Abstract
During capacitation, sperm acquire the ability to undergo the acrosome reaction (AR), an essential step in fertilization. Progesterone produced by cumulus cells has been associated with various physiological processes in sperm, including stimulation of AR. An increase in intracellular Ca2+ ([Ca2+]i) is necessary for AR to occur. In this study, we investigated the spatiotemporal correlation between the changes in [Ca2+]i and AR in single mouse spermatozoa in response to progesterone. We found that progesterone stimulates an [Ca2+]i increase in five different patterns: gradual increase, oscillatory, late transitory, immediate transitory, and sustained. We also observed that the [Ca2+]i increase promoted by progesterone starts at either the flagellum or the head. We validated the use of FM4-64 as an indicator for the occurrence of the AR by simultaneously detecting its fluorescence increase and the loss of EGFP in transgenic EGFPAcr sperm. For the first time, we have simultaneously visualized the rise in [Ca2+]i and the process of exocytosis in response to progesterone and found that only a specific transitory increase in [Ca2+]i originating in the sperm head promotes the initiation of AR.
Keywords: acrosomal exocytosis, calcium, progesterone, sperm
INTRODUCTION
Mammalian sperm must first undergo a complex process called capacitation in the female reproductive tract to acquire fertilizing capacity [1, 2]. During capacitation, sperm gain the ability to develop hyperactivated motility and to undergo acrosome reaction (AR) in response to specific stimuli [3–5]. Both processes are absolutely essential for fertilization, and the AR is mandatory for proper relocalization of specific fusion proteins [6]. The AR shares similarities with exocytotic secretory mechanisms in other cells though it is a unique single controlled secretory event [7, 8].
For many years, it was considered that sperm undergo AR upon interaction with the egg's zona pellucida (ZP). Most of our knowledge of this process is derived from in vitro studies using solubilized ZP [9–11]. However, recent evidence suggests that most fertilizing mouse sperm undergo AR before binding to the ZP [12, 13]. Thus, it is still controversial where fertilizing mouse spermatozoa initiate AR and what stimuli trigger it. These are key events that remain to be determined. In this scenario, progesterone secreted by the cumulus cells is a well-known stimulant of AR [14, 15]. Progesterone could play a physiological role in inducing the AR because the follicular fluid is incorporated into the oviduct upon ovulation and sperm pass through the cumulus oophorus to reach ovulated eggs.
At concentrations found in the female reproductive tract, progesterone has also been implicated in other aspects of sperm physiology such as capacitation, chemotaxis, motility, and hyperactivation [16–21], although the molecular mechanisms or signaling pathways involved in these processes are poorly understood. In addition, the identity of the progesterone receptor(s) is not well established in mammalian sperm, particularly in mouse. It is known that the effect of progesterone in mammalian sperm does not involve the classical regulation by nuclear receptors. Various candidate membrane receptors for progesterone have emerged, and some of them have been reported to be present in mammalian sperm [22, 23].
The AR is driven by an increase in intracellular Ca2+ ([Ca2+]i). The nature of the channels involved in the progesterone-induced increase in [Ca2+]i appears to be species dependent and may involve a combination of CatSper, voltage-gated calcium channels, store-operated channels, and intracellular channels in an intertwined network of signaling cascades [24]. In human sperm, it was recently demonstrated that progesterone induces a Ca2+ influx mediated by CatSper channels [17, 18] although in mouse, CatSper-null sperm undergo AR normally, suggesting that this channel is not essential for this process [25, 26]. In addition, there is still controversy about where Ca2+ is stored in sperm. Some authors postulate that the acrosome is a Ca2+ store and that its depletion from the acrosome activates store-operated channels that allow a sustained entry of Ca2+ from the medium [27, 28]. Similarly, Ho and Suarez [29] identified the redundant nuclear envelope at the posterior end of the sperm head as a Ca2+ store. Along these lines, Xia and Ren [26] have shown controversial results indicating that the ZP-induced Ca2+ increase starts in the sperm tail and propagates toward the head (anterograde direction; flagella to head). Ca2+-imaging studies by Fukami and colleagues [30] revealed that the Ca2+ increase induced by ZP or progesterone started at different sites within the sperm head, indicating that these agonists trigger acrosomal exocytosis via different mechanisms. The anterograde wave of Ca2+ observed by Fukami and colleagues [30] and Xia and Ren [26] in response to soluble ZP suggests that a Ca2+ store near the head-tail junction may be associated with the AR. The anterograde direction of this wave from the base of the head is reminiscent of the loss of acrosomal GFP previously demonstrated [31]. However, none of these studies had simultaneously recorded exocytosis and [Ca2+]i changes in single sperm to analyze progesterone responses. Recently, Sánchez Cárdenas and coworkers [32] found that FM4-64 labeled the human sperm plasma membrane and that its fluorescence increased in the sperm head upon induction of the AR. Because of its fluorescence properties, FM4-64 imaging can be simultaneously combined with [Ca2+]i imaging probes such as Fluo-4 AM. In this work, we aim to study the spatiotemporal [Ca2+]i changes and AR in single mouse spermatozoa after stimulation with progesterone.
