Abstract
Background
Involved field radiation therapy (IFRT) is integral in curative therapy for Hodgkin lymphoma (HL), although primarily used in patients with intermediate/high-risk HL. We present failure patterns and clinical outcomes in a cohort of pediatric and young adult patients with HL treated with IFRT at the Johns Hopkins Hospital.
Procedure
Patients ≤40 years old with intermediate/high-risk HL who received chemotherapy and IFRT from 1997 to 2012 were included in this retrospective analysis. Patients were evaluated for failure patterns, overall survival (OS), and event-free survival (EFS) using Kaplan–Meier curves, descriptive statistics, and Cox proportional hazard regressions.
Results
We reviewed 74 patients (45 pediatric and 29 young adult) with a median follow-up of 4.4 years. The mean age at diagnosis was 21.4 years. Patients received a median of 29.75 Gy of IFRT (range 15–39.6 Gy). The majority of pediatric patients received ABVE-PC chemotherapy (n=25) and <30 Gy of radiation (n=33) while most young adults received ABVD chemotherapy (n=24) and ≥30 Gy (n=25). Estimated 5-year OS and EFS were 96% and 81%, respectively. Thirteen patients had recurrence; eight were pediatric. Distant relapse alone comprised 83% of failures in patients receiving ≥30 Gy. Of the seven patients who received <30 Gy and had recurrence, six had local failure as a component of their recurrence. Caucasian race (P=0.02) and nodular sclerosing histology (P=0.01) predicted for increased EFS. Late effects were minimal and all deaths (n=4) were from HL.
Conclusions
In this series, pediatric and young adult patients were treated with differing chemoradiation and had distinct recurrence patterns.
Keywords: hematology/oncology, Hodgkin lymphoma, involved field radiation therapy
INTRODUCTION
Hodgkin lymphoma (HL) comprises 6% of pediatric malignancies in the United States. The 5-year overall survival with modern treatment strategies is 97% for children; it is 87% for both adults and children combined [1]. HL has a bimodal age distribution with at least 2 etiologically distinct forms of disease based on epidemiological studies in developed countries: the childhood form with an onset at 10–14 years and the young adult form with an onset between 15 and 35 years [1,2].
Approximately 95% of children and young adults with early-stage and 85% with advanced-stage HL are cured with combined modality therapy in the form of chemotherapy and radiation therapy [3]. Today, the advent of PET/CT as the primary staging modality for HL has allowed oncologists to customize chemoradiation treatment plans based on response to therapy. While chemotherapy regimens differ for children and adults in the modern era, radiation therapy in the form of involved field radiation therapy (IFRT) continues to be an important component of therapy for all patients with intermediate and high-risk disease [4–6]. A reduction from the historical extended field radiation therapy (EFRT), IFRT targets the involved nodal regions of disease [5]. More recently, involved site radiation therapy (ISRT) has evolved to further reduce treatment field size [6].
Historically, the treatment for HL did not depend on age of diagnosis. Both pediatric and adult patients were treated with the same chemotherapy regimens followed by EFRT to 35–44 Gy. Despite encouraging rates of overall and event-free survival, children who received high doses (35–44 Gy) of EFRT demonstrated impaired bone growth and soft tissue development, cardiopulmonary toxicities, and secondary benign and malignant neoplasms [7–9]. These morbidities prompted the evolution of treatment strategies for pediatric Hodgkin lymphoma, including a reduction in field size from the extended field (EFRT) to the involved field (IFRT), as well as lowering the total dose from 35 to 44 Gy to approximately 20 Gy [10]. Subsequent studies showed excellent overall and event-free survival in the majority of pediatric patients who were given low dose IFRT treatments (15–25.5 Gy) following chemotherapy [11–14].
While overall survival and event-free survival for children, adolescents, and young adults with HL is excellent, the optimal treatment strategy is still a matter of debate. In a study examining patients with HL in northern England, patients 15–19 years of age had a superior 5-year survival (86%) when compared with patients aged 20–24 (79%) [15]. Here we compare patterns of failure and clinical outcomes for pediatric and young adult patients (≤40 years of age) with intermediate- and high-risk HL who were treated with radiation therapy at our institution.
MATERIALS AND METHODS
Patients
Seventy-four patients with HL who were consecutively treated at the Johns Hopkins Hospital (JHH) from June 1997 to October 2012 were identified from the Sidney Kimmel Comprehensive Cancer Center (SKCCC) database following Institutional Review Board (IRB) approval for the study. We identified 74 pediatric and young adult patients 40 years of age or younger with intermediate and high-risk HL (Stages II–IV) who received chemotherapy followed by consolidative involved field radiation therapy (IFRT) to be included in this retrospective analysis. Pediatric patients were defined as patients <21 years old at diagnosis, while patients were considered to be young adult if they were ≥21 at diagnosis.
Data were abstracted from electronic medical records and paper charts. Variables analyzed included age at diagnosis, sex, presenting symptoms, presence of bulky disease (defined as disease ≥10 cm or disease encompassing >33% of the chest diameter on chest x-ray), location of disease, disease stage, method of staging, date and type of chemotherapy, RT dose and volume, imaging, and failure patterns.
Definition of Endpoints
Patients were evaluated for overall survival (OS), event-free survival (EFS), and patterns of recurrence. OS was measured from the date of diagnosis to the date of death or last follow-up. EFS was measured from date of diagnosis to date of first recurrence or progression of disease. Recurrences were defined as local (within a site receiving IFRT), distant (outside the radiation field), or combined (recurrences seen both within and outside of the radiation field).
Statistical Analysis
Stata 12.1 (StataCorp LP, College Station, TX) software was used to calculate survival rates based on the Kaplan-Meier method. Descriptive statistics were also used. A P value of ≤0.05 was considered to be statistically significant. A multivariate Cox proportional hazards regression analysis was performed to determine which parameters were significant.
RESULTS
Patient Characteristics
In our cohort of 74 patients, 45 patients were pediatric (<21) and 29 patients were young adult (21–40 years of age). Patient characteristics are listed in Table I. The mean age at diagnosis was 21.4 years (range, 4.1 to 39.9). 43 patients were male (58%). The median follow-up time for the entire cohort was 4.4 years (range, 0.5–11.8 years). Forty-five patients were staged by PET/CT between 2000 and 2011. Nineteen patients were staged via CT scans between 1997 and 2003. The remaining 10 patients were staged via Gallium-67 citrate scans between 1999 and 2002.
TABLE I.
Demographic and Clinical Characteristics
| All patients (n=74) |
Pediatric (n=45) |
Young adult (n=29) |
|
|---|---|---|---|
| Age at diagnosis (y) | |||
| Mean | 21.4 | 15.2 | 31.2 |
| Range | 4.1–39.9 | 4.1–20.6 | 22.6–39.9 |
| Male (n, %) | 43 (58%) | 30 (67%) | 13 (45%) |
| Race (n, %) | |||
| Caucasian | 51 (69%) | 31 (69%) | 20 (69%) |
| Black | 15 (20%) | 9 (20%) | 6 (21%) |
| Asian | 1 (1%) | 1 (2%) | 0 (0%) |
| Hispanic | 2 (3%) | 1 (2%) | 1 (3%) |
| Other/unknown | 5 (7%) | 3 (7%) | 2 (7%) |
| Clinical staging (n, %) | |||
| II | 46 (62%) | 22 (49%) | 24 (83%) |
| III | 12 (16%) | 10 (22%) | 2 (7%) |
| IV | 16 (22%) | 13 (29%) | 3 (10%) |
| “B” symptoms (n, %) | 32 (43%) | 20 (44%) | 12 (41%) |
| Bulky disease (n, %) | 20 (27%) | 12 (27%) | 8 (28%) |
| Histology (n, %) | |||
| Nodular sclerosing | 62 (84%) | 36 (80%) | 26 (91%) |
| Mixed cellularity | 8 (11%) | 7 (16%) | 1 (3%) |
| Lymphocyte predominant | 1 (1%) | 0 (0%) | 1 (3%) |
| Other | 3 (4%) | 2 (4%) | 1 (3%) |
| Chemotherapy (n, %) | |||
| ABVD | 28 (38%) | 4 (9%) | 24 (83%) |
| ABVE-PC | 25 (34%) | 25 (56%) | 0 (0%) |
| Other | 21 (28%) | 16 (35%) | 5 (17%) |
| Radiation therapy (n, %) | |||
| Conventional | 68 (92%) | 41 (91%) | 27 (93%) |
| 3D-conformal | 1 (1%) | 0 (0%) | 1 (3%) |
| Intensity modulated | 1 (1%) | 1 (2%) | 0 (0%) |
| Unspecified | 4 (6%) | 3 (7%) | 1 (3%) |
| Radiation doses (n, %) | |||
| <30 Gy | 37 (50%) | 33 (73%) | 4 (14%) |
| ≥30 Gy | 37 (50%) | 12 (27%) | 25 (86%) |
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; ABVE-PC, doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide.
Chemotherapy and Radiation Treatment
All patients in this study underwent chemotherapy followed by consolidative IFRT. Twenty eight (38%) patients in the cohort received ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and 25 (34%) patients received ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide) chemotherapy. The majority of the pediatric patients received ABVE-PC chemotherapy (n=25, 56%) while the majority of the young adult patients received ABVD (n=24, 83%).
The median dose of adjuvant radiation received was 29.75 Gy (range 15–39.6 Gy). Four of the 74 patients received their initial radiation therapy at an outside institution and were referred to JHH for subsequent treatment. Nearly all patients received conventional radiation (n=68, 92%), one received 3D-conformal radiation therapy (1%), one received intensity modulated radiation therapy (1%), and four patients had unspecified radiation treatments (6%).
OS and EFS
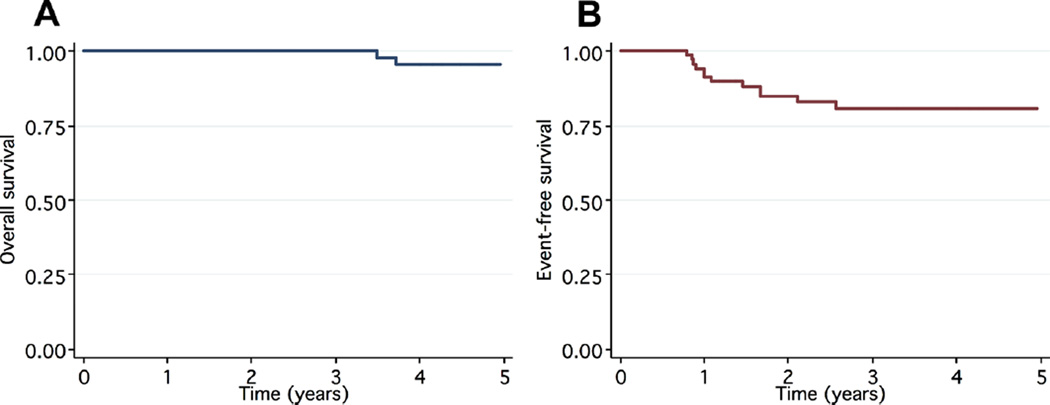
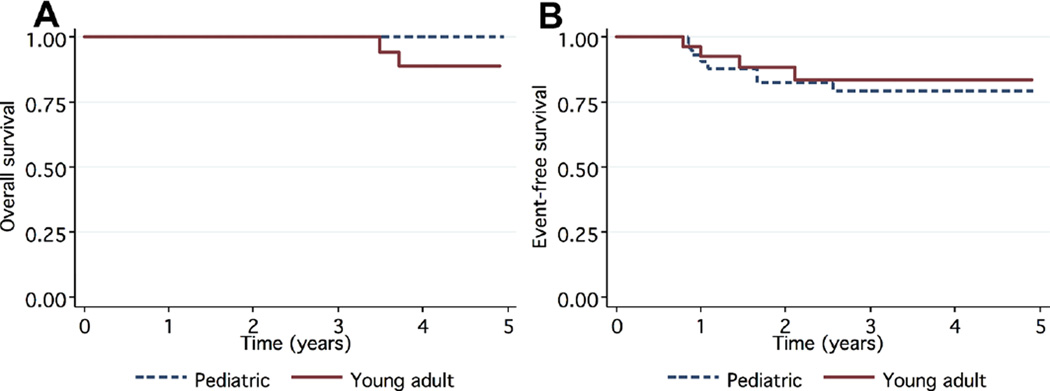
The estimated 5-year OS was 96%, while the estimated EFS at 5 years for the entire cohort was 81% (Fig. 1). All deaths (n=4) were due to HL. All four were young adult and each had recurrence of HL. The pediatric patients had a significantly higher 5-year OS (100%) compared to the young adults (89%), P=0.007 (Fig. 2A). However, there was no significant difference in 5-year EFS in the pediatric cohort (79%) compared to the estimated 5-year EFS of 83% in the young adult cohort (P=0.93, Fig. 2B).
Fig. 1.
Kaplan–Meier 5-year estimates of OS (A) and EFS (B) of the entire cohort.
Fig. 2.
Kaplan–Meier 5-year estimates of OS (A, P=0.007) and EFS (B, P=0.93) stratified by age at diagnosis.
Patterns of Failure
Thirteen patients had recurrences of their HL. Having recurrence of HL was associated with significantly lower survival; among the 13 patients who recurred, 5-year OS was 80%, compared to 100% in the 61 patients without recurrence (P=0.001).
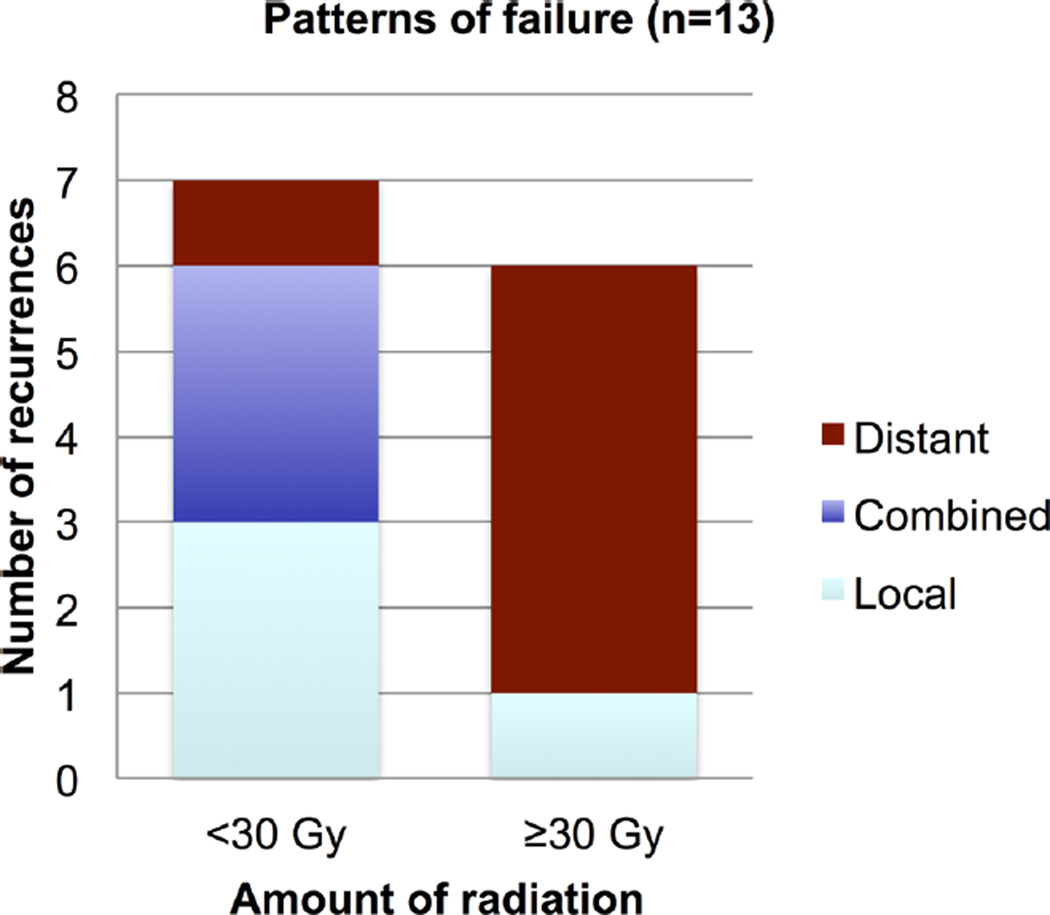
Six of the patients who recurred received ≥30 Gy of radiation while seven received <30 Gy (Fig. 3). Four patients had local failure alone, six had distant failures, and three had combined failures. Distant relapse alone comprised 83% of failures in patients who received ≥30 Gy.
Fig. 3.
Patterns of failure in patients who had recurrence of HL.
Three of four patients with a local recurrence alone (75%) received <30 Gy of IFRT (Fig. 3). Of the seven patients who received <30 Gy and had a recurrence, six (86%) had local failure as a component of their relapse. Three of these six patients had local disease failures, while the other three had combined disease failures.
Of the thirteen patients who recurred, eight were pediatric and five were young adults. Six of the eight pediatric patients who recurred received <30 Gy of radiation. Of these six pediatric patients, three had local recurrences and two had combined recurrences. In contrast, of the five young adults who recurred, four received ≥30 Gy and three of these four had distant recurrences.
Prognostic Factors for Event-Free Survival
Univariate analysis of factors predicting treatment failure is shown in Table II. Caucasian race (P=0.02) and nodular sclerosing histology (P=0.01) predicted for increased EFS. No significant differences were seen in the estimated 5-year EFS when stratified by age, chemotherapy regimen, radiation dose, IFRT modality used, presence of bulky disease, or clinical stage. A multivariate Cox regression model on the variables gender, age, ethnicity, histology, cancer stage, bulky disease, and radiation dose did not identify any significant predictors of EFS.
TABLE II.
Univariate Analysis of Patient, Tumor, and Treatment Parameters and Influence on EFS
| Characteristic | 5-year EFS | P-value |
|---|---|---|
| Age | ||
| <21 (pediatric) | 0.79 | 0.93 |
| ≥21 (young adult) | 0.83 | |
| Gender | ||
| Male | 0.87 | 0.15 |
| Female | 0.73 | |
| Race | ||
| Caucasian | 0.87 | 0.02 |
| Other | 0.53 | |
| Histologic type | ||
| Nodular sclerosing | 0.84 | 0.01 |
| Other | 0.63 | |
| Clinical stage | ||
| II | 0.77 | 0.36 |
| III | 0.81 | |
| IV | 0.92 | |
| Systemic “B” symptoms | ||
| Present | 0.74 | 0.40 |
| Absent | 0.86 | |
| Bulky disease | ||
| Yes | 0.76 | 0.21 |
| No | 0.82 | |
| Chemotherapy | ||
| ABVD | 0.76 | 0.63 |
| ABVE-PC | 0.87 | |
| Other | 0.80 | |
| Radiation dose | ||
| <30 Gy | 0.79 | 0.69 |
| ≥30 Gy | 0.84 |
One secondary malignancy was identified. This pediatric patient developed an infield mucoepidermoid carcinoma of the parotid gland 6 years after mantle radiation to 30.6 Gy. The carcinoma was treated with complete surgical resection without significant morbidity. At the time of last follow-up, 10 years after the initial diagnosis, the patient was still alive and disease-free without further complications from therapy.
DISCUSSION
Overall survival rates of patients with HL are excellent; however, there remains a cohort of patients who develop recurrent or progressive disease and ultimately die of HL. Optimizing treatment in young patients is challenging, particularly given the incidence of potential late effects. This study focused on failure patterns after IFRT in pediatric and young adult patients with HL ≤40 years old at diagnosis and includes patients with stages II–IV disease. In general, the pediatric patients in our cohort received doses of IFRT to <30 Gy while the young adult patients mainly received IFRT to ≥30 Gy. The pediatric patients were also given differing chemotherapy regimens before RT, with the majority of the pediatric patients receiving ABVE-PC chemotherapy and the majority of young adults receiving ABVD chemotherapy. Many patients in our cohort had unfavorable characteristics such as bulk and B symptoms. Thirteen patients went on to develop recurrent disease after receiving consolidative IFRT.
The patients with HL in this series had different patterns of failure when analyzed with respect to age and radiation dose. Eight of the thirteen patients who had recurrences of HL after IFRT were pediatric. Overall, patients who recurred after IFRT had a significantly lower 5-year OS, and only 1 of the 13 patients who experienced relapse were alive at last follow-up. Interestingly, the patients in our cohort who were treated with higher doses of radiation (≥30 Gy) demonstrated a trend toward improved local control of their disease (Fig. 3); this pattern held true in both the pediatric and young adult cohorts. Five out of the six patients who received ≥30 Gy had distant recurrences; only one had a local recurrence. On the other hand, of the seven patients who received <30 Gy and recurred, six had local failure as a component of their relapse. Prior literature on the subject of HL failure patterns has suggested that combined modality treatments provide excellent local disease control in children and young adults. The St. Jude’s HOD90 and HOD94 prospective studies analyzed 195 patients with HL and found that 27 (14%) of these patients experienced failure; 22 of these 27 patients had local failure [16]. An earlier retrospective study on local disease control did not demonstrate statistically significant evidence of increasing tumor control in HL with radiation doses >30 Gy, but noted that combined chemoradiation may improve the local control of large tumors >6 cm[17]. While our failure pattern data are in concordance with the idea that combined chemoradiation may increase local control rates, our data suggest that higher doses of radiation may be necessary to prevent local failure in select patients.
Differing failure patterns between the pediatric and young adult patients leads to the question of optimum management of HL in these age groups. Previous studies have shown excellent OS and EFS in the majority of pediatric patients given low dose IFRT treatments (15–25.5 Gy) following chemotherapy [11–14]. While it has been shown that IFRT improves outcomes in children, the literature is inconclusive for patients who fall into the young adult group. All patients who died in our cohort were young adults; to date, all pediatric patients are alive without evidence of disease. Although overall mortality in our series was low at 5%, HL was the primary cause of all four deaths. At many institutions, patients are treated according to pediatric or adult radiation paradigms depending on the physician to whom they are referred. Two prior studies reported that adolescents and young adults with HL have similar outcomes when treated with the same protocols [18,19]. However, a separate study reported that for patients <18 years of age, survival appeared to be increased with the use of pediatric treatment regimens [20]. Further elucidation of the most effective treatments for the young adult cohort is needed.
Our findings suggest that doses of radiation >30 Gy are important for disease control in a subset of patients with HL (Fig. 3). As our standard RT fields are again decreasing in size, treating higher risk patients to higher doses of radiation may be more acceptable. Today, the traditional IFRT fields have been replaced in the National Comprehensive Cancer Network guidelines with involved site radiation therapy (ISRT). ISRT treats only lymph node areas that contained detectable disease at the time of diagnosis rather than entire lymph node regions potentially at risk [5]. Given the potential significant reduction in the volume of the radiation field with ISRT, it would be expected that the incidence of late effects such as cardiovascular disease and secondary malignancies would be meaningfully decreased [6]. This move from IFRT to ISRT is a result of modern advances in imaging modalities (both for tumor staging purposes as well as tumor contouring in RT) and improvements in RT delivery techniques.
We see a change in the tumor staging methodology in our cohort over the period of study. Patients diagnosed around the turn of the 21st century were generally staged with older techniques such as Gallium-67 citrate spectroscopy or from CT scans alone. After 2000, there was a shift toward the use of combined PET/CT scans for tumor staging. PET is a standard part of the staging workup for HL and is critical in the determination of a patient’s response to therapy [21]. Therefore, it is a limitation of our study that not all patients in this series were staged with the samemodality using PET/CT.As our treatment paradigms in HL move toward limiting the role of RT to those patients with the most unfavorable characteristics, there is a need to identify the subset of patients (via the use of PET/CT) who are likely to derive the most significant benefit from radiation. In this cohort of patients, it may be possible to treat a smaller volume of disease to a higher dose of radiation to improve the therapeutic ratio and limit treatment failures. Optimizing radiation with respect to dose and field size warrants further study particularly for those patients in whom radiation may be most advantageous to maximize local control (e.g., bulky disease).
Our study is limited by its retrospective nature. We were unable to define our failure patterns as marginal failures beyond local failure; we were restricted by the availability of the radiology reports and the radiation treatment plans. Going forward, it will be critical to have CT-based planning with dosimetry evaluation to precisely identify the location of failure with respect to treatment field design particularly as our fields decrease in size. Patients receiving chemotherapy alone were excluded from this study, a limitation that impacts the reported OS and EFS. Additionally, as in all retrospective studies, our study includes patients with heterogeneous disease characteristics and treatment regimens. As a result of this heterogeneity, we were unable to assess outcomes as they relate to early- and mid-treatment response, which has been found in recent years to be an important prognosticator of patient outcomes. However, this heterogeneity does allow for the analysis of the relationships between outcomes and age, chemoradiation, and general treatment approaches for pediatric and young adult patients. We found that our patients experienced different patterns of failure as a function of their age and resultant IFRT paradigm.
CONCLUSION
Our study demonstrates that pediatric and young adult patients with HL differ in recurrence patterns. Pediatric patients received lower doses of IFRT. Additionally, more local recurrences were seen in patients who received lower doses of radiation. This analysis supports the need for further study toward the optimum therapy for young adult patients. Therapeutic optimization of combination chemotherapy and radiation is essential for further improvement of clinical outcomes. Treatment paradigms are moving toward response-adapted therapy, isolating those patients at highest risk for relapse. Nevertheless, radiation continues to play an important role in the treatment of HL, particularly with the potential use of smaller volumes possible with ISRT. Ultimately, however, the approach to radiation for patients with high-risk HL will have to be balanced with risk of late effects for long-term survivors.
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Grufferman S, Delzell E. Epidemiology of Hodgkin’s disease. Epidemiol Rev. 1984;6:76–106. doi: 10.1093/oxfordjournals.epirev.a036276. [DOI] [PubMed] [Google Scholar]
- 3.Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20:3765–3771. doi: 10.1200/JCO.2002.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Yahalom J, Mauch P. The involved field is back: Issues in delineating the radiation field in Hodgkin’s disease. Ann Oncol. 2002;13:79–83. doi: 10.1093/annonc/13.s1.79. [DOI] [PubMed] [Google Scholar]
- 5.Yahalom J. Changing role and decreasing size: Current trends in radiotherapy for Hodgkin’s disease. Current Onc Reports. 2002;4:415–423. doi: 10.1007/s11912-002-0036-9. [DOI] [PubMed] [Google Scholar]
- 6.Specht L, Yahalom J, Illedge T, et al. Modern radiation therapy for hodgkin lymphoma: Field and dose guidelines from the international lymphoma radiation oncology group (ILROG) Int J Radiat Oncol Biol Phys. 2013 doi: 10.1016/j.ijrobp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Mauch PM, Weinstein H, Botnick L, et al. An evaluation of long-term survival and treatment complications in children with Hodgkin’s disease. Cancer. 1983;51:925–932. doi: 10.1002/1097-0142(19830301)51:5<925::aid-cncr2820510527>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 1993;11:1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson DC, Hudson MM, Constine LS. Pediatric hodgkin lymphoma: Maximizing efficacy and minimizing toxicity. Semin Radiat Oncol. 2007;17:230–242. doi: 10.1016/j.semradonc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Oberlin O, Leverger G, Pacquement H, et al. Low-dose radiation therapy and reduced chemotherapy in childhood Hodgkin’s disease: The experience of the French Society of Pediatric Oncology. J Clin Oncol. 1992;10:1602–1608. doi: 10.1200/JCO.1992.10.10.1602. [DOI] [PubMed] [Google Scholar]
- 12.Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol. 1993;11:100–108. doi: 10.1200/JCO.1993.11.1.100. [DOI] [PubMed] [Google Scholar]
- 13.Hunger SP, Link MP, Donaldson SS. ABVD/MOPP and low-dose involved-field radiotherapy in pediatric Hodgkin’s disease: The Stanford experience. J Clin Oncol. 1994;12:2160–2166. doi: 10.1200/JCO.1994.12.10.2160. [DOI] [PubMed] [Google Scholar]
- 14.Weiner MA, Leventhal BG, Marcus R, et al. Intensive chemotherapy and low-dose radiotherapy for the treatment of advanced-stage Hodgkin’s disease in pediatric patients: A Pediatric Oncology Group study. J Clin Oncol. 1991;9:1591–1598. doi: 10.1200/JCO.1991.9.9.1591. [DOI] [PubMed] [Google Scholar]
- 15.Pearce MS, Parker L, Windebank KP, et al. Cancer in adolescents and young adults aged 15–24 years: A report from the North of England young person’s malignant disease registry, UK. Pediatr Blood Cancer. 2005;45:687–693. doi: 10.1002/pbc.20444. [DOI] [PubMed] [Google Scholar]
- 16.Krasin MJ, Rai SN, Kun LE, et al. Patterns of treatment failure in pediatric and young adult patients with Hodgkin’s disease: Local disease control with combined-modality ttherapy. J Clin Oncol. 2005;23:8406–8413. doi: 10.1200/JCO.2004.00.8763. [DOI] [PubMed] [Google Scholar]
- 17.Mendenhall NP, Rodrigue LL, Moore-Higgs GJ, et al. The optimal dose or radiation in Hodgkin’s disease: An analysis of clinical and treatment factors affecting in-field disease control. Int J Rad Biol Phys. 1999;44:551–561. doi: 10.1016/s0360-3016(99)00087-5. [DOI] [PubMed] [Google Scholar]
- 18.Foltz LM, Song KW, Connors JM. Hodgkin’s lymphoma in adolescents. J Clin Oncol. 2006;24:2520–2526. doi: 10.1200/JCO.2005.04.5823. [DOI] [PubMed] [Google Scholar]
- 19.Eichenauer DA, Bredenfeld H, Haverkamp H, et al. Hodgkin’s lymphoma in adolescents treated with adult protocols: A report from the German Hodgkin study group. J Clin Oncol. 2009;27:6079–6085. doi: 10.1200/JCO.2008.20.2655. [DOI] [PubMed] [Google Scholar]
- 20.Muller J, Illes A, Molnar Z, et al. Adolescent Hodgkin lymphoma: Are treatment results more favorable with pediatric than with adult reigmens? J Pediatr Hematol Oncol. 2011;33:e60–e63. doi: 10.1097/MPH.0b013e3181f4686e. [DOI] [PubMed] [Google Scholar]
- 21.Evans WC, Gilmore D, English J. The role of PET and PET/CT in managing the care of lymphoma patients. J Nucl Med Technol. 2001;39:190–194. doi: 10.2967/jnmt.110.082982. [DOI] [PubMed] [Google Scholar]