Abstract
Background
We previously reported that enhanced nuclear factor kappa B (NFκB) activity is responsible for resistance arteries dysfunction in type 2 diabetic mice.
Methods
In this study we aimed to determine whether augmented NFkB activity also impairs conductance artery (thoracic aorta) function in type 2 diabetic mice. We treated type 2 diabetic (db−/db−) and control (db−/db+) mice with two NFκB inhibitors (DHMEQ, 6 mg/kg, twice a week and IKK-NBD peptide, 500 μg/kg/day) for four weeks.
Results
As expected, the NFκB inhibition did not affect blood glucose level and body weight. Thoracic aorta vascular endothelium-dependent relaxation (EDR), determined by the wire myograph, was impaired in diabetic mice compared to control, and was significantly improved after NFκB inhibition. Interestingly, thoracic EDR was also rescued in db−/db-p50NFκB−/− and db−/db-PARP-1−/− double knockout mice compared to db−/db− mice. Similarly, the acute in vitro down regulation of NFκB-p65 using p65 shRNA lentiviral particles in arteries from db−/db− mice also improved thoracic aorta EDR. Western blot analysis showed that the p65NFκB phosphorylation, cleaved PARP-1 and COX-2 expression were increased in thoracic aorta from diabetic mice, which were restored after NFκB inhibition and in db−/db-p-50NFκB−/− and db−/db-PARP-1−/− mice.
Conclusions
The present results indicate that in male type 2 diabetic mice, the augmented NFκB activity also impairs conductance artery function through PARP-1 and COX-2-dependent mechanisms.
Keywords: Type 2 diabetes, NFκB, PARP-1, COX-2, Thoracic aorta, Endothelial function
INTRODUCTION
Diabetes is an established cardiovascular risk factor, affecting millions of people worldwide. Several factors such as metabolic factors, inflammation, glycation products and endothelial dysfunction, contribute to diabetes-induced cardiovascular diseases. The complication of microvascular and macrovascular function is a major cause of morbidity and mortality in diabetic patients [ 1 , 2 ]. Endothelial vascular dysfunction is mostly characterized by the impairment of eNOS signaling and/or decreased nitric oxide bioavailability [3, 4, 5] associated with increases in activity of the pro-inflammatory transcription factor nuclear factor kappa B (NFκB) [3]. It was reported that enhanced NFκB activity is an important factor involved in the development of cardiovascular diseases [6].
Previous studies indicate that hyperglycemia can trigger the induction of cyclooxygenases 2 (COX-2) and enhance NFκB activity [7]. Additionally, studies reported that COX-2 induction is primarily mediated through the activation of the NFκB pathway [ 8 , 9 ]. Furthermore, hyperglycemia also enhances Poly(ADP-ribose) Polymerase-1 (PARP-1)-dependent NFκB activation that is implicated in the expression of pro-inflammatory genes that are dependent on this transcription factor [10]. We recently elucidated that PARP-1 inhibition improves vascular function in type 2 diabetes indicating that PARP-1 could be a potential target for overcoming diabetic micro-vascular complications [11]. Additionally, previous studies suggested a role for PARP-1 activation in vascular dysfunction in type 2 diabetes [ 12, 13] Thus, the role and mechanism of NFκB in conductance artery dysfunction in type 2 diabetes is an important question that remains unanswered. We recently demonstrated that enhanced NFκB in type 2 diabetic mice impairs resistance arteries endothelium-dependent relaxation [ 14 ]. Since the complication of vasculature in diabetes includes resistance and conductance arteries, we aimed to determine whether enhanced NFkB activity in type 2 diabetes also impairs endothelium-dependent relaxation in conductance arteries such thoracic aorta.
MATERIALS AND METHODS
General protocol in mice
All experiments were performed according to the American Guidelines for the Ethical Care of Animals and were approved by Tulane University Health Sciences Center Animal Care and Use Committee. Type 2 diabetic male mice (db−/db−, 8 to 10 weeks-old males) and their homologous control were purchased from Jackson Laboratories (Bar Harbor, ME), housed in groups of five mice, and maintained at a temperature of 23 ºC with 12 h light/dark cycles. The mice were fed with Harlan Global 18% Protein Rodent Diet (2018) containing 6.0% fat, 18.0% protein and 3.1Kcal/g in content. Mice were divided in 6 groups: 1) control mice infused with saline, n=10); 2) control mice who received DHMEQ (NFκB inhibitor, 6 mg/kg intra-peritoneal injection twice a week) for 4 weeks (Control + DHMEQ, n=10); 3) control mice who received IKK-NBD peptide (NFκB inhibitor, 500 μg/kg/day daily intra-peritoneal injection) for 4 weeks (Control + Peptide, n=10); 4) diabetic mice (db−/db− mice infused with saline, n=10); 5) Diabetic mice treated with DHMEQ for 4 weeks (db−/db− + DHMEQ, n=10); 6) Diabetic mice treated with IKK-NBD peptide for 4 weeks (db−/db− + Peptide, n=10). The body weight and blood glucose levels were recorded weekly during the experimental period. Blood glucose measurements were obtained from tail blood samples using a blood glucose meter (Prestige Smart System HDI; Home Diagnostic, Fort Lauderdale, FL) in all groups of mice after 6 hours fast as previously described [15].
At the end of the treatment period, mice were anaesthetized with isoflurane and blood samples were collected from carotid artery into containing heparin tubes. Then, thoracic aorta was harvested immediately, placed in Physiological Salt Solution (PSS, composition in mM: NaCl 118; KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4x7H2O 1.2; NaHCO3 25 and glucose 11, pH=7.4) and processed appropriately for further studies.
In other series of experiment, we used 8 weeks-old double knock out between db−/db− and p50NFκB male mice (db−/db-p50NFκB−/−, n=5) and between db−/db− and PARP-1 male mice (db−/db-PARP-1−/−, n=5). The PARP-1 knockout mice were kindly provided by Dr. Alexandar Bishop (Department of Cellular and Structural Biology, University of Texas Health Science Center at San Antonio, San Antonio, TX 78229, USA). The p50NFκB knockout mice were purchased from Jackson laboratory. To generate double knockout, we have bred heterozygote db−/db+ with PARP-1 or p50NFκB knockout mice. Before sacrificing the animals, the body weight and blood glucose levels were measured. Then mice were anaesthetized with isoflurane and then thoracic aortas were immediately harvested and placed in PSS solution and processed appropriately for further studies.
Vascular Reactivity
Ex vivo experiments
Isometric tension recording
Thoracic aorta from control and diabetic mice were carefully cleaned of fat and connective tissue and then cut into rings (2 mm in length) and mounted in a small vessel dual chamber myograph for measurement of isometric tension. After a 30 min equilibration period in PSS solution bubbled with carbogen, at 37°C and pH=7.4, arteries were stretched to their optimal lumen diameter for active tension development. After a second 30 min equilibration period, the vessels were exposed to phenylephrine (PE, 10-5 M) and the presence of functional endothelium was assessed by the ability of acetylcholine (ACh, 10−6 M) to induce relaxation.
To determine the role of eNOS and COX-2 in the impaired endothelium-dependent relaxation in diabetic mice, aortas were incubated with NS 398 (10 μM), a COX-2 selective inhibitor, for 1h and then endothelium-dependent relaxation was performed after pre-contraction with PE.
To determine the role of eNOS and NADPH oxidase in the impaired endothelium-dependent relaxation in diabetic mice, aortas were incubated with L-NAME (100 μM) and apocynin (100 μM) for 30 minutes and then endothelium-dependent relaxation was performed after pre-contraction with PE.
The same protocol was used for double knockout db−/db− and p50 NFκB (db−/db-p50NFκB−/−), db−/db− and PARP-1 (db−/db-PARP-1−/−) male mice. After pre-contraction with PE (10−5 M) and the steady maximal contraction, cumulative dose-response curves were obtained for ACh (10−8−10−5 M) and SNP (10−8−10−5 M) in the presence or absence of NS-398, L-NAME and apocynin.
In vitro experiments
Thoracic aortas from diabetic mice were carefully cleaned of fat and connective tissue and then cut into rings (2 mm in length) and were mounted in a small vessel dual chamber myograph for measurement of isometric tension. Arteries were incubated with either p65NFκB shRNA lentiviral particle (Santa Cruz, Santa Cruz, CA) to down-regulate p65NFκB expression or AG1478 (LC Laboratories, Woburn, MA), EGFR tyrosine kinase inhibitor for 4 hours. After pre-contraction with PE (10−5 M) and the steady maximal contraction, cumulative dose-response curves were obtained for ACh (10−8−10−5 M) and SNP (10−8−10−5 M) in the presence or absence of L-NAME as described above.
Western blot analysis
Freshly isolated aortas from all groups were immediately frozen in liquid nitrogen and then homogenized in ice-cold lysis buffer as described previously [14, 8] Western blot analysis was performed for phosphorylated-Ser1177 and total eNOS (1:1,000 dilution; Cell Signaling, Boston, MA), phosphorylated and total p65NFκB (1:1,000 dilution; Cell Signaling, Boston, MA), cleaved and total PARP-1 (1:1,000 dilution; Cell Signaling, Boston, MA) and COX-2 (1:500 dilution, Santa Cruz, Santa Cruz, CA) using specific antibodies. Blots were stripped and then reprobed with either β-actin (1:2000 dilution, Santa Cruz, Santa Cruz, CA) or GAPDH (1:2000 dilution, Santa Cruz, Santa Cruz, CA) antibodies to verify the equal loading among the samples.
Drugs
Phenylephrine hydrochloride, acetylcholine, L-NAME, apocynin, NS398 were obtained from Sigma-Aldrich (St. Louis, MO). IKK-NBD peptide was purchased from Enzo Life sciences (Farmingdale, NY). The DHMEQ was synthesized by Dr. Kazuo Umezawa (Aichi Medical University, Nagakute, Japan).
Statistical analysis
Results are expressed as mean ± SEM. Concentration-response curves were analyzed using the GraphPad Prism 4.0 software (GraphPad software, La Jolla, CA). One-way or 2-way ANOVA was used to compare each parameter when appropriate. Comparisons between groups were performed with t- tests when the ANOVA test was statistically significant. Values of P < 0.05 were considered significant. Differences between specified groups were analyzed using the Student's t test (two-tailed) for comparing two groups with P < 0.05 considered statistically significant.
RESULTS
General parameters
Blood glucose levels and body weight were higher in db−/db− mice (393.7 ± 20.17mg/dl, 42.29 ± 0.57g respectively) with and without NFκB inhibitors, and in double knockout mice (db−/db-p50NFκB−/− and db−/db-PARP-1−/−) compared to db−/db+ mice (132.3 ± 0.89 mg/dl, 24.19 ± 0.48 g respectively) (Table 1).
Table 1.
Blood glucose and body weight measurements
| Body weight (g) | Blood Glucose (mg/dl) | |
|---|---|---|
| Control | 24.19 ± 0.48* | 132.3 ± 0.89* |
| Control + DHMEQ | 25.88 ± 1.06* | 141.6 ± 3.28* |
| Control + IKK-NBD peptide | 25.52 ± 0.81* | 140 ± 7.0* |
| db−/db− | 42.29 ± 0.57 | 393.7 ± 20.17 |
| db−/db− + DHMEQ | 41.33 ± 0.63 | 401.6 ± 20.12 |
| db−/db− + IKK-NBD peptide | 38.16 ± 0.42 | 400 ± 14.8 |
| db−/db-p-50NFkB-/- | 38.58 ± 1.90 | 340.03 ± 18.12 |
| db−/db-PARP-1-/- | 38.81 ± 2.11 | 380.42±11.44 |
Data (means ± SE)
Blood glucose and body weight measurements from control, type 2 diabetic mice (db−/db−) treated with and without DHMEQ or IKK-NBD peptide, double knockout mice db−/db-p50NFκB and double knockout db−/db-PARP-1.
P < 0.05 for control, control treated with DHMEQ or IKK-NBD vs. db−/db−, db−/db− treated with DHMEQ or IKK-NBD, db−/db-p50NFκB and double knockout db−/db-PARP-1.
NFκB and endothelium-dependent relaxation (EDR) in thoracic aorta
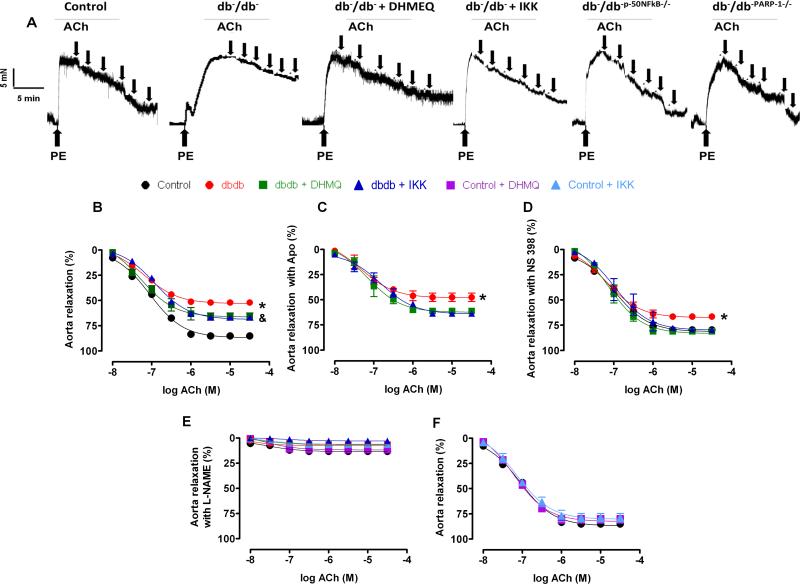
The endothelium-dependent relaxation (EDR) in thoracic aorta was impaired in db−/db− mice compared to db−/db+ mice (Figure 1A, B). Interestingly the inhibition of NFκB improved EDR in thoracic aorta from db−/db− mice (Figure 1A, B Table 2A). We did not observe any effect on EDR in thoracic aorta in db−/db+ mice after the inhibition of NFκB (Figure 1F). The inhibition of eNOS with L-NAME reduced EDR in all groups of mice (Figure 1E).
Figure 1. Effect of the NFκB inhibition on endothelium-dependent relaxation in thoracic aorta (n=10).
Key representative traces showing endothelium-dependent relaxation curves to ACh from control, and type 2 diabetic mice (db−/db−) treated with or without DHMEQ or IKK-NBD peptide and double knockout mice between db−/db− and p-50NFκB (db−/db-p-50NFκB−/−) and double knockout mice between db−/db− and PARP-1 male mice (db−/db-PARP-1−/−) (A). Endothelium-dependent relaxation in response to cumulative doses of ACh (10−8−3.10−5 M) in rings from aorta, pre-contracted with phenylephrine (PE, 10−5 M), from control, db−/db− treated with or without DHMEQ or IKK-NBD peptide (B) and incubated with apocynin (APO, NADPH oxidase inhibitor) (C) or NS 398 (COX-2 inhibitor) (D) or L-NAME (eNOS inhibitor) (E). Control, control treated with or without DHMEQ or IKK-NBD peptide (F). *P < 0.05 for db−/db− vs. control, db−/db− treated with DHMEQ or IKK-NBD. &P < 0.05 for db−/db− treated with DHMEQ or IKK-NBD vs. control.
Table II.
pD2 and Emax
| A | Aorta | + NS398 | Aorta | |||
|---|---|---|---|---|---|---|
| pD2 | Emax | pD2 | Emax | |||
| Control | 7.01 ± 0.05 | 85.21 ± 2.01 | Control | 6.98 ± 0.07 | 79.7 0 ± 1.90 | |
| db−/db− | 7.22 ± 0.06 | 52.21 ± 1.01 | db−/db− | 7.19 ± 0.08 | 66.47 ± 1.46 | |
| db−/db− + DHMEQ | 7.21 ± 0.12 | 65.91 ± 3.60 | db−/db− + DHMEQ | 7.14 ± 0.08 | 81.03 ± 1.26 | |
| db−/db− + IKK-NBD peptide | 6.93 ± 0.06 | 66.81 ± 1.81 | db−/db− + IKK-NBD peptide | 7.02 ± 0.07 | 79.59 ± 1.63 | |
| db−/db-p-50NFkB-/- | 6.97 ± 0.08 | 92.19 ± 2.51 | db−/db-p-50NFkB-/- | 7.17 ± 0.12 | 85.52 ± 0.99 | |
| db−/db-PARP-1-/- | 7.45 ± 0.10 | 73.14 ± 1.17 | db−/db-PARP-1-/- | 7.10 ± 0.08 | 79.59 ± 1.67 | |
| B | + APO | Aorta | Aorta | |||
|---|---|---|---|---|---|---|
| pD2 | Emax | pD2 | Emax | |||
| Control | 7.41 ± 0.15 | 66.10 ± 4.07 | db−/db− | 7.31 ± 0.10 | 37.10 ± 1.07 | |
| db−/db− | 7.33 ± 0.16 | 47.57 ± 4.16 | db−/db− + AG1478 | 7.23 ± 0.09 | 54.75 ± 2.76 | |
| db−/db− + DHMEQ | 7.21 ± 0.02 | 61.13 ± 0.06 | db−/db− + P65 shRNA | 7.41 ± 0.12 | 66.00 ± 1.96 | |
| db−/db− + IKK-NBD peptide | 6.83 ± 0.09 | 63.59 ± 0.73 | db−/db− + Scramb | 7.86 ± 0.27 | 40.97 ± 0.54 | |
Data (means ± SE)
PD2 and Emax for thoracic aorta from control, type 2 diabetic mice (db−/db−) treated with and without DHMEQ or IKK-NBD peptide, double knockout mice db−/db-p50NFκB and double knockout db−/db-PARP-1 in the absence (A) or presence of apocynin (APO) (B) and COX-2 inhibitor (NS398) (C).
PD2 and Emax for thoracic aorta from type 2 diabetic mice (db−/db−) in vitro incubated with EGFRtk inhibitor (AG1478) or p65shRNA or scramble shRNA (D).
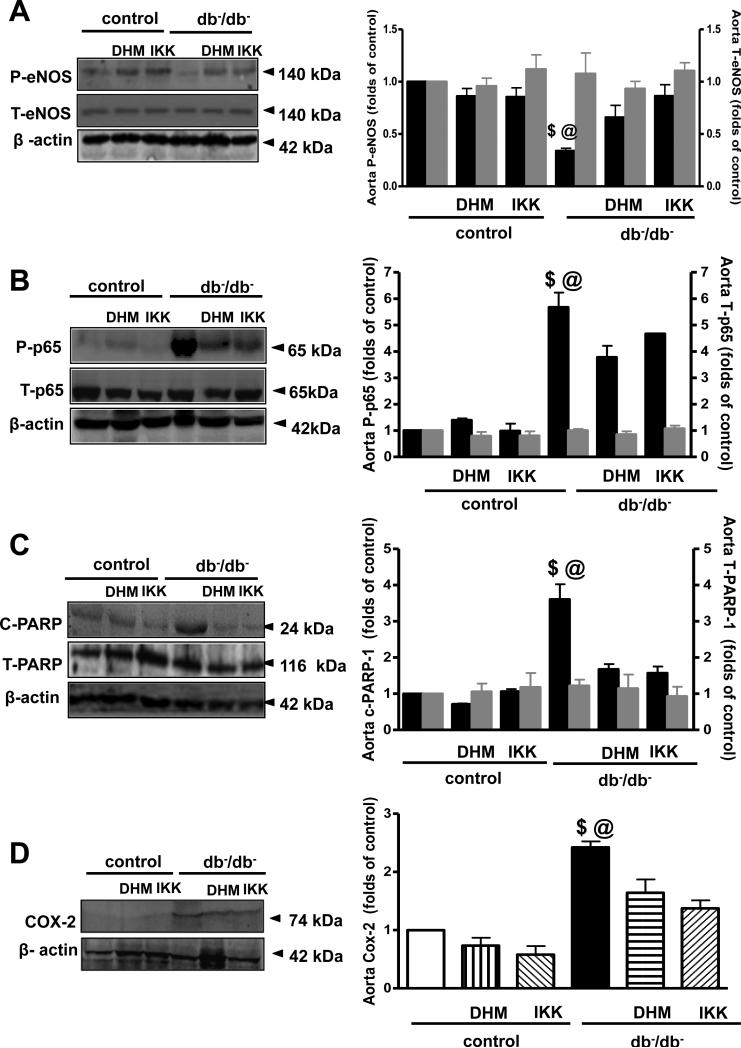
Our data demonstrated that the inhibition of NADPH oxidases by apocynin did not affect the EDR in the thoracic aorta (Figure 1C, Table 2B). However, the inhibition of COX-2 by NS398 significantly improved the EDR in thoracic aorta (Figure 1D, Table 2C). Western blot analysis revealed that phosphorylated eNOS was significantly reduced, while phosphorylated NFκB-p65 and cleaved PARP-1 and COX-2 expression were significantly augmented in thoracic aorta from db−/db− mice (Figure 2A, B, C, D). Interestingly, diabetic mice treated with NFκB inhibitors (DHMEQ and IKK-NBD peptide) enhanced eNOS phosphorylation but reduced NFκB-p65 phosphorylation, cleaved PARP-1 and COX-2 expression (Figure 2A, B, C, D).
Figure 2.
Western blot analysis and quantitative data (n=5), in homogenized aorta from control and type 2 diabetic mice (db−/db−) treated with or without DHMEQ or IKK-NBD, showing phosphorylated P-eNOS, total T-eNOS (A) phosphorylated P-p-65, total T-p-65 (B), cleaved c-PARP-1 and total T-PARP-1 (C), COX-2 (D) and β-actin. $P< 0.05 for db−/db− vs. control, control treated with DHMEQ or IKK-NBD, db−/db− treated with DHMEQ or IKK-NBD.
@P< 0.05 for db−/db− vs. db−/db− treated with DHMEQ or IKK-NBD
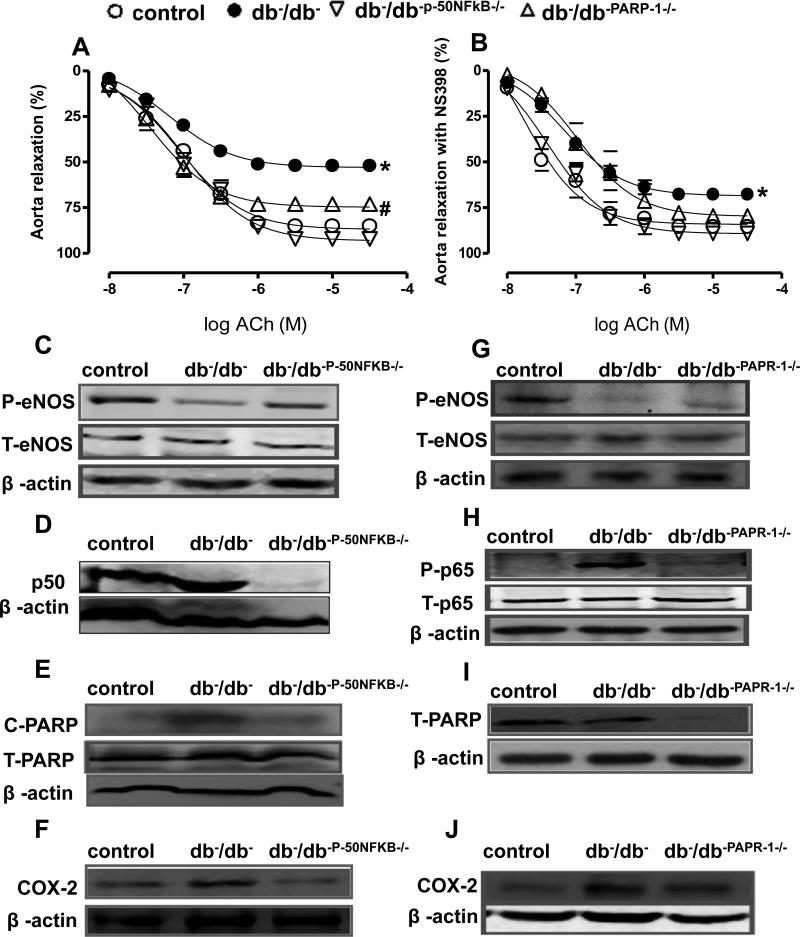
To strengthen our results, we examined the EDR in thoracic aorta from double knockout mice (db−/db- p50NFκB−/−). Western blot analysis confirmed the absence of p50NFκB in aorta from db−/db−p50NFκB−/− mice (Figure 3D). Furthermore, our data demonstrated that the EDR was significantly improved in the thoracic aorta isolated from db−/db- p50NFκB−/− mice associated with increased eNOS phosphorylation (Figure 3A, C Table 2A). In addition we observed that the EDR in thoracic aorta was significantly improved in double knockout db−/db-PARP-1−/− mice and arteries from db−/db− mice treated with COX-2 inhibitor (Figure 3A, B, Table 2C). These results were also associated with a reduction in cleaved PARP-1 and COX-2 expression in thoracic aorta from db−/db- p50NFκB−/− (Figure 3E, F). Furthermore, western blot analysis revealed that in the thoracic aorta from db−/db- PARP-1−/− mice, PARP-1 was absent and this was associated with reduced p65NFκB phosphorylation and COX-2 expression and an increase in eNOS phosphorylation (Figure 3G, H, I, J). Endothelium-independent relaxation in response to sodium nitroprusside (SNP) in thoracic aorta was similar in all groups (control, db−/db−, db−/db- p50NFκB−/− and db−/db−PARP-1−/− (data not shown) indicating that impaired EDR is related to bioavailability of nitric oxide rather than response of smooth muscle cells to nitric oxide.
Figure 3. Effect of the NFκB inhibition on endothelium-dependent relaxation in thoracic aorta (n=10).
Endothelium-dependent relaxation in response to cumulative doses of ACh (10−8−3.10−5 M) in rings from aorta, pre-contracted with phenylephrine (PE, 10−5 M), from Control, db−/db−, db−/db-p- 50NFκB−/− and db−/db-PARP-1−/−, (A) and incubated with NS 398 (COX-2 inhibitor)(B) *P < 0.05 for db−/db− vs. control, db−/db-p-50NFκB−/− or db−/db-PARP-1−/− #P < 0.05 for db−/db-PARP-1 vs. control, db−/dbp-50NFκB−/−.
Western blot analysis and quantitative data (n=5) in homogenized thoracic aorta from control, db−/db−, and double knockout mice between db−/db− and p50NFκB (db−/db-p-50NFκB−/−) showing phosphorylated (P)-eNOS, total (T)-eNOS (C) p50NFκB (D), cleaved (c)-PARP-1 and total (T)-PARP-1 (E), COX-2 (F) and β-actin, and double knockout mice between db−/db− and PARP-1 male mice (db−/db-PARP-1−/−), showing phosphorylated (P)-eNOS, total (T)-eNOS (G) phosphorylated (P)-p-65 and total (T)-p-65 (H), total (T)-PARP-1 (I), COX-2 (J) and β-actin.
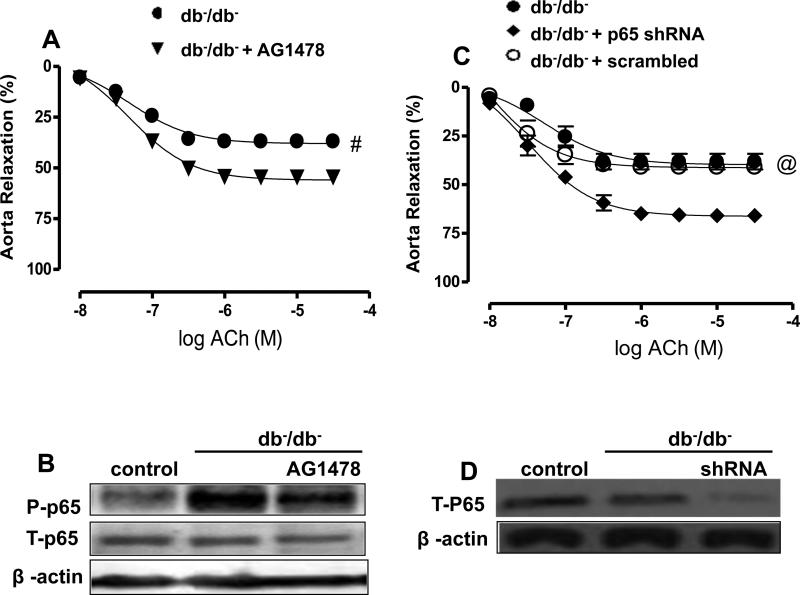
Effect of acute EGFR tyrosine kinase (TK) inhibition and down regulation of p65NFκB on thoracic aorta reactivity
The acute inhibition of EGFRtk significantly improved the EDR in isolated thoracic aorta from db−/db− mice (Figure 4A, Table 2D). The p65NFκB phosphorylation was reduced in thoracic aorta after the inhibition of EGFRtk (Figure 4B) indicating that p65NFκB is down stream to EGFRtk. The acute down regulation of p65NFκB in isolated aorta from db−/db− mice transfected with lentivirus-shRNA-p65NFκB significantly improved the EDR (Figure 4C, Table 2D). Western blot analysis confirmed the down regulation of p65NFκB in thoracic aorta transfected with lentivirus-shRNA-p65NFκB (Figure 4D). Endothelium-independent relaxation in response to sodium nitroprusside (SNP) in thoracic aorta showed no difference among groups (control, db-/db− and db−/db− transfected with p65NFκB shRNA lentiviral particles) (data not shown).
Figure 4. Effect of the EGFRtk inhibitor and p-65NFκB shRNA lenti-viral particles (n=5) on.
-Endothelium-dependent relaxation in thoracic aorta in response to cumulative doses of ACh (10−8−3.10−5 M) in rings from aorta, pre-contracted with phenylephrine (PE, 10−5 M), from db−/db− incubated with either AG1478 (EGFRtk inhibitor) or p-65NFκB shRNA lenti-viral particles (A, C). #P < 0.05 for db−/db− vs. db−/db− + AG1478. @P < 0.05 for db−/db−, db−/db− + scrambled vs. db-/db− + P 65 shRNA
Western blot analysis and quantitative data (n=5) in homogenized thoracic aorta from db−/db− incubated with either AG1478 (EGFRtk inhibitor) or p-65NFκB shRNA lenti-viral particles, showing P-p-65, T-p-65 (B, D) and β-actin.
DISCUSSION
In this study, we determined that enhanced NFκB pathway impairs conductance arteries endothelium-dependent relaxation in type 2 diabetic male mice. Thus, the inhibition of NFκB using pharmacology, molecular and genetic approaches significantly improved EDR in db−/db− mice. We also delineated that enhanced NFκB impaired endothelium-dependent relaxation in thoracic aorta by PARP-1 and COX-2-dependent mechanisms. These observations suggest that NFκB could be an important target to improve the conductance artery function in type 2 diabetes.
Diabetic vascular dysfunction is a major clinical problem that predisposes patients to a variety of cardiovascular diseases [16, 17, 18, 19]. Hyperglycemic episodes play a major part in the development of vascular endothelial dysfunction, which is an early factor preceding the arterial structural wall remodeling [20]. It has been documented that altered cyclooxygenases activity [21], increased oxidative stress levels [22], insulin resistance and inflammation are potentially through the induction of NFκB [ 23 ]. Although significant progress has been advanced in understanding the importance of NFκB [24, 25], less is known about NFκB in thoracic aorta endothelial dysfunction in type 2 diabetes.
Endothelium is an important organ regulating the vascular reactivity. Our results indicate that endothelium-dependent relaxation in thoracic aorta, which is highly dependent on the bioavailability of nitric oxide [26], was impaired in type 2 diabetes. These data are in agreement with previous studies [27, 28]. Bussy et al. showed that NFκB activity is increased after loss of nitric oxide [29]. Our data are in accordance with this study since we observed that impairment in endothelial function was associated with an increase in vascular NFκB activity. Importantly, the inhibition of NFκB activity significantly improved endothelium dependent relaxation in thoracic aorta associated with increased eNOS phosphorylation. The inhibition of NFκB did not affect blood glucose, insulin levels or body weight indicating that enhanced NFκB in conductance arteries is a consequence of type 2 diabetes.
Previous studies reported that active NFκB trans-locates into the nucleus, interacts with PARP-1 and binds to DNA [30]. We previously reported that the inhibition of PARP-1 activity significantly improved vascular function [11] in type 2 diabetes. Moreover, Dr. Veves group's showed activated PARP in subjects at risk for developing type 2 diabetes and impaired vascular reactivity [31]. Furthermore, it has been shown that PARP-1 inhibition exert beneficial effect against the development of cardiovascular complications in type 1 diabetes [32]. Interestingly, the inhibition of NFκB signaling reduced cleaved PARP-1, indicating the cross-talk between NFκB and PARP-1. Our data are in accordance with another study showing that, PARP-1 activation plays an important role in the diabetes-induced death of retinal capillary cells, at least in part via NF-kappaB [32]. Additionally, the inhibition of PARP-1 activity in db−/db− mice significantly improved vascular function. All together, these data reveal that NFκB regulates vascular function in type 2 diabetes by PARP-1-dependent mechanism. These data were strengthened with the use of double knockout db−/db-PARP-1−/−. Moreover, several studies, supporting our results, demonstrated the formation of a complex between NFκB and PARP-1 in the nucleus, which binds to DNA to modulate gene expression [33].
Another factor that plays an important role in vascular endothelial dysfunction in type 2 diabetes is COX-2. Our data indicate that vascular COX-2 expression was enhanced in thoracic aorta in db−/db− mice, which is in accordance with previous studies [34, 35]. It is well established that NFκB controls the expression of genes involved in the inflammatory response, such as COX-2. We observed that the inhibition of NFκB in db−/db− mice reduced COX-2 expression suggesting that COX-2 is downstream to NFκB. In addition, our in vitro study showed that acute inhibition of COX-2 improves thoracic aorta endothelium-dependent relaxation in db−/db− mice. These data indicate that the inhibition of NFκB improved thoracic aorta function by a COX-2-dependent mechanism in db−/db− mice. The role of COX-2 in vascular dysfunction in diabetes can also be independent of eNOS. Thus, previous studies reported a significant up-regulation of COX-2 in thoracic aortic VSMCs that contributes to enhanced contractile responses likely through TXA2 in type 2 diabetic mice [36]. Our data demonstrated that the inhibition of NFkB reduced COX-2 expression and improved thoracic aorta endothelium-dependent relaxation. These results indicate that COX-2 plays a role in impaired endothelium-dependent relaxation in thoracic aorta in diabetes.
Additionally others and we showed that epidermal growth factor receptor tyrosine kinase (EGFRtk) plays an important role in the regulation of resistance artery myogenic tone. Thus, elevated EGFRtk phosphorylation contributes to resistance arteries dysfunction in type 2 and type 1 diabetes [37, 38]. Our data indicate that in vitro acute inhibition of EGFRtk improved EDR and reduced p65NFκB phosphorylation, indicating that EGFRtk is upstream to NFκB.
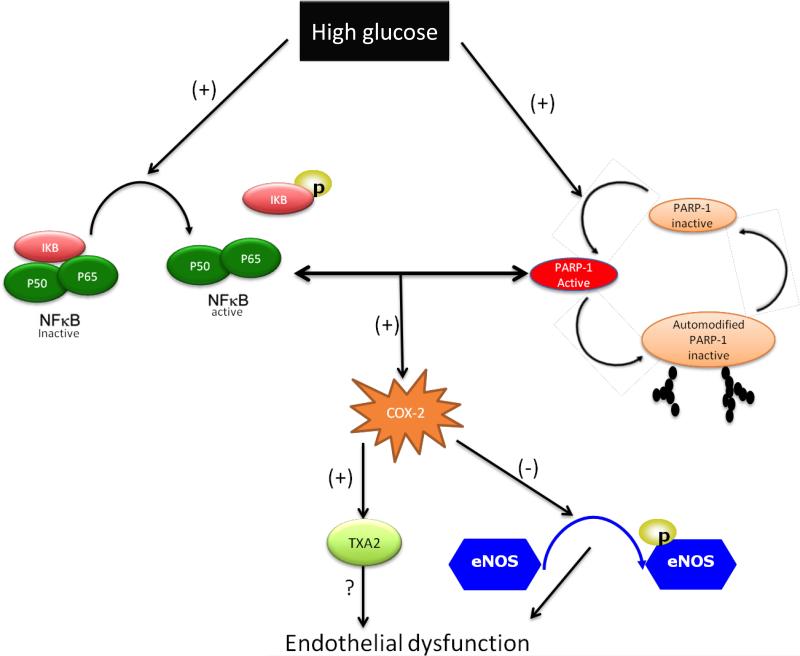
In conclusion, our in vivo and in vitro data clearly indicate that enhanced NFκB pathway impairs thoracic aorta endothelium-dependent relaxation in type 2 diabetes. We also previously demonstrated that augmented NFκB impairs resistance arteries endothelium-dependent relaxation in type 2 diabetic mice. Taken all together these evidences indicate that the NFκB pathway is not specific to one vascular bed and could be a potential target for a novel therapeutic strategy to reverse diabetes-induced vascular complication. Figure 5 showed the proposed mechanism by which enhanced NFκB causes endothelial dysfunction in thoracic aorta in type 2 diabetes.
Figure 5.
Representative schematic diagram of the proposed mechanism by which NFκB impairs thoracic aorta endothelium-dependent relaxation in type 2 diabetic mice.
PERSPECTIVES
Type 2 diabetes is a metabolic disease, characterized by hyperglycemia and insulin resistance, associated with vascular dysfunction. Diabetes induced-vascular complication is still growing. Therefore, the development of novel effective treatments for diabetic patients with vascular complications remains critical. Our data indicate that NFkB plays an important role in vascular dysfunction in type 2 diabetic mice. Importantly, the inhibition of NFkB activity improved vascular function by PARP-1 and COX-2 dependent mechanisms. Therefore, NFkB and its down stream signaling (PARP-1 and COX-2) could be potential targets for novel therapeutic strategies to overcome diabetes-induced vascular complications.
LIMITATION
The endothelial nitric-oxide synthase (eNOS) activity is regulated by multiple phosphorylation sites. The coordinated phosphorylation of eNOS at Ser1177 and dephosphorylation at Thr495 activates the enzyme, whereas inhibition results when Thr495 is phosphorylated and Ser1177 is dephosphorylated. However, Ser1177 can be phosphorylated along with other inhibitory residues that prevent the enzyme from being active and as consequence it reduces the amount of NO bioavailability. Recently other phosphorylation sites of eNOS have been reported, including the stimulatory Ser 635, Ser 617 sites, and the inhibitory Thr 495 and Ser 116 sites. Therefore, the eNOS phosphorylation should be associated with the measurement of NO bioavailability and vascular endothelium-dependent relaxation.
Another limitation of this work is the use of genetic model mouse (db−/db−). The human diabetes is typically a result of obesity, sedentary lifestyle, and uncontrolled diet. Diet-induced obesity and/or diabetes would be a more representative model. Therefore, the role of NFκB inhibition in mice fed with diet-induced obesity/diabetes is needed.
Acknowledgements
N/A
Sources of Funding
We acknowledge grant support from National Institutes of Health (1R01HL095566; PI: Dr. Matrougui) and (5R01HL097111; PI: Dr. Trebak)
Footnotes
Disclosures
None
REFERENCES
- 1.Ruderman NB, Williamson JR, Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992;6(11):S2905–2914. doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- 2.Cosentino F, Lüscher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol. 1998;32(3):S54–61. [PubMed] [Google Scholar]
- 3.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100(9):2153–2177. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4(1):53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59(2):415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- 7.Shanmugam N, Gaw Gonzalo IT, Natarajan R. Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes. 2004;53(3):795–802. doi: 10.2337/diabetes.53.3.795. [DOI] [PubMed] [Google Scholar]
- 8.Crofford LJ, Tan B, McCarthy CJ, Hla T. Involvement of nuclear factor kappa B in the regulation of cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum. 1997;40(2):226–236. doi: 10.1002/art.1780400207. [DOI] [PubMed] [Google Scholar]
- 9.Charalambous MP, Lightfoot T, Speirs V, Horgan K, Gooderham NJ. Expression of COX-2, NF-kappaB-p65, NF-kappaB-p50 and IKKalpha in malignant and adjacent normal human colorectal tissue. Br J Cancer. 2009;101(1):106–115. doi: 10.1038/sj.bjc.6605120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, Gersbach M, Imhof R, Hottiger MO. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280(49):40450–40464. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- 11.Choi SK, Galán M, Kassan M, Partyka M, Trbak M, Matrougui M. Poly(ADP-Ribose) Polymerase 1 Inhibition Improves Coronary Arteriole Function in Type 2 Diabetes Mellitus. Hypertension. 2012;59(5):1060–1068. doi: 10.1161/HYPERTENSIONAHA.111.190140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soriano FG, Pacher P, Mabley J, Liaudet L, Szabó C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- 13.Pacher P, Szabó C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal. 2005;7:1568–1580. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassan M, Choi SK, Galan M, Bishop A, Umezawa K, Trebak M, Belmadani S, Matrougui K. Enhanced NFκB Activity Impairs Vascular Function through PARP-1, SP-1 and COX2-Dependent Mechanisms in Type 2 Diabetes. Diabetes. 2013 doi: 10.2337/db12-1374. doi: 10.2337/db12-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi SK, Galán M, Partyka M, Trebak M, Belmadani S, Matrougui K. Chronic inhibition of epidermal growth factor receptor tyrosine kinase and extracellular signal-regulated kinases 1 and 2 (ERK1/2) augments vascular response to limb ischemia in type 2 diabetic mice. Am J Pathol. 2012;180(1):410–418. doi: 10.1016/j.ajpath.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 2005;109(2):143–59. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 17.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26(6):1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 18.Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46(6):760–765. doi: 10.1007/s00125-003-1116-6. [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 20.Tooke JE, Goh KL. Vascular function in Type 2 diabetes mellitus and pre-diabetes: the case for intrinsic endotheiopathy. Diabet Med. 1999;16(9):710–715. doi: 10.1046/j.1464-5491.1999.00099.x. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez A, Contreras C, Martínez P, Villalba N, Benedito S, Garcia-Sacristan A, Salaices M, Hernandez M, Prieto D. Enhanced cyclooxygenase 2-mediated vasorelaxation in coronary arteries from insulin-resistant obese Zucker rats. Atherosclerosis. 2010;213(2):392–399. doi: 10.1016/j.atherosclerosis.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Schuhmacher S, Oelze M, Bollmann F, Kleinert H, Otto C, Heeren T, Steven S, Hausding M, Knorr M, Pautz A, Reifenberg K, Schulz E, Gori T, Wenzel P, Munzel T, Daiber A. Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes. 2011;60(10):2608–2616. doi: 10.2337/db10-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Sacca L, Ferrannini E. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55(4):1133–1140. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzo O, Picatoste B, Ares-Carrasco S, Ramirez E, Egido J, Tunon J. Potential role of nuclear factor κB in diabetic cardiomyopathy. Mediators Inflamm. 2011;2011:652097. doi: 10.1155/2011/652097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Ayala E, Anderstam B, Suliman ME, Seeberger A, Heimburger O, Lindholm B, Stenvinkel P. Enhanced RAGE-mediated NFkappaB stimulation in inflamed hemodialysis patients. Atherosclerosis. 2005;180(2):333–340. doi: 10.1016/j.atherosclerosis.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 27.Palen DI, Matrougui K. Role of elevated EGFR phosphorylation in the induction of structural remodelling and altered mechanical properties of resistance artery from type 2 diabetic mice. Diabetes Metab Res Rev. 2008;24(8):651–656. doi: 10.1002/dmrr.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su J, Lucchesi PA, Gonzalez-Villalobos RA, Palen DI, Rezk BM, Suzuki Y, Boulares HA, Matrougui K. Role of advanced glycation end products with oxidative stress in resistance artery dysfunction in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28(8):1432–1438. doi: 10.1161/ATVBAHA.108.167205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995;76(6):980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- 30.Zerfaoui M, Errami Y, Naura AS, Suzuki Y, Kim H, Ju J, Liu T, Hans CP, Kim JG, Abd Elmageed ZY, Koochekpour S, Catling A, Boulares AH. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J Immunol. 2010;185(3):1894–1902. doi: 10.4049/jimmunol.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabó C, Zanchi A, Komjáti K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabó E, Szabó C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 33.Ménissier de Murcia J, Ricoul M, Tartier L, Niedergang C, Huber A, Dantzer F, Schreiber V, Ame JC, Dierich A, Le Meur M, Sabatier L, Chambon P, de Murcia G. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003;22(9):2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagi Z, Erdei N, Toth A, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol. 2005;25(8):1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Lei T, Chen X, Peng Y, Long H, Zhou L, Huang J, Chen Z, Long Q, Yang Z. Resistin up-regulates COX-2 expression via TAK1-IKK-NF-kappaB signaling pathway. Inflammation. 2010;33(1):25–33. doi: 10.1007/s10753-009-9155-x. [DOI] [PubMed] [Google Scholar]
- 36.Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, Gong MC. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67(4):723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57(6):1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benter IF, Yousif MH, Griffiths SM, Benboubetra M, Akhtar S. Epidermal growth factor receptor tyrosine kinase-mediated signalling contributes to diabetes-induced vascular dysfunction in the mesenteric bed. Br J Pharmacol. 2005;145(6):829–836. doi: 10.1038/sj.bjp.0706238. [DOI] [PMC free article] [PubMed] [Google Scholar]