 1.82) over the study period.
Table 2.
Rapid Kidney Function Decline among SPS3 Participants Randomized to Higher (130-149 mmHg) vs. Lower SBP (<130 mmHg) Target- Intention to Treat
| Higher SBP | Lower SBP | |
|---|---|---|
| Overall Study Period (n=2610) | ||
|
| ||
| N with rapid decline (%) | 247 (19%) | 313 (24%) |
| OR for lower vs. higher (95% Cl) | REF | 1.4 (1.1, 1.6) |
|
| ||
| Baseline to Year 1 (n=2489) | ||
|
| ||
| N with rapid decline (%) | 101 (8%) | 133 (11%) |
| OR for lower vs. higher (95% Cl) | REF | 1.4 (1.1, 1.8) |
|
| ||
| From Year One to Five (n=2085) | ||
|
| ||
| N with rapid decline (%) | 83 (8%) | 83 (8%) |
| OR for lower vs. higher (95% Cl) | REF | 1.0 (0.73, 1.4) |
OR = odds ratio comparing the effect of higher vs. lower BP target on rapid kidney function decline, defined as eGFR decline ≥30% from baseline to any annual follow up visit. Analyses are presented over entire follow up (overall) and stratified by study period. For analyses of baseline to year 1, rapid decline is assessed from baseline to 12 months. For analyses of year 1 to end of study, rapid decline is assessed from year 1 to any yearly follow up visit.
After adjustment for treatment arm, characteristics associated with rapid decline included older age, current smoking, diabetes, higher SBP, use of BB and ACE/ARB. (Supplemental Table 2) Higher achieved SBP was associated with rapid decline in the higher target arm (OR 1.3, 1.1 to 1.6), but the association was not statistically significant in the lower BP arm (OR 1.1, 0.9 to 1.3), p for interaction 0.07.
Kidney Function Decline and Study Outcomes
We estimated the association of lower vs. higher BP target with stroke, major vascular event or the composite outcome (death, MVE, MI or stroke), before and after adjustment for eGFR decline (slope) or rapid decline at year 1. In unadjusted models, the HR for intensive treatment, compared to usual, was 0.84 for stroke (95% CI: 0.65 to 1.09), 0.87 for MVE (0.69 to 1.10), and 0.91 for the composite outcome (0.75 to 1.12). Adjustment for eGFR decline only slightly increased the strength of the point estimates, but these did not become statistically significant. Specifically, in models that controlled for rapid decline in year 1, the HR for intensive treatment vs. usual was 0.81 for stroke (95% CI: 0.62 to 1.05), 0.83 for MVE (0.65 to 1.06), and 0.89 for the composite outcome (0.72 to 1.09).
Finally, we evaluated the association of rapid decline in year 1 with each clinical outcome, separately. We found that the association of rapid decline with outcomes varied by treatment arm. Specifically, among persons randomized to the higher target group, participants with rapid decline during the first year had approximately 2-fold risk for stroke, MVE and composite outcome, compared with no rapid decline. Associations remained significant after adjustment for potential confounders. In contrast, rapid decline did not appear to be associated with increased risk for stroke, MVE or the composite outcome among participants randomized to the lower BP target. (Table 3)
Table 3. The Association of Rapid Kidney Function Decline at Year 1 with Clinical Endpoints in SPS3 by Treatment Arm.
| Higher Target | Lower Target | P Interaction Treatment × Decline | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Rate per 1000 PY | Unadjusted HR (95% CI) | Adjusted* HR (95% CI) | Rate per 1000 PY | Unadjusted HR (95% CI) | Adjusted* HR (95% CI) | ||
| All Stroke | |||||||
|
| |||||||
| No Rapid Decline Year 1 | 24.1 | ref | ref | 21.5 | ref | ref | |
| Rapid Decline Year 1 | 51.6 | 2.13 (1.29, 3.52) | 1.93 (1.15, 3.21) | 21.4 | 0.99 (0.53, 1.86) | 0.93 (0.50, 1.75) | 0.06 |
|
| |||||||
| Major Vascular Event | |||||||
|
| |||||||
| No Rapid Decline Year 1 | 30.1 | ref | ref | 27.5 | ref | ref | |
| Rapid Decline Year 1 | 58.6 | 1.95 (1.22, 3.13) | 1.76 (1.09, 2.85) | 25.4 | 0.92 (0.52, 1.63) | 0.86 (0.48, 1.53) | 0.05 |
|
| |||||||
| Composite (death, MVE, MI or stroke) | |||||||
|
| |||||||
| No Rapid Decline Year 1 | 39.2 | ref | ref | 38.0 | ref | ref | |
| Rapid Decline Year 1 | 70.3 | 1.84 (1.20, 2.82) | 1.62 (1.05, 2.51) | 35.2 | 0.90 (0.55, 1.47) | 0.83 (0.51, 1.35) | 0.03 |
Hazard Ratios (HR) and 95% Confidence Intervals (CI) from Cox proportional hazard regression models
Adjusted for age, gender, ethnicity, smoking, diabetes, hypertension, use of ACE/ARB, baseline SBP, baseline eGFR
MVE (major vascular event) = Stroke, MI or vascular death
Rapid kidney function decline defined as eGFR decline ≥30% from baseline to year
Discussion
We showed that, among persons with prior lacunar stroke who had a mean age of 63±11 years and relatively preserved kidney function, compared with treating to a higher SBP target (130-149 mmHg), a lower SBP target (<130 mmHg) was associated with greater reduction in eGFR and higher risk of having rapid kidney function decline during follow up. We found that differences in kidney function loss were primarily observed during the first year of anti-hypertensive treatment intensification. In longer follow up, kidney function did not improve in the lower BP target group, but rather continued to decline in parallel with the higher BP group. Rapid kidney function decline during the first year was associated with a higher risk for stroke, major vascular events and the composite outcome only among persons randomized to the higher target group. Rapid kidney function decline was not associated with higher risk for the clinical endpoints among persons randomized to the lower BP arm.
The optimal BP target remains one of the most critical issues in the management of persons with hypertension and high cardiovascular risk. Data from placebo-controlled randomized trials have examined SBP goals of <160 26 or <150 mmHg,27 and the recommendations on lowering SBP beyond 140 mmHg remain conflicting.28-30. The recent SPRINT trial showed lower rates of death and cardiovascular events with more intensive BP lowering in persons at high cardiovascular risk but without a history of stroke or diabetes.15 Among persons with diabetes, there was no difference in clinical outcomes among persons randomized to lower vs. usual BP targets in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.31 Whether or not there may be renal benefit or harm from more intensive BP lowering in persons at high cardiovascular risk without established CKD remains less clear. In a meta-analysis of anti-hypertensive treatment trials conducted in the 1970s to 1990s, designed with higher BP targets and before widespread use of ACE/ARBs, treatment was not associated with a reduction in the incidence of renal dysfunction.32 The ONTARGET trial showed faster rates of kidney function decline among persons treated with dual ACE/ARB therapy, compared with either alone, but whether this was due to lower achieved BP is unclear. 14 SPRINT and ACCORD have both reported higher rates of kidney function decline with intensification of anti-hypertensive therapy.15, 31 In the setting of established CKD, randomized trials show a benefit from lower BP levels only among persons with proteinuria,5-7, 9 and SPRINT did not show a difference in CKD progression to ESRD by study arm.15 Our study suggests that, among persons with cerebrovascular disease and relatively preserved eGFR, intensive BP lowering results in the expected sharper eGFR decline in the first year, but is not followed by recovery or eventual renal benefit during the study period. Our findings that SPS3 participants in the lower BP arm had increased use of most of the anti-hypertensive medication classes makes it less likely that the renal function decline is attributable to one class alone, but rather to the BP lowering from a combination of the drugs.
We also found that rapid kidney function decline was associated with an increased risk for stroke, MVE and the composite outcome among persons randomized to the higher BP target, but not the lower BP target. The reasons for these observed differences are not certain. One possibility is that more intensive lowering of BP may have some benefit in reducing the likelihood of the SPS3 clinical outcomes, which offsets the higher cardiovascular risk associated with kidney dysfunction. In this scenario, intensive BP lowering in persons with existing small vessel disease may be cardio-protective, but it could also result in renal hypo-perfusion due to decreased effective circulating volume, over-diuresis, or an intrinsic impairment in adequate renal auto-regulation; concomitant use of inhibitors of the renin-angiotensin system or diuretics may exacerbate this risk. 33 In our study, adjustment for rapid kidney function decline did not substantially alter the comparisons of higher vs. lower BP target with risk of clinical outcomes in SPS3. A second possibility is that the physiologic mechanisms leading to rapid kidney function decline differs by study arm. That is, among persons randomized to the lower BP target, eGFR loss represents a hemodynamic phenomenon that may be reversible and does not increase future cardiovascular risk. In contrast, among persons in the higher BP target, kidney function decline may represent true kidney disease progression, perhaps due to persistent BP levels above the threshold needed for nephroprotection34, 35. A hemodynamic renal effect with intensive BP lowering is supported by previous clinical trial data in persons with established CKD which show a sharp, acute reduction in eGFR when BP is lowered, followed by eventual renal protection among persons with proteinuria.5, 6, 25, 36 In the setting of ACE use, it is suggested by some experts that an initial reduction in eGFR typically occurs early (within weeks), followed by stabilization (within months) and eventual attenuation of renal function loss.33 Hemodynamic effects on renal function with calcium channel blockers have also been suggested.37 However, in a recent meta-analysis of 37 trials among persons with kidney disease of varying etiologies, rapid decline was strongly associated with higher risk for ESRD. 24 Taken together, our findings suggest that the clinical significance of rapid kidney function decline may vary based on whether or not the eGFR change is observed in the setting of active, aggressive BP lowering. Given the results of the SPRINT trial, many patients may find their anti-hypertensive treatment intensified in the near future. Our findings are especially important in this context because patients may have their therapy de-intensified due to concerns about increases in creatinine levels in the setting of BP lowering. Future studies are required to understand whether or not changes in renal function during intensification of anti-hypertensive therapy are associated with electrolyte disturbances, patient-centered outcomes, resource utilization and clinical events.
In addition to the randomized design, this study has other strengths. SPS3 maintained a significant difference in SBP between arms throughout the study period, which allows examination of a sustained lower BP level. Since the overall trial was null, differences in renal function decline are less likely influenced by bias due to differential loss to follow up. The clinical outcomes used were adjudicated by blinded reviewers. We must also note important limitations. The study was not designed or powered to detect differences in incident CKD or ESRD. However, rapid decline is considered an appropriate surrogate renal outcome for clinical trials. SPS3 did not measure serum creatinine repeatedly during the first year. While we are unable to assess very acute changes in eGFR, intensification of anti-hypertensive medications in SPS3 happened early in the first year (first three months) with most persons achieving BP control within 6 months. Nonetheless, the renal function measure at year 1 allows ample time for eGFR stabilization described in other studies.25, 38, 39 While SPS3 had a relatively short follow up time, it is consistent with the follow up time of other studies that have suggested renal protection with intensive BP lowering in persons with proteinuria.25 We are unable to ascertain whether or not rapid decline in the usual arm is reversible, as this would require withdrawal of medication. Whether or not there is renal benefit eventually from lower BP targets requires longer follow-up. While creatinine values were not specifically calibrated by the study, we expect variations to be randomly allocated by study arm. We were unable to study the effect of intensive BP lowering among persons with significant proteinuria. Since SPS3 was not enriched for kidney disease, it is likely that the overall prevalence of significant proteinuria was low.
In summary, we found that, in this population of persons with an average age of 63±11, with previous lacunar stroke and relatively preserved kidney function, intensive BP lowering was associated with somewhat greater eGFR drop. This difference was most pronounced during the first year, and we found no evidence for renal protection over the follow up period. The clinical significance of rapid kidney function decline varied by treatment arm, as it was associated with increased risk of clinical outcomes only among the higher target group. Rapid decline was not associated with higher risk for clinical events among persons undergoing intensive BP lowering.
Supplementary Material
Clinical Perspectives.
The effect of intensive BP lowering on kidney function among persons at high cardiovascular risk and relatively preserved kidney function has been unclear. Among participants in the SPS3 randomized trial with prior lacunar stroke, we found that persons undergoing active, intensive BP lowering had faster rates of kidney function decline, compared with persons undergoing usual treatment to established goals. In the intensive treatment group, rapid kidney function decline was not associated with a higher risk for stroke, death, or major vascular events. In contrast, among persons undergoing usual anti-hypertensive treatment, rapid decline was associated with a higher risk for all the outcomes, as has been observed in many prior observational studies. Taken together, our findings could guide clinicians to understand that decline in kidney function is common and expected among persons undergoing intensive BP lowering. In this specific setting, kidney function decline does not appear to pose significant clinical risk for adverse cardiovascular outcomes. Further studies are needed to understand whether rapid kidney function decline in the setting of intensive BP lowering is associated with end stage renal disease in longer follow up.
Acknowledgments
Funding Sources: CP was funded by grant 1R01AG046206-01A1.
Disclosures: CP was sponsored by NIH-NINDS. LM, CW, MGS, MO, PP, OB have no conflict of interest to disclose. RS received an honorarium from Merck for participating in a Renal Expert Input Forum; this honorarium was donated to NCIRE to support kidney research. CP had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Stevens PE, Levin A Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 2.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277:1293–8. [PubMed] [Google Scholar]
- 3.Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, Charleston J, He J, Kallem R, Lash JP, Miller ER, 3rd, Rahman M, Steigerwalt S, Weir M, Wright JT, Jr, Feldman HI Chronic Renal Insufficiency Cohort Study I. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162:258–65. doi: 10.7326/M14-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peralta CA, Norris KC, Li S, Chang TI, Tamura MK, Jolly SE, Bakris G, McCullough PA, Shlipak M, Investigators K. Blood pressure components and end-stage renal disease in persons with chronic kidney disease: the Kidney Early Evaluation Program (KEEP) Arch Intern Med. 2012;172:41–7. doi: 10.1001/archinternmed.2011.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–51. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X, Group ACR. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–29. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011;154:541–8. doi: 10.7326/0003-4819-154-8-201104190-00335. [DOI] [PubMed] [Google Scholar]
- 8.Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Moller K, Wigger M, Peruzzi L, Mehls O, Schaefer F. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–50. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, Garini G, Sessa A, Basile C, Alpa M, Scanziani R, Sorba G, Zoccali C, Remuzzi G, Group RS. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–46. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 10.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–40. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. Am J Kidney Dis. 1999;34:809–17. doi: 10.1016/s0272-6386(99)70036-3. [DOI] [PubMed] [Google Scholar]
- 12.Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799–810. doi: 10.1161/CIRCULATIONAHA.110.016337. 9 p following 810. [DOI] [PubMed] [Google Scholar]
- 13.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–97. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 14.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–53. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 15.The SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;0:1–14. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G Cardio-Sis i. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–33. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 17.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson AM, Hu T, Eshelbrenner CL, Reynolds K, He J, Bazzano LA. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta-analysis. JAMA. 2011;305:913–22. doi: 10.1001/jama.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–15. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, Coffey C, McClure LA, Szychowski JM, Conwit R, Heberling PA, Howard G, Bazan C, Vidal-Pergola G, Talbert R, Hart RG. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke. 2011;6:164–75. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA, Lambers Heerspink HJ, Mondal H, Schmid CH, Tighiouart H, Noubary F, Coresh J, Greene T, Levey AS. GFR decline as an alternative end point to kidney failure in clinical trials: a meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis. 2014;64:848–59. doi: 10.1053/j.ajkd.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–35. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Lambers Heerspink HJ, Tighiouart H, Sang Y, Ballew S, Mondal H, Matsushita K, Coresh J, Levey AS, Inker LA. GFR decline and subsequent risk of established kidney outcomes: a meta-analysis of 37 randomized controlled trials. Am J Kidney Dis. 2014;64:860–6. doi: 10.1053/j.ajkd.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG African American Study of Kidney D, Hypertension Study G. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 26.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 27.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ. Treatment of Hypertension in Patients 80 Years of Age or Older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F Task Force for the Management of Arterial Hypertension of the European Society of H and the European Society of C. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014;23:3–16. doi: 10.3109/08037051.2014.868629. [DOI] [PubMed] [Google Scholar]
- 29.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 30.Wright JT, Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. doi: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 31.The ACCORD Study Group. Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu CY. Does treatment of non-malignant hypertension reduce the incidence of renal dysfunction? A meta-analysis of 10 randomised, controlled trials. J Hum Hypertens. 2001;15:99–106. doi: 10.1038/sj.jhh.1001128. [DOI] [PubMed] [Google Scholar]
- 33.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–93. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 34.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens. 2013;22:1–9. doi: 10.1097/MNH.0b013e32835b36c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 36.Short-term effects of protein intake, blood pressure, and antihypertensive therapy on glomerular filtration rate in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol. 1996;7:2097–109. doi: 10.1681/ASN.V7102097. [DOI] [PubMed] [Google Scholar]
- 37.Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT, Jr, Whelton PK, Barzilay J, Batuman V, Eckfeldt JH, Farber M, Henriquez M, Kopyt N, Louis GT, Saklayen M, Stanford C, Walworth C, Ward H, Wiegmann T. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2005;165:936–46. doi: 10.1001/archinte.165.8.936. [DOI] [PubMed] [Google Scholar]
- 38.Weir MR, Bakris GL, Weber MA, Dahlof B, Devereux RB, Kjeldsen SE, Pitt B, Wright JT, Kelly RY, Hua TA, Hester RA, Velazquez E, Jamerson KA. Renal outcomes in hypertensive Black patients at high cardiovascular risk. Kidney Int. 2012;81:568–76. doi: 10.1038/ki.2011.417. [DOI] [PubMed] [Google Scholar]
- 39.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–7. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