MATERIALS AND METHODS
Reagents and Chemicals
FM4-64, Fluo-4 AM, pluronic acid, and laminin were obtained from Life Technologies Corporation (Invitrogen). Bovine serum albumin, concanavalin A, and progesterone were purchased from Sigma-Aldrich Chemical Co. Ionomycin was from Alomone Labs. All other chemicals were of reagent grade.
Animals
CD1 mature (10- to 12-wk old) and BDF1-Tg (CAG- mtDsRed2, Acr-EGFP) RBGS0020sb male mice were used. These transgenic mice express EGFP and Ds-Red2 fluorescence in acrosomal vesicles and the midpiece, respectively [33]. Animals were maintained at 23°C with a 12L:12D cycle. Animal experimental procedures were reviewed and approved by the Ethical Committee of Instituto de Biotecnología/UNAM, the Committee for the Care and Use of Laboratory animals IFC/UNAM (CICUAL AHC54-14), and by the Instituto de Biología y Medicina Experimental.
Sperm Preparation
In all the experiments, cauda epididymal mouse sperm were collected from retired male breeders by placing minced cauda epididymis in a modified Krebs-Ringer medium (Whitten-HEPES-buffered [WH] medium) [31]. For capacitated medium experiments, 5 mg/ml bovine serum albumin and 24 mM NaHCO3 were added. The pH was maintained at 7.4. Epididymal motile mice sperm were collected by swim-up in WH medium at 37°C for 30 min.
Live Imaging of [Ca2+]i in Individual Mice Spermatozoa
For Ca2+ recordings, the obtained motile cells were incubated with 2 μM Fluo-4 AM and 0.05% pluronic acid during 60 min in WH medium supplemented with bovine serum albumin and NaHCO3 according to experimental capacitating conditions. Once loaded, sperm were immobilized on mouse laminin (1 mg/ml)- or concanavalin A (1 mg/ml)-coated cover slips to allow recordings. Unattached spermatozoa were removed by gentle washing, and the chamber was filled with the recording medium (nonsupplemented WH medium). Recordings were performed at 37°C using a temperature controller model 202A (Harvard Apparatus). Progesterone (stock concentration 100 mM dissolved in dimethyl sulfoxide) or vehicle (dimethyl sulfoxide) prepared in the recording medium were applied gently using a micropipette. Sperm were viewed with an inverted microscope (Nikon Eclipse Ti-U) and an oil immersion fluorescence objective (Nikon plan Apo TIRF DIC H/N2 60x/1.45 NA). A precentered fiber illuminator Nikon Intensilight-CHGFI (Nikon) was used as the light source. For excitation and emission collection of Fluo-4 AM, the filter set GFP 96343, D: 495, Exc: 470/40, barrier 525/50 (Nikon) was used. Fluorescence images were acquired with an Andor Ixon 3 EMCCD camera model DU-8970-C00#B (Andor Technology) under protocols written in Andor iQ 1.10.2 software version 4.0. For these experiments, we recorded one image every 2 or 5 sec with an exposure/illumination time of 50 msec for periods of 5–30 min. For faster time resolution images, sperm were exposed to light continuously for 1 min and 8–10 images per second were acquired.
Live Imaging of Transgenic Mice Sperm Undergoing AR
Transgenic mice sperm were incubated in recording medium containing 10 μM FM4-64 for 5 min. For FM4-64 measurements, the dye was present in the extracellular medium throughout the experiments. Recordings were done as described above. For excitation and emission collection of FM4-64, a filter set Wide Green 11007v2, D: 565 dcxt, Exc: 535/50, Em: 590 lpv2 (Chroma Technology Corporation) was used. For GFP, the same filter set described above for Fluo-4 AM was used. The time interval to change between FM4-64/GFP filter cubes was 400 msec. Images were acquired at every 2 or 5 sec with an exposure/illumination time of 50 msec for periods of 30 min.
Live Imaging Recording of [Ca2+]i and AR in Individual Mouse Sperm
For alternated Ca2+/AR imaging, Fluo-4 AM-loaded mouse sperm were exposed for 5 min to recording medium containing 10 μM FM4-64. Fluo-4 AM and FM4-64 recordings were performed as described above. The time interval to change between Fluo-4 AM/FM4-64 filter cubes was 400 msec. Images were acquired at every 2 or 5 sec with an exposure/illumination time of 50 msec for periods of 5–30 min. For simultaneous Ca2+/AR imaging, recordings were performed at 37°C using a TC-202 Bipolar temperature controller (Medical Systems Corp). Sperm were viewed with an inverted microscope Nikon Eclipse TE 300 and an oil immersion fluorescence objective Nikon Plan Apo 60x/1.40 NA. Fluorescence illumination was supplied by a Luxeon V Star Lambertian Cyan LED par (LXHL-LE5C; Lumileds Lighting LLC) attached to a custom-built stroboscopic control box. For dual-emission imaging using Fluo-4 AM and FM4-64 (Ca2+ and AR, respectively), an Optosplit II image splitter (Cairn Research) was used. For excitation of both fluorophores (Fluo-4 AM and FM4-64) a D485/25X filter (Chroma Technology Corporation) was used. Dual-emission was achieved using a 610LP dichroic mirror and collected with a HQ535/50 and ET610LP filters (Chroma Technology Corporation) for Fluo-4 AM, and FM4-64 respectively. Fluorescence images were recorded on an EMCCD Andor camera (DV887, Andor iXon). Images were acquired at two images per second with an exposure/illumination time of 2 msec for periods of 5 min.
Analysis of Images
During all the experiments, 16 bit images were obtained, and movies were processed and analyzed with macros written in Image J (version 1.4.3.67, National Institutes of Health). Regions of interest were drawn on each sperm in the movie for quantification. A plot of the fluorescence intensity of each spermatozoon versus time was generated in Origin 6.0 (Microcal Software). Fluorescence is expressed as (F − F0)/F0. When brightness and contrast were adjusted, this was done equally in all images or movies taken under the same conditions.
Statistical Analysis
All numerical data are presented as the mean ± standard error of the mean. All tests were two-tailed with a statistical significance assessed at the P < 0.05 level. Results obtained were compared by one-way analysis of variance (ANOVA) with a Tukey multiple comparison test with the exception of Figures 1 and 6, where the Student t-test was used. Statistical analysis was performed using standard statistical software (Graphpad Software).
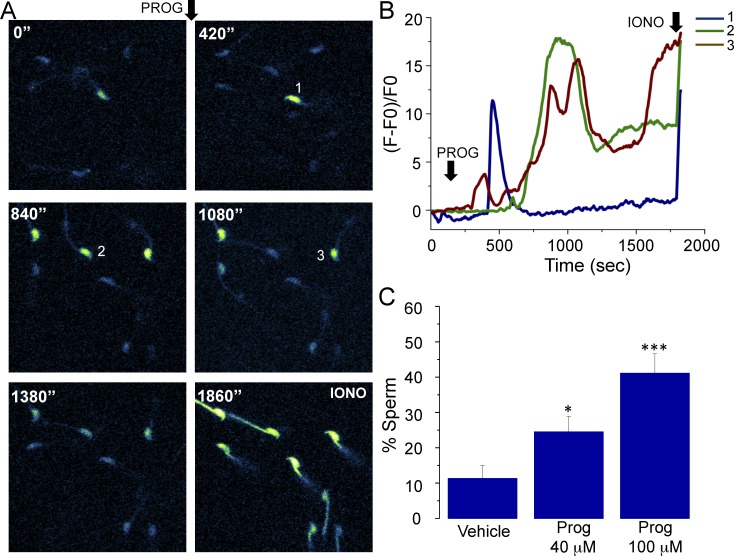
FIG. 1.
Progesterone promotes an [Ca2+]i increase in Fluo-4 AM-loaded mice sperm. A) Image sequence showing sperm exposed to 40 μM progesterone (PROG) and recorded for 30 min. Sperm displayed an [Ca2+]i increase at different times during the experiment. At the end of the recording, ionomycin (IONO) was added as a viability control. B) Fluorescence changes corresponding to sperm 1–3 of the field recorded in A. C) Percentage of sperm displaying an [Ca2+]i increase in response to vehicle, 40 μM, and 100 μM progesterone addition. Black arrows indicate the time where progesterone and ionomycin were applied. These results were obtained by analyzing 319 sperm from 25 mice; statistically significant differences at *P < 0.05 and ***P < 0.001 when compared to vehicle.
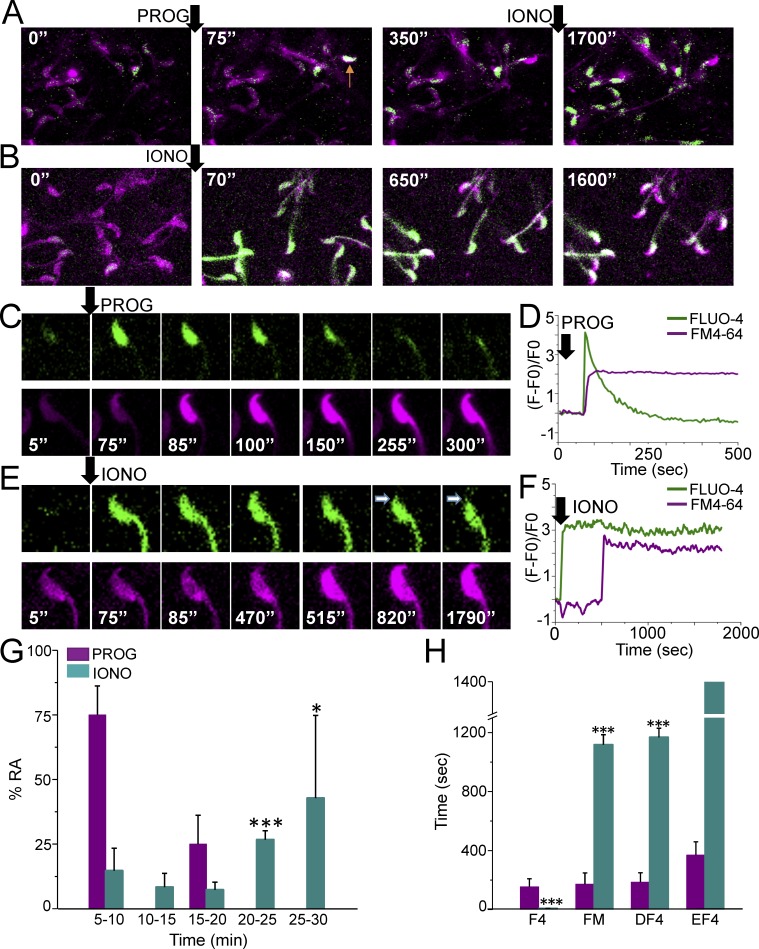
FIG. 6.
[Ca2+]i and AR alternate measurements in mouse sperm. A and B) Fluo-4 AM and FM4-64 fluorescence from sperm exposed to progesterone (PROG) or ionomycin (IONO), respectively. The arrow in the panel corresponding to 75 sec indicates a sperm that increased its [Ca2+]i and underwent AR (arrow in panel corresponding to 350 sec). C) Representative alternate Fluo-4 AM and FM4-64 fluorescence images of a sperm undergoing a progesterone response. C) Fluo-4 AM and FM4-64 fluorescence changes observed in the sperm displayed in D. E) Alternate Fluo-4 AM and FM4-64 fluorescence images of a representative sperm responding to ionomycin. F) Fluo-4 AM and FM4-64 fluorescence changes observed during the recordings of the sperm shown in E. G) Percentage of AR stimulated by progesterone or ionomycin at different times (AR time was established as the time that FM4-64 fluorescence significantly increased); n = 8 mice, 21 reacted cells and n = 4 mice, 43 reacted cells for progesterone and ionomycin experiments, respectively. Statistically significant differences between the progesterone and ionomycin response at *P < 0.05 and **P < 0.001. H) Average time at which the indicated processes occurred after progesterone or ionomycin stimulation: F4 (delay for Fluo-4 AM fluorescence increase), FM (delay for FM4-64 fluorescence increase), DF4 (delay for Fluo-4 AM fluorescence decrease), EF4 (delay for complete loss of Fluo-4 fluorescence in the head); n = 8 mice, 21 reacted cells and n = 4 mice, 43 reacted cells for progesterone and ionomycin experiments, respectively. Statistically significant differences between progesterone and ionomycin responses (***P < 0.001).
RESULTS
Progesterone Promotes [Ca2+]i Increases in Mouse Sperm
Progesterone has been proposed as the physiological inductor of the AR in human sperm. However, in mouse sperm, its role in this process is still uncertain. In the first set of experiments we observed that progesterone was able to promote an [Ca2+]i increase in mouse sperm as previously reported [15, 34, 35]. This steroid induced an [Ca2+]i increase in some sperm and at different times (Fig. 1A). Fluorescence traces in Figure 1B illustrate the [Ca2+]i responses promoted by progesterone in the three sperm of Figure 1A, which displayed different patterns in terms of amplitude and kinetics. The percentage of sperm responding to 40 and 100 μM progesterone was 24.5% ± 4.3% and 41.1% ± 5.4% respectively, which was statistically different compared to vehicle (11.3% ± 3.6%) (Fig. 1C). By using 100 μM progesterone, a higher number of cells displayed a calcium response, and because of this, we continued our experiments using this concentration.
Main Patterns of Progesterone-Induced [Ca2+]i Changes
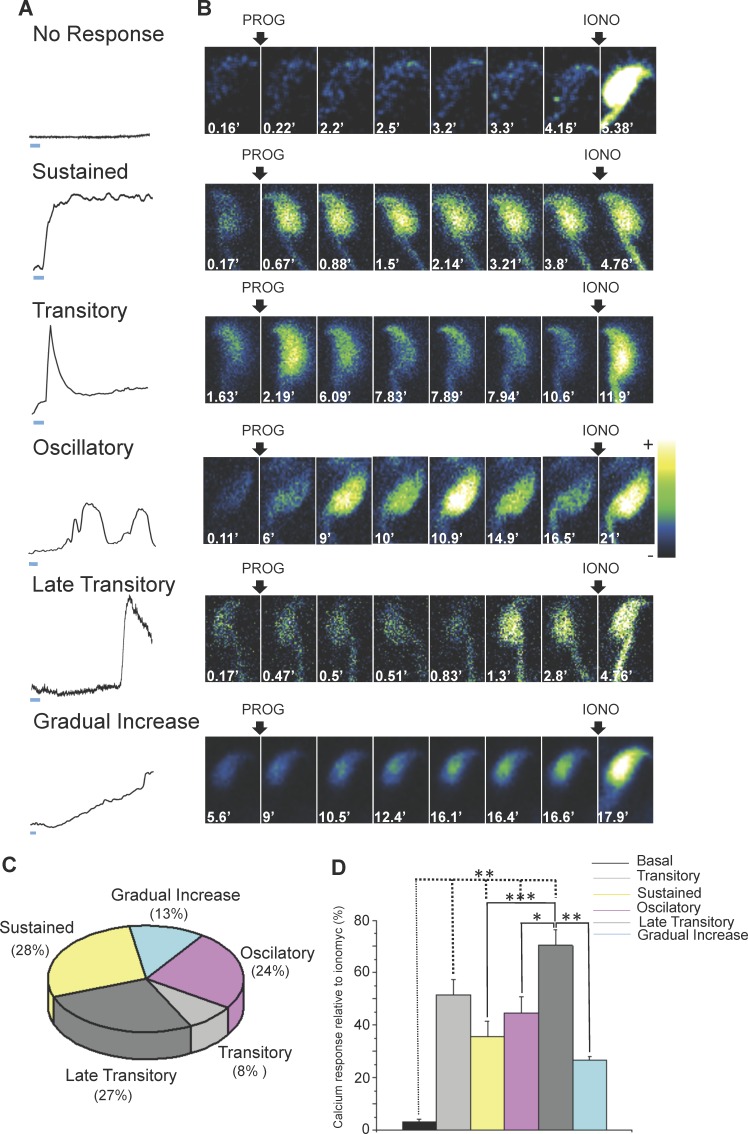
Progesterone stimulation resulted in mainly five different patterns of [Ca2+]i increase in the sperm head: sustained, immediate transitory, oscillatory, late transitory, and gradual increase (Fig. 2A). Figure 2B illustrates representative image sequences of each of these patterns. Most sperm displayed the sustained (28%) and the late transitory (27%) pattern in response to 100 μM progesterone (Fig. 2C).
FIG. 2.
Mouse sperm displayed different patterns of [Ca2+]i increase in response to progesterone. A) Graphics representing the different patterns of [Ca2+]i increase observed in the sperm head as a result of the addition of 100 μM progesterone (PROG). According to the calcium response, the patterns of increase were classified as sustained, transitory, oscillatory, late transitory, and gradual increase. The bar below the traces represents the time scale = 1 min. B) Representative time lapse images of Fluo-4 AM-loaded sperm following the addition of 100 μM progesterone and 10 μM ionomycin (IONO). C) Percentage of sperm displaying each of the patterns observed. These results were obtained by analyzing 184 sperm from 10 mice. D) Comparison of the calcium increase in response to progesterone normalized by the ionomycin response. These results were obtained by analyzing 184 sperm from 10 mice; statistically significant differences at *P < 0.05, **P < 0.01, and ***P < 0.001.
The mean progesterone response of each pattern was normalized against the maximum ionomycin response (to minimize variation among experiments) and compared to the baseline response. The magnitude of the response of each pattern was statistically different compared to the basal response except for the gradual increase response. In addition, when all the patterns were compared, the late transitory response was statistically different in comparison with the oscillatory, sustained, and gradual increase responses (Fig. 2D).
In Most Sperm, Progesterone Elevated [Ca2+]i First in the Flagellum and Then This Elevation Propagated in the Anterograde Direction
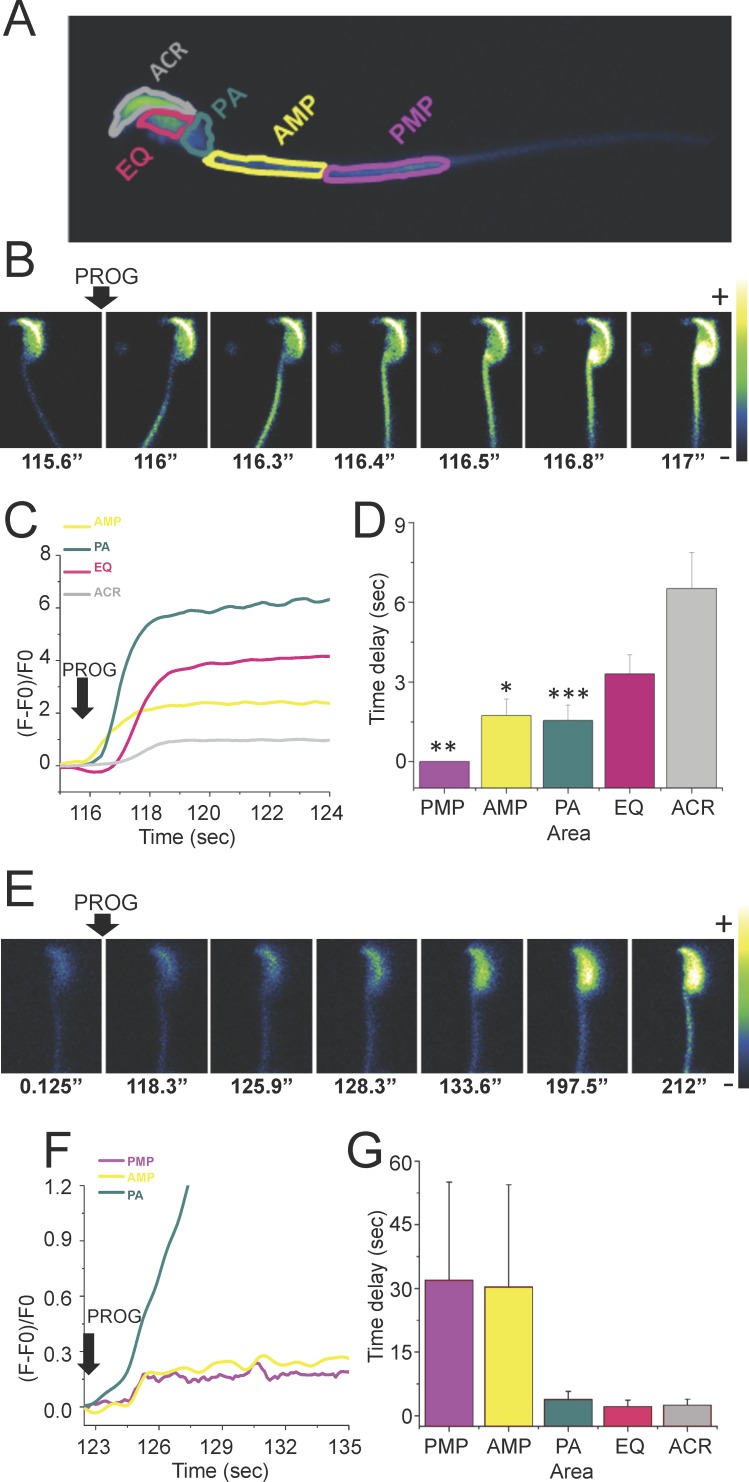
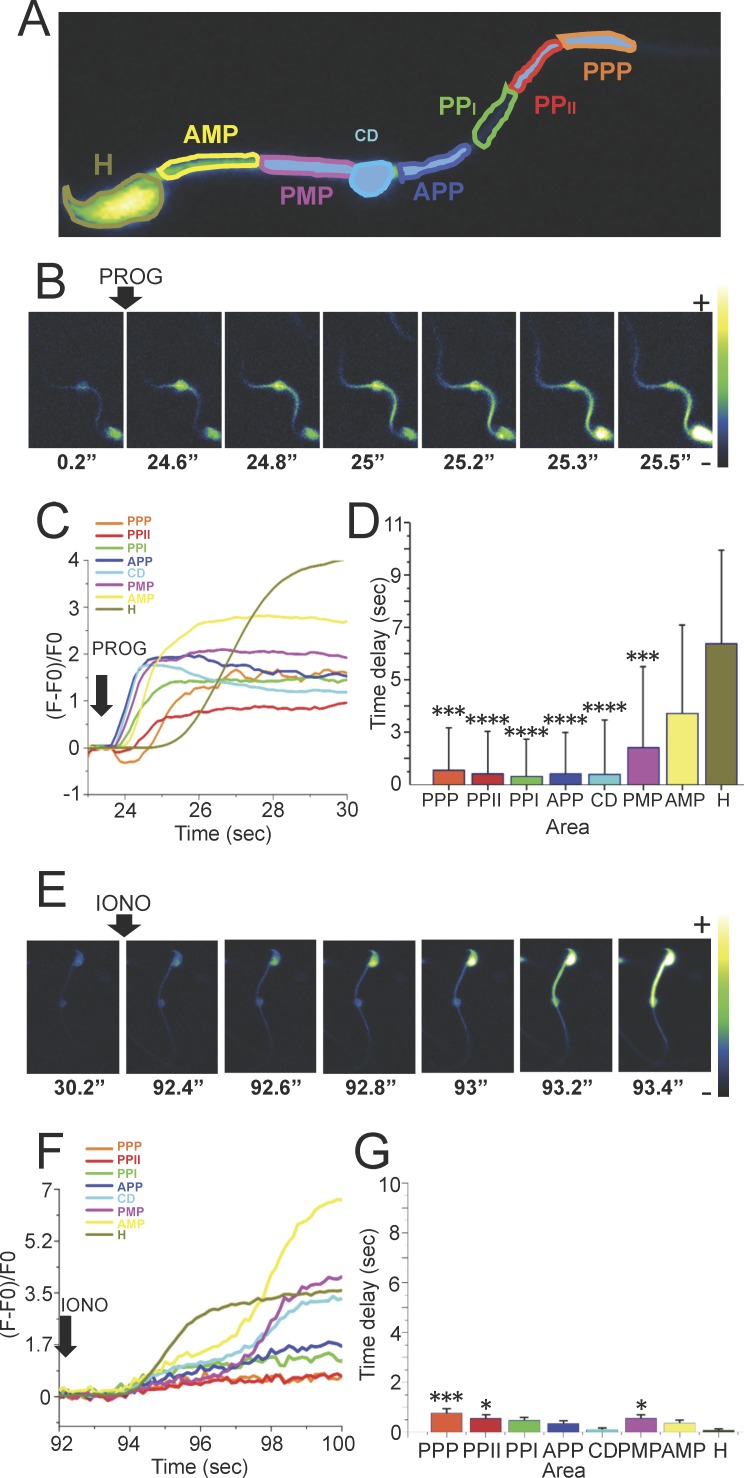
Directionality and kinetic experiments of the [Ca2+]i changes triggered by progesterone performed in laminin-coated slides, the optimal method to visualize changes in the sperm head and occasionally the midpiece, but not in the flagellar principal piece, revealed three different patterns: anterograde (from the flagellum midpiece toward the head), retrograde (from the head to the flagellum midpiece), and others (not accurately determined). Representative image sequences of a sperm where progesterone induced [Ca2+]i increases that propagated in an anterograde direction are shown in Figure 3B. The [Ca2+]i increase was first noticeable at the anterior midpiece (AMP) (see panel t = 116.3 sec) followed by its propagation in a posterior to anterior direction (see panel t = 117 sec). Time traces corresponding to this experiment are illustrated in Figure 3C. The time delays of the different analyzed areas are shown in Figure 3D. A representative sequence of images corresponding to a sperm displaying a retrograde direction of an [Ca2+]i increase is shown in Figure 3E. In this case, progesterone first elevated the [Ca2+]i at the postacrosomal region (see panel t = 133.6 sec) followed by a propagation in an anterior to posterior direction (see panel t = 212 sec). Time traces corresponding to this experiment are shown in Figure 3F. The time delays of the different analyzed areas are shown in Figure 3G. The anterograde directionality was much more frequent than retrograde propagation (85% vs. 10%, respectively).
FIG. 3.
Directionality of the [Ca2+]i increase wave propagation in response to progesterone in mouse sperm immobilized with laminin. A) Representative diagram of the different sperm areas evaluated in this experiment. PMP, posterior midpiece; AMP, anterior midpiece; PA, postacrosomal region; EQ, equatorial segment; ACR, acrosome. B) Representative images of the time course of Fluo-4 AM-labeled sperm after addition of 100 μM progesterone showing an anterograde direction of the [Ca2+]i increase wave propagation. C) Time course of the Fluo-4 AM fluorescence changes in each of the evaluated areas of the sperm shown in B. D) Different time delays of the analyzed areas in sperm with an anterograde direction of the [Ca2+]i increase propagation (n = 25). Results are expressed as the mean ± SEM. Statistically significant differences at *P < 0.05, **P < 0.01, and ****P < 0.001 when compared to ACR. E) Representative time course images of Fluo-4 AM-labeled sperm after addition of 100 μM progesterone showing a retrograde direction of the [Ca2+]i increase propagation. F) Time course of the Fluo-4 AM fluorescence changes in each of the evaluated areas of the sperm shown in E. G) Different time delays of the analyzed areas in sperm with a retrograde direction of the [Ca2+]i increase propagation (n = 25). Results are expressed as the mean ± SEM. The comparisons between all the areas were not statistically different.
To analyze the propagation characteristics of the progesterone-induced [Ca2+]i changes in the principal piece of the flagellum, sperm were attached to concanavalin A-coated slides, which immobilized most of this region (Fig. 4A). Figure 4B depicts a representative sequence of images obtained during these experiments. Progesterone first increased [Ca2+]i in the anterior principal piece (see panel t = 24.6 sec), which propagated in both anterior and posterior directions, toward the head and posterior principal piece. Time traces corresponding to this experiment are illustrated in Figure 4C. The time delays of the different analyzed areas are shown in Figure 4D.
FIG. 4.
Directionality of the [Ca2+]i increase wave propagation in response to progesterone in mouse sperm immobilized with concanavalin A. A) Representative diagram of the different sperm areas evaluated in sperm attached to concanavalin A-coated slides. PPP, posterior principal piece; PPPII, posterior principal piece II; PPPI, posterior principal piece I; APP, anterior principal piece; CD, cytoplasmic droplet; PMP, posterior midpiece; AMP, anterior midpiece; H, head. B and E) Representative images of the time course of Fluo-4 AM-labeled sperm after addition of 100 μM progesterone or 10 μM ionomycin as a control, respectively. C and F) Time course of the fluorescence changes in each of the areas of the sperm shown in B and E, respectively. D and G) Different time delays of the analyzed areas of the [Ca2+]i increase propagation in sperm stimulated with progesterone or ionomycin, respectively (n = 8). Statistically significant differences at *P < 0.05, **P < 0.001, and ***P < 0.0001 when compared to head (H).
Because the propagation differences of the [Ca2+]i changes observed could be originated due to a progesterone diffusion artifact, the same experimental protocol was performed using ionomycin, a widely used nonphysiological potent inductor of [Ca2+]i increases and the AR. Ionomycin stimulation resulted in a strong increase in [Ca2+]i at t = 92.4 sec (Fig. 4E), but in contrast to progesterone, there was no preferred initiation point site for the [Ca2+]i elevation that increased uniformly throughout the cell. The fluorescence traces corresponding to this experiment are illustrated in Figure 4F. Interestingly, it was also observed that certain regions of the sperm middle piece displayed a second and substantial calcium increase. The reason for this ionomycin-induced second increase is unknown and will require further investigations. The delays observed with ionomycin between regions were minimal compared to those recorded with progesterone and are summarized in Figure 4G.
The Mouse Sperm AR Can Be Monitored Using the FM4-64 Dye
The dye FM4-64 was recently shown to be useful to detect the AR in single human sperm [32], and its fluorescence characteristics are compatible with simultaneous recording of Fluo-4 AM fluorescence. Therefore, we validated the use of FM4-64 as an alternative method to detect the AR in single mouse sperm. We simultaneously detected EGFP and FM4-64 fluorescence in transgenic EGFP sperm exposed to 10 μM ionomycin. Those sperm that remained intact during the analysis, as judged by presence of the acrosomal EGFP content, did not increase their FM4-64 fluorescence (Fig. 5, A and B). In contrast, those sperm that underwent AR displayed the loss of EGFP and an increase in FM4-64 fluorescence (Fig. 5, C and D). We found a total correlation between the results of both methods to measure AR (n = 3 mice, 46 analyzed cells). The percentage of sperm attached to laminin-coated slides that underwent AR with progesterone and ionomycin were 8% ± 2% and 73% ± 25%, respectively (n = 10, 368 cells analyzed for progesterone; and n = 5, 105 cell analyzed for ionomycin). The percentage of cells that underwent spontaneous AR was 0.5% ± 0.5% (n = 4, 81 cells analyzed). There was a delay of 4.6 ± 0.7 sec between the loss of EGFP and the increase in FM4-64 fluorescence (n = 3 mice, 8 reacted sperm). The validation of FM4-64 as an AR indicator in mouse sperm allowed us to simultaneously visualize the rise in the levels of [Ca2+]i and the process of exocytosis in response to progesterone.
FIG. 5.
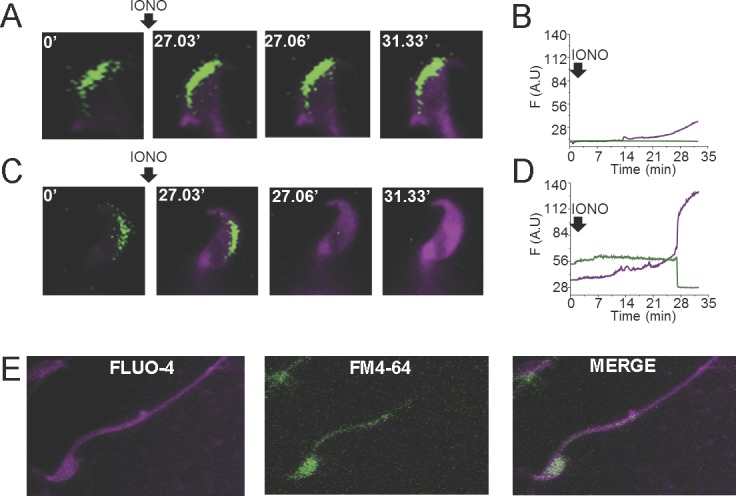
Validation of the plasma membrane probe FM4-64 as an AR indicator and its simultaneous observation with the calcium indicator Fluo-4 AM. A) Representative fluorescence images of the time course of an FM4-64-labeled EGFP-sperm that does not undergo AR after addition of 10 μM ionomycin (IONO). B) EGFP and FM4-64 fluorescence intensities (in arbitrary units) of the sperm shown in A. C) Representative fluorescence images of the time course of FM4-64-labeled EGFP-sperm undergoing AR after addition of 10 μM ionomycin. D) EGFP and FM4-64 fluorescence intensities (in arbitrary units) of the sperm shown in C. E) Fluorