Summary
Our global hypothesis is that atopic conditions and asthma develop because an individual’s immune system is not able to appropriately resolve inflammation resulting from allergen exposures. We propose that the failure to appropriately down-regulate inflammation and produce a toleragenic state results primarily from less robust immune homeostatic processes rather than from a tendency to over-respond to allergenic stimuli. An individual with lower immune homeostatic capacity is unable to rapidly and completely terminate, on average over time, immune responses to innocuous allergens, increasing risk of allergic disease. A lack of robust homeostasis also increases the risk of other inflammatory conditions, such as prolonged respiratory viral infections and obesity, leading to the common co-occurrence of these conditions. Further, we posit that the development of vigorous immune homeostatic mechanisms is an evolutionary adaptation strongly influenced by both 1) exposure to a diverse maternal microbiota through the prenatal period, labor and delivery, and, 2) an orderly assemblage process of the infant’s gut microbiota ecosystem shaped by breastfeeding and early exposure to a wide variety of ingested foods and environmental microbes. This early succession of microbial communities together with early allergen exposures orchestrate the development of an immune system with a robust ability to optimally control inflammatory responses and a lowered risk for atopic disorders.
Keywords: allergy, homeostasis, immune response, environment, microbes, microbiota, dog, cat, home, birth, gastrointestinal tract, delivery
Introduction
There has been an increasing research focus in recent years on the prenatal and post-natal period as being fundamentally critical for not just the child’s immunological, neurological and metabolic development but also for the later occurrence of adult diseases and healthy aging. The developmental origins of health and disease (DOHaD) hypothesis suggests that a child’s environment from conception through 1000 days (approximately age 2 years), together with her or his genetic makeup, greatly influences a child’s subsequent risk for chronic disease.1
The most radical and rapid changes in the human immune system take place in the first two years of life. The infant immune system is exquisitely adapted to shift rapidly from a more toleragenic status in utero to a state capable of discriminating between helpful and harmful microbes post-delivery. The post-natal immune system must allow mutualistic organisms to assemble and colonize the various body niches while at the same time recognizing pathogens and fostering the latter’s containment and destruction during development and beyond.2, 3 The initial tasks of the neonate’s developing immune system are to: 1) distinguish self from non-self, 2) distinguish potentially damaging from innocuous or helpful antigens (such as food-related molecules and mutualistic microbes), and 3) develop an optimal inflammatory response for protection against pathogens. There is increasing recognition that in addition to rapidly and effectively responding to pathogens, the immune system must also develop an optimal capacity to rapidly and effectively halt and resolve inflammatory responses.4
Immense efforts have been made to better understand the immune mechanisms responsible for allergic diseases, especially allergic asthma. These efforts have been largely based upon an assumption that a specific pattern of immune response leads to an allergic phenotype. This allergic phenotype is often described as an atopic march from early food allergen sensitization to atopic dermatitis, and subsequently to allergic asthma and allergic rhinitis.5, 6 We believe that this assumption is incorrect, leading to results difficult to reproduce or even conflicting between well done studies. Our central hypothesis is that while the immune systems of humans may vary in the strength of initial immune responses to any neo-antigen, it is more important to understand the variation in the rate and extent to which new immune responses are modified and resolved.7 We theorize that it is this capacity, or more accurately, diminished capacity, that is the underlying mechanism that leads to one or more of the “allergic march” outcomes, rather than a sequential causal pathway of one atopic condition following another.
Much research into the development of early allergic sensitization and atopic disorders thus far has focused on a potential tendency during childhood to over-react to antigen exposure and the risk factors or immune pathways contributing to the propensity to over-react. However, the results of these studies have not provided clear answers to the enigma of allergic disease. All humans regularly encounter multiple immune stimuli every day including many allergens. Everyone’s immune system will initially recognize an allergen as foreign, stimulating an initial innate immune response. We propose that it is not primarily the tendency to over-react with a Th2 type response that distinguishes allergic from non-allergic children but rather a relative deficiency in ability to return to immune homeostasis. This deficiency results in a failure to halt an initial Th2 response in a timely fashion, which allows continuing production of IgE. The critical factor determining whether an allergic disease will follow such stimulation is how quickly and effectively the immune response is modified and resolved. Prompt resolution depends on the strength of homeostatic mechanisms and the absence of injury signals.7 In addition, a sub-optimal capacity for an immune reaction to return to a homeostatic state renders a child to be more susceptible to other consequences of sustained inflammation that have been associated with allergic diseases, such as prolonged and severe symptoms related to respiratory infections.
We hypothesize that the development of this immunologic capacity is strongly influenced by the carefully timed assemblage and composition of the early infant gut microbiota, which produces multiple metabolites capable of influencing the function of different types of immune cells. And what drives this evolutionarily designed microbiota ontogeny? The formation of the infant gut microbiota appears to be most strongly influenced by birth characteristics, postnatal diet and environmental exposures.8–10 Investigators are now capitalizing on recent advances in 16S rRNA gene sequencing and array technologies, which allow culture independent analyses of microbiotas on a large scale basis,11, 12 to explore what factors influence the human microbiota and how the human microbiota impacts human physiology. The large data sets resulting from these analyses bring complex analytic challenges but are already yielding scientific advances related to health and disease.
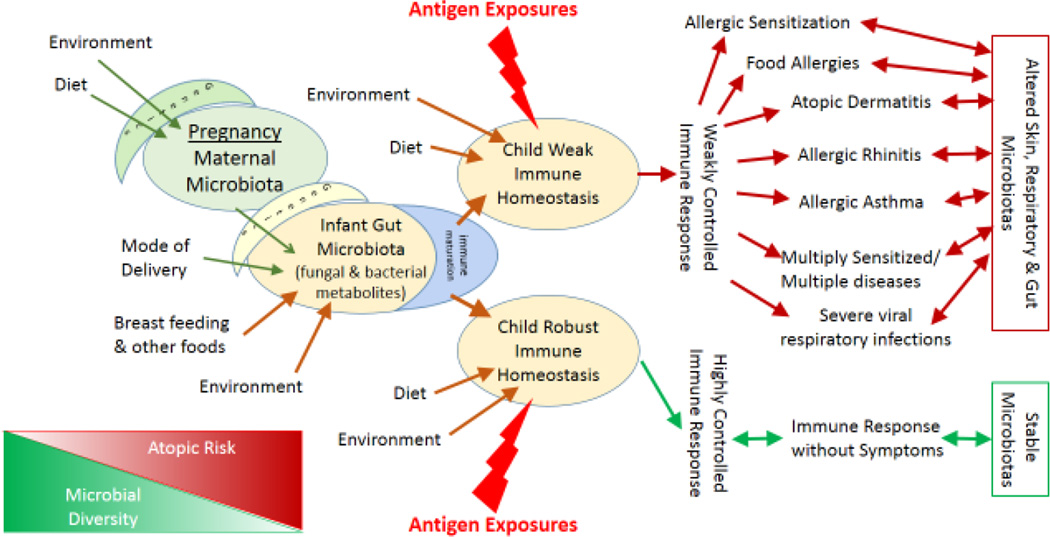
This perspective will focus on prenatal and early postpartum exposures, the infant gut microbiota, and the subsequent development of immune homeostasis which reduces allergic disease incidence. A conceptual diagram depicting our hypothesis is presented in Figure 1. We will proceed herein to elaborate on the various steps in this proposed causal pathway. The largest immune organ in the body, with constant exposure to non-self antigens, is the gastrointestinal tract, and it contains about 65% of the body’s immunologic tissues and an estimated 80% of the immunoglobulin producing tissues.13 Multiple studies have suggested that an infant’s early life microbiota ecology may be linked to the development of allergic disorders,14–20 with the first emerging from Scandinavia over a decade ago.21, 22 We and others believe that this complex pathway can be influenced by multiple environmental and dietary exposures. Many of these exposures, which have been implicated as allergy-related in various epidemiological studies, can likely shift the trajectory of a child’s immune development. Perhaps the consistency of inconsistencies across previous studies can be partially accounted for by the differences in the degree to which the various analyses have considered these influential exposures. Equally possible is the hypothesis that some exposures may only be important with respect to the infant’s microbiota during certain developmental stages, meaning that variable timing of observations may have led to different findings.
Figure 1.
Conceptual Causal Diagram: Factors related to Early Life Infant Gut Microbiota and Immune Homeostasis and Relationships to Outcomes
Environment Microbiota and Diet: Impact on the Maternal Gut and Vaginal Microbiotas
The left side of Figure 1 indicates that the pregnant mother’s genetics, environment and diet each impact her gastrointestinal and vaginal microbiotas, which later provide the de novo colonizers for the newborn, at least through the delivery mode to which humans have adapted.23 The adult human gut microbiota is comprised primarily of bacteria representing a relatively small number of highly conserved phyla, yet varies substantially by individual and is relatively resistant to perturbation.24, 25 Murine and human studies have demonstrated that the host’s genetic traits can influence gut microbiota composition.25, 26 Other studies have evaluated the vaginal microbiota in healthy pregnant and non-pregnant women, with pregnant women demonstrating a relatively stable bacterial community composition dominated by lactobacilli throughout pregnancy.23, 27 Studies have also found that characteristics of the home environment and its residents can affect the microbial and fungal load in that environment,28–33 but few studies have examined how the environmental micro- and mycobiotas relate to the maternal human microbiota. Recent reviews focus on the plasticity of the adult microbiota and on studies that demonstrate that diet and bacteria in the environment can impact gut microbial community composition, in particular at the lower phylogenetic level.24, 34, 35 For example, a recent intervention study has shown that cigarette smoking has a powerful effect on the human gut microbiota.36
Maternal Microbiota, Mode of Delivery, Breast Feeding, Food Introduction, Medications and the Environment: Impact on the Baby’s Gut Microbiota
As one editorial cleverly stated, when a baby is born, the mother delivers twice: the baby and the pioneer species of the baby’s de novo microbiota.37 Transformations in medical and cultural practices and the environment in the last 30–60 years likely have resulted in dramatic alterations in the patterns of microbial residents in the baby’s gastrointestinal tract, leading to what has been termed a gut “Microbial Dysbiosis”38, 39 or an altered gut microbial ecosystem. The alteration of the balance refers to a comparison with the microbes that humans have evolved with over millennia and that are still the norm in less well developed societies.12
Moving to the right in Figure 1, mode of delivery has been shown to have a primary influence on the baby’s gut microbiota in several studies, with one study demonstrating that 72% of the species in the baby’s stool matched the mother’s fecal microbiota in vaginal deliveries compared to 41% in babies delivered by cesarean section.9 Newborns delivered vaginally have initial microbial inocula resembling maternal vaginal and fecal microbes but those delivered by caesarean section show microbes predominantly coming from skin and environmental sources.40, 41 The initial differences in microbial community composition found with C-section babies were a more heterogeneous gut microbiota with fewer Bacteriodes. This difference was sustained through 12 months of age.9
Breast milk is an exquisite example of Darwinian dynamics since it provides many different elements to enhance infant survival. Breast milk serves as a buffer to keep the baby’s gastrointestinal tract at a higher pH, allowing for greater absorption of nutrients and survival of bacteria passing through to the lower gut.8 Human milk is additionally composed of a human milk microbiota, primarily Proteobacteria and Firmicutes, and pre-biotic components (such as human milk oligosaccharides) that stimulate bacterial growth and are bifidogenic, as well as antimicrobials (such as IgG, IgM and secretory immunoglobulin A).8, 13 Recent work suggests that it is not the initiation of solid food but the cessation of breast feeding that begins to shift the infant’s gut microbiota to an adult pattern.9
Antibiotic use has the potential of resulting in a transient or permanent change in the infant’s gut microbiota composition, which, depending on timing, could have long term consequences on the immune system and health.8 Penders et al. found that antibiotic use resulted in a decreased number of bifidobacterium and Bacteroides.42
Interestingly, and contrary to what seems intuitive, it appears that more bacterially diverse environments and exposures promote a less diverse initial neonatal gut microbiota that is dominated by species from the phyla Proteobacter and Actinobacter. From these initial colonizers, the gut microbiota community assembles over the next 2–3 years to a highly diverse adult microbiota dwarfed by bacteria belonging to the phyla Bacteroidetes and Firmicutes.9 Nylund et al, using microarrays, were able to show in a small sample that children with atopic dermatitis were more likely to have an early microbiota at 18 months that was more diverse.43
Baby’s Gut Microbiota: Impact on Baby’s Immune Homeostasis Development
The role of the gut microbiota in the development of both immune responsiveness and homeostasis is being studied both in humans and in mice. Germ-free mice have long been known to have poorly developed immune systems.44 Intestinal mucosa defenses including gut associated lymphoid tissues (GALT), as well as the cellular and molecular immune functions of the gastrointestinal system, are compromised in germ-free animals.45 Extra-intestinal immune responses are also less robust, implying effects on the entire immune system and not just effects on the GALT.46 Germ-free mice also have reduced numbers and function of T regulatory (Treg) cells in the mesenteric lymph nodes and Peyer’s patches. These reductions in Treg cells are thought to represent a decrease in induced Treg cells associated with lower levels of IL10.47, 48 Thus germ-free mice show deficiencies in both their ability to produce robust immune responses and in their ability to stop and resolve responses.
In studies using IL4ra gain-of-function mutant mice, which have an increased susceptibility to allergic sensitization, these mice were found to have different gut microbiota than allergy resistant mice.49 Upon moving gut microbiota from the allergic mice to the allergy-resistant mice, food allergy could be generated in the latter. Transferring Treg cells into the IL4ra mice suppressed sensitization. Yamashita et al. introduced Treg cells from allergy-resistant mice into naïve mice and found that subsequent attempts at sensitization were suppressed, suggesting that the gut microbiota’s impact is on Treg capacity.50
Studies of immune mechanisms in humans are more limited for many reasons. However, epidemiologic data has typically agreed with and supported murine studies showing strong interactions between the gut microbiota and the immune system. In addition to the early reports showing large reductions in allergic risk associated with early animal or endotoxin exposure are studies comparing allergic outcomes between children delivered vaginally and by C-section.51–53 Culture-independent studies have shown that the gut microbiotas of children differ by mode of delivery. Those born vaginally have initial gut microbiotas resembling the maternal vaginal and fecal microbiotas, while those born by C-section have microbiotas resembling maternal skin microbiotas.40 Children born by C-section also have a higher risk of childhood asthma suggesting that the difference in early microbial acquisition influences the risk of asthma. A study by Havstad et al.54 demonstrated that maternal exposure to indoor pets during pregnancy influenced the trajectory of IgE development during the first 2 years of life with pet exposure leading to a lower trajectory. This protective relationship of pet exposure related to total IgE was more marked among children delivered by C-section, suggesting that the diverse environmental microbiota associated with pets might have a greater impact in children lacking the large maternal inoculum of vaginal and fecal microbes.
Another study of probiotics supports the concept that children born by C-section are more easily influenced by external sources of bacteria.55 In a double-blind, placebo-controlled intent-to-treat analysis of prebiotic and probiotic supplementation in 1018 mothers with infants at high risk of allergy, no significant treatment changes were observed in the total population of infants for IgE sensitization, eczema, allergic rhinitis and asthma at 5 years of age. However, among children delivered by C-section there was a significant reduction in IgE-associated disease (24.3% versus 40.5%, 95% CI 23% to 96%, P=.035). This study again suggests that the initial inoculum from the mother’s vaginal and gut microbes is a dominant factor controlling the development of the gut microbiota.
The critical nature of the perinatal period has been shown in some studies. An examination of the relationship between pet exposure and IgE outcomes in a birth cohort followed to 18 years of age found that pet exposure only affected IgE if the pet was present during the first year of life.56 Pet exposure during the first year was still associated with IgE production at age 18 years. The necessity for exposure to important gut microbes in early life was also shown in a study of germfree mice who were conventionalized and compared to conventionally-raised mice.46
A critical question is whether the reductions in allergic disease associated with animal or probiotic exposure are really the result of bacterial transfer? A study strongly suggesting that the effects are related to bacterial exposure was done in mice. Mice were gavaged with either dust from homes with or without dogs before being immunized using a protocol designed to produce allergic airway disease.57 Mice given dust from homes with dogs were significantly protected from airway disease as assessed in multiple ways including airway resistance, cytokine production and inflammatory cell numbers. Culture-independent analysis of the bacteria from the caeca of the mice showed that a small number of bacterial taxa were dramatically increased in the mice given the dust from homes with dogs. One of the bacteria showing a dramatic increase was Lactobacillus johnsonii. When live but not killed L. johnsonii was given to mice much of the protection from sensitization was present. These studies suggest that the protective effect related to the dust from homes with dogs is due to live bacteria in the dust. The studies also suggest that live bacteria produce some metabolites capable of modulating immune responses in young adult mice.
Studies demonstrating protection of mice from allergic sensitization by gavage with live bacteria immediately raise of the question of how bacteria influence the immune system. Multiple possible mechanisms have been suggested concerning the link between gut bacteria and immune development. Murine studies have demonstrated that a prominent human gut commensal, Bacteroides fragilis, produces polysaccharide A which suppresses inflammation by down-regulating Il-17 production and promoting the development of inducible Tregs that produce IL10 in the gut during commensal colonization.58 This same bacteria species was found to be inversely associated with TLR4 mRNA expression among a population of 64 Swedish infants, and correspondingly, the down-regulation of PBMC response to LPS, with lower production of the inflammatory cytokines and chemokines IL-6, IL-8, IL-17 and CCL4.59 In this same study, Bifidobacterium colonization was directly associated with salivary secretory IgA levels, which have been associated with lower risk for allergy.59 Increased diversity of bifidobacteria in the first months of life was associated with higher levels of salivary secretory IgE and down-regulation of LPS-induced production of inflammatory cytokines and chemokines.59
Another possible source of immune modulation is short-chain fatty acids (SCFAs). SCFAs are produced by multiple microbes in the process of fermenting dietary fiber.44 SCFAs bind the G-protein coupled receptor 43 (GPR43, also referred to as FFAR2) and profoundly affect inflammatory responses. A recent study demonstrated that stimulation of GPR43 by SCFAs was necessary for normal resolution of some inflammatory responses because GPR43-deficient (Gpr43−/−) mice failed to resolve inflammation in models of asthma, arthritis and colitis.60 GPR43 deficient mice had dramatically significantly higher composite scores of inflammation in lungs after ova albumin sensitization.
Another study implicating SCFAs found that the production of cathelicidin-related antimicrobial peptide (CRAMP) was defective in non-obese diabetic (NOD) mice.61 The administration of CRAMP to NOD mice still in the pre-diabetic stage induced regulatory immune cells dampening the incidence of autoimmune diabetes. In the NOD mice the production of CRAMP was controlled by SCFAs. A second SCFA produced by gut fermentation of fiber, butyrate, was found to induce the differentiation of colonic Treg cells.62 Butyrate was found to induce differentiation of Treg cells in vitro and in vivo and to dampen colitis induced by cell transfer into Rag1−/− mice. Exposure of naïve T cells to butyrate enhanced histone H3 acetylation in the promoter region of the Foxp3 locus. Each of these studies demonstrates that metabolic products from gut bacteria can enhance immune homeostasis and that the loss of these products or the inability of the animal to utilize these products is associated with enhanced inflammation.
These relationships between bacterial fermentation products and immune homeostasis lead to the question of whether increasing dietary fermentable fiber would alter immune function. In a study in which mice were fed either high or low fiber diets, the mice given the high fiber diets were protected against allergic inflammation in the lung.63 The gut and lung microbiota were altered by the dietary change as were circulating levels of SCFAs. The investigators also demonstrated that treatment of mice with the SCFA propionate led to alterations in bone marrow hematopoiesis, with increased production of macrophage and dendritic cell (DC) precursors. As these increased DCs moved into the lungs of the mice they had high phagocytic capacity but a reduced ability to promote T helper type 2 (Th2) cell function. Interestingly the effects of propionate on allergic inflammation were dependent on G protein-coupled receptor 41 (GPR41) but not GPR43. Together the findings of this study show that the quantity of dietary fermentable fiber and SCFAs can shape the immunological environment in the lung and influence the severity of allergic inflammation. These findings from mice are interesting and suggestive considering that over the last decades a low fiber diet has been strongly associated with populations residing in well-developed countries.64
Certain T regulatory cells express the transcription factor Foxp3 (Foxp3+ Tregs). These Tregs are important for promoting tissue homeostasis in several settings, however, a recent study found that symbiotic members of the human gut microbiota induce a distinct Treg population in the mouse colon through the transcription factor Rorγ.65 This is unexpected because Rorγ has been though to antagonize Foxp3 and to promote T helper 17 (Th17) cell differentiation. The Rorγ Tregs are important in the control of Th1/Th17 inflammation in the colon.
Another exposure common to well-developed countries where allergic disorders have been more prevalent is the use of antibiotics. Many children receive antibiotics during the first year of life and many of the courses of antibiotics are for inappropriate indications, such as viral respiratory infections.66 Studies examining the relationship of antibiotic receipt in the first year of life and allergic disease outcomes have not been entirely concordant but they do consistently suggest an increased risk of disease associated with antibiotics. A study of antibiotic prescriptions during the first 6 months of life in a birth cohort of 725 children found that atopy increased with antibiotic use approached statistical significance (aOR 1.48; 95% CI 0.94–2.34, P=.09).67 However, in children with less than two pets in the home the risk of antibiotics was significant (aOR 1.73: 95% CI 1.07–2, 80, P=.024). Similarly, a retrospective study using data from a large employer health plan (n=62,576) found that antibiotic use in the first year of life was associated with a significant increase in transient wheezing and persistent asthma (OR 1.6; 95% CI 1.5–1.7, P<.001).68 A smaller population-based study of 1206 seven and eight year old children found that antibiotics in the first year of life was significantly associated with asthma (OR 1.7, 95% CI 1.0–3.1), hay fever and eczema.69 These results all support the hypothesis that interfering or altering the formation of the gut microbiota during the first year of life with antibiotics increases the risk of later asthma at least in some subsets of children.
Baby’s Immune Homeostasis: Impact on Atopic Disease Development, Incidence of Severe Respiratory Infections and Obesity
Moving to the right side of Figure 1 and to the cornerstone of our hypothesis, we propose that a weak immune homeostasis underlies not only allergic sensitization and asthma but also other health problems that are often thought to be intertwined with allergy. Children with allergic disease often appear to have more and more severe viral respiratory infections.70 In the previously mentioned study in which mice were gavaged with dust either from homes with or without dogs, the mice given the dust from homes with dogs were not only protected against allergic asthma but also they developed much less lung pathology after infection with respiratory syncytial virus (RSV).57 Whether the reduction in RSV-related pathology was due to greater immune homeostasis or a secondary effect of reduced serum IgE was not examined. Others have shown that the persistence of some viral infections is dependent on interferons that are in turn produced in association with the correct gut microbiota.71
Obesity is another increasingly common problem that can be related to the gut microbiota.72, 73 Multiple studies in both humans and mice show strong relationships between the gut microbiota and obesity.
EXPERT COMMENTARY
We believe that data strongly support an important causal link between the initial development of the gut microbiota in humans and the development of robust immune homeostasis. If this twin development of gut microbiota and immune homeostasis does not occur in the first year of life an individual is left at higher risk for adverse health outcomes. At this stage it is not possible to directly evaluate the gut microbiota and predict a health outcome. Clearly this is too simplistic a view since genetics, environmental exposures, and possibly many other variables come in to play to elicit a health outcome years later. The important message is that many adverse outcomes, including allergic disorders, may all have a central pathogenic factor; a dysbiotic gut microbiota in very early life. The contribution of these adverse outcomes to a reduced quality of life over the lifespan justifies the current intense study of the relationships between environmental and human microbiotas and health.
It is important to translate research findings into clinical recommendations as rapidly as prudently possible. While it seems that efforts to broaden microbial exposure in early life might help prevent allergic disease, there has been little effort to examine potential risks of certain microbial exposures associated with reduced allergy, such as unpasteurized milk.74, 75 Based on the major effects of the gut microbiota and the current paucity of understanding, it is impossible as yet to provide well substantiated recommendations related to using the rapidly accumulating findings of gut-microbiota interactions to prevent disease in humans.
5 YEAR VIEW
The immense costs of allergies and asthma to society provide strong incentives to develop a comprehensive understanding of the pathogenesis of these maladies, which will allow both better therapeutic approaches but, importantly, successful preventive methods. Increasingly refined explanations of how the immune system interacts with gut microbes offers great hope for new preventive strategies. Current clinical trials of pre- and pro-biotics for prevention of allergic disease and asthma will multiply in number and sophistication as knowledge builds. As understanding escalates it will also lead to attempts to influence immune homeostasis by supplementing the diet with anti-inflammatory substances produced by externally cultured microbes. Vast new fields of inquiry will surround not just bacterial interactions but also the interactions between fungi and viruses in the gut with their own kind and with all of the other microbes in the gut environment. Discernment of the microbial interactions in different segments of the gastrointestinal tract will also expand. Other areas likely to be critically important will be insights as to how human genetics and epigenetics influence the interactions between the gut microbiota and the immune system. Also, our recognition of how external influences such as housing and social characteristics, cigarette smoking, antibiotics and other medications and diet affect microbe-immune interactions will intensify. Many of these findings related to allergies and asthma will provide extensive cross-fertilization with the elucidation of other disease processes such as: obesity, diabetes (types 1 & 2), inflammatory bowel disease, response to vaccines and perhaps autism and some forms of cancer. The accumulation of new knowledge related to immune-microbiota interactions will radically transform efforts to maintain health and to treat disease. This will be a major new epoch in medicine perhaps surpassing the development of antibiotics.
KEY ISSUES.
Immune homeostasis, or the ability to halt and resolve immune responses, is critical to health. If the immune system fails to adequately stop and resolve an inflammatory response, chronic disease may result.
The balance between immune responsiveness and homeostasis is strongly influenced by the gut microbiota established during infancy when the immune system is developing and relatively plastic.
During infancy the gut microbiota is unstable and highly susceptible to external influences. The strongest influences on the developing gut microbiota are the intensity and diversity of microbial exposures including: mode of delivery, diet (especially breast feeding), and exposure both to foods with high live microbial content and a home environment with high microbial diversity.
The immune plasticity of infancy steadily declines as a child grows into adulthood, but the strong interactions between the gut microbiota and immune functions remain.
There are multiple ways in which the gut microbiota and immune system interact. Dominant interactions appear to be through many microbial metabolites that directly influence the gut environment, such as mucous production, and others that influence immune responses by influencing the activities of cells initiating immune responses, such as dendritic cells.
The lungs are constantly exposed to a wide variety of external irritants and immune stimuli. When homeostatic functions are inadequate to completely resolve responses to innocuous stimuli, such as allergens, chronic inflammation can develop leading to disease, such as asthma.
Even after the gut microbiota is established external influences such as diet and antibiotics may alter the interactions between the gut microbiota and the immune system. Diet can shift the relative proportions of specific bacteria and also alter their metabolism; increasing or decreasing the production of specific metabolites.
A better understanding of the mechanisms in which the gut microbiota alters immune function will lead to many therapeutic options for inflammatory diseases such as asthma and type 1 diabetes.
Acknowledgments
This work was funded by the National Institutes of Health, USA (P01 AI089473).
References
- 1.Barker D, Barker M, Fleming T, Lampl M. Developmental biology: Support mothers to secure future public health. Nature. 2013 Dec 12;504(7479):209–211. doi: 10.1038/504209a. [DOI] [PubMed] [Google Scholar]
- 2.Goenka A, Kollmann TR. Development of immunity in early life. Journal of Infection. 71:S112–S120. doi: 10.1016/j.jinf.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 3.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014 Jul;35(7):299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 2010 Aug;105(2):99–106. doi: 10.1016/j.anai.2009.10.002. quiz 07-9, 17. [DOI] [PubMed] [Google Scholar]
- 6.Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. 2014 Jan;69(1):17–27. doi: 10.1111/all.12268. [DOI] [PubMed] [Google Scholar]
- 7.Ohnmacht C, Marques R, Presley L, Sawa S, Lochner M, Eberl G. Intestinal microbiota, evolution of the immune system and the bad reputation of pro-inflammatory immunity. Cell Microbiol. 2011 May;13(5):653–659. doi: 10.1111/j.1462-5822.2011.01577.x. [DOI] [PubMed] [Google Scholar]
- 8.Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015 May 13;17(5):553–564. doi: 10.1016/j.chom.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015 May 13;17(5):690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013 Apr;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 11.Ye Y. Identification and Quantification of Abundant Species from Pyrosequences of 16S rRNA by Consensus Alignment. Proceedings (IEEE Int Conf Bioinformatics Biomed) 2011 Feb 4;2010:153–157. doi: 10.1109/BIBM.2010.5706555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012 Jun;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeurink PV, van Esch BC, Rijnierse A, Garssen J, Knippels LM. Mechanisms underlying immune effects of dietary oligosaccharides. Am J Clin Nutr. 2013 Aug;98(2):572S–577S. doi: 10.3945/ajcn.112.038596. [DOI] [PubMed] [Google Scholar]
- 14.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007 Nov;62(11):1223–1236. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 15.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007 May;56(5):661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sjogren YM, Jenmalm MC, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009 Apr;39(4):518–526. doi: 10.1111/j.1365-2222.2008.03156.x. [DOI] [PubMed] [Google Scholar]
- 17.Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011 Sep;128(3):646–652. e1–e5. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Johansson MA, Sjogren YM, Persson JO, Nilsson C, Sverremark-Ekstrom E. Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One. 2011;6(8):e23031. doi: 10.1371/journal.pone.0023031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012 Feb;129(2):434–440. 40, e1–e2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014 Jun;44(6):842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 21.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001 Oct;108(4):516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 22.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001 Jan;107(1):129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 23.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011 Mar 15;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power SE, O'Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr. 2014 Feb;111(3):387–402. doi: 10.1017/S0007114513002560. [DOI] [PubMed] [Google Scholar]
- 25.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivares M, Laparra JM, Sanz Y. Host genotype, intestinal microbiota and inflammatory disorders. Br J Nutr. 2013 Jan;109(Suppl 2):S76–S80. doi: 10.1017/S0007114512005521. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tischer C, Zock JP, Valkonen M, Doekes G, Guerra S, Heederik D, et al. Predictors of microbial agents in dust and respiratory health in the Ecrhs. BMC Pulm Med. 2015;15:48. doi: 10.1186/s12890-015-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn RR, Fierer N, Henley JB, Leff JW, Menninger HL. Home life: factors structuring the bacterial diversity found within and between homes. PLoS One. 2013;8(5):e64133. doi: 10.1371/journal.pone.0064133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man's best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010 Aug;126(2):410–412. 12, e1–e3. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sordillo JE, Alwis UK, Hoffman E, Gold DR, Milton DK. Home characteristics as predictors of bacterial and fungal microbial biomarkers in house dust. Environ Health Perspect. 2011 Feb;119(2):189–195. doi: 10.1289/ehp.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barberán A, Dunn RR, Reich BJ, Pacifici K, Laber EB, Menninger HL, et al. The ecology of microscopic life in household dust. Proc Biol Sci. 2015 Sep;282(1814) doi: 10.1098/rspb.2015.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kettleson EM, Adhikari A, Vesper S, Coombs K, Indugula R, Reponen T. Key determinants of the fungal and bacterial microbiomes in homes. Environ Res. 2015 Apr;138:130–135. doi: 10.1016/j.envres.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Candela M, Biagi E, Maccaferri S, Turroni S, Brigidi P. Intestinal microbiota is a plastic factor responding to environmental changes. Trends Microbiol. 2012 Aug;20(8):385–391. doi: 10.1016/j.tim.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Zoetendal EG, de Vos WM. Effect of diet on the intestinal microbiota and its activity. Curr Opin Gastroenterol. 2014 Mar;30(2):189–195. doi: 10.1097/MOG.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 36.Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8(3):e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frese SA, Mills DA. Birth of the infant gut microbiome: moms deliver twice. Cell Host Microbe. 2015 May 13;17(5):543–544. doi: 10.1016/j.chom.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010 Apr;8(4):435–454. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr. 2015 Feb;174(2):151–167. doi: 10.1007/s00431-014-2476-2. [DOI] [PubMed] [Google Scholar]
- 40.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014 Apr;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 42.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006 Aug;118(2):511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 43.Nylund L, Satokari R, Nikkilä J, Rajilić-Stojanović M, Kalliomäki M, Isolauri E, et al. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol. 2013;13:12. doi: 10.1186/1471-2180-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molloy J, Allen K, Collier F, Tang ML, Ward AC, Vuillermin P. The potential link between gut microbiota and IgE-mediated food allergy in early life. Int J Environ Res Public Health. 2013 Dec;10(12):7235–7256. doi: 10.3390/ijerph10127235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010 Dec 24;330(6012):1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Aidy S, Hooiveld G, Tremaroli V, Backhed F, Kleerebezem M. The gut microbiota and mucosal homeostasis: colonized at birth or at adulthood, does it matter? Gut Microbes. 2013 Mar-Apr;4(2):118–124. doi: 10.4161/gmic.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishikawa H, Tanaka K, Maeda Y, Aiba Y, Hata A, Tsuji NM, et al. Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells. Clin Exp Immunol. 2008 Jul;153(1):127–135. doi: 10.1111/j.1365-2249.2008.03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006 Sep;36(9):2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 49.Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013 Jan;131(1):201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita H, Takahashi K, Tanaka H, Nagai H, Inagaki N. Overcoming food allergy through acquired tolerance conferred by transfer of Tregs in a murine model. Allergy. 2012 Feb;67(2):201–209. doi: 10.1111/j.1398-9995.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 51.Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002 Sep;347(12):869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 52.Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001 Oct;358(9288):1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 53.Renz-Polster H, David MR, Buist AS, Vollmer WM, O'Connor EA, Frazier EA, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005 Nov;35(11):1466–1472. doi: 10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 54.Havstad S, Wegienka G, Zoratti EM, Lynch SV, Boushey HA, Nicholas C, et al. Effect of prenatal indoor pet exposure on the trajectory of total IgE levels in early childhood. J Allergy Clin Immunol. 2011 Oct;128(4):880.e4–885.e4. doi: 10.1016/j.jaci.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol. 2009 Feb;123(2):335–341. doi: 10.1016/j.jaci.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 56.Wegienka G, Johnson CC, Havstad S, Ownby DR, Zoratti EM. Indoor pet exposure and the outcomes of total IgE and sensitization at age 18 years. J Allergy Clin Immunol. 2010 Aug;126(2):274–279. 79.e1–79.e5. doi: 10.1016/j.jaci.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014 Jan;111(2):805–810. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010 Jul 6;107(27):12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sjogren YM, Tomicic S, Lundberg A, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009 Dec;39(12):1842–1851. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 60.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009 Oct;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, et al. Pancreatic beta-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity. 2015 Aug 18;43(2):304–317. doi: 10.1016/j.immuni.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013 Dec;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 63.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014 Feb;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 64.Imamura F, Micha R, Khatibzadeh S, Fahimi S, Shi P, Powles J, et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. The Lancet Global Health. 3(3):e132–e42. doi: 10.1016/S2214-109X(14)70381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015 Aug 28;349(6251):993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015 Jul;27:51–56. doi: 10.1016/j.mib.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson CC, Ownby DR, Alford SH, Havstad SL, Williams LK, Zoratti EM, et al. Antibiotic exposure in early infancy and risk for childhood atopy. J Allergy Clin Immunol. 2005 Jun;115(6):1218–1224. doi: 10.1016/j.jaci.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 68.Ong MS, Umetsu DT, Mandl KD. Consequences of antibiotics and infections in infancy: bugs, drugs, and wheezing. Ann Allergy Asthma Immunol. 2014 May;112(5):441.e1–445.e1. doi: 10.1016/j.anai.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 69.Droste JH, Wieringa MH, Weyler JJ, Nelen VJ, Vermeire PA, Van Bever HP. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin Exp Allergy. 2000 Nov;30(11):1547–1553. doi: 10.1046/j.1365-2222.2000.00939.x. [DOI] [PubMed] [Google Scholar]
- 70.Yoo J, Tcheurekdjian H, Lynch SV, Cabana M, Boushey HA. Microbial manipulation of immune function for asthma prevention: inferences from clinical trials. Proc Am Thorac Soc. 2007 Jul;4(3):277–282. doi: 10.1513/pats.200702-033AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science. 2015 Jan;347(6219):266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallace JG, Gohir W, Sloboda DM. The impact of early life gut colonization on metabolic and obesogenic outcomes: what have animal models shown us? J Dev Orig Health Dis. 2015 Sep;:1–10. doi: 10.1017/S2040174415001518. [DOI] [PubMed] [Google Scholar]
- 73.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010 Jan;26(1):5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 74.Lejeune JT, Rajala-Schultz PJ. Food safety: unpasteurized milk: a continued public health threat. Clin Infect Dis. 2009 Jan;48(1):93–100. doi: 10.1086/595007. [DOI] [PubMed] [Google Scholar]
- 75.Perkin MR, Strachan DP. Which aspects of the farming lifestyle explain the inverse association with childhood allergy? J Allergy Clin Immunol. 2006 Jun;117(6):1374–1381. doi: 10.1016/j.jaci.2006.03.008. [DOI] [PubMed] [Google Scholar]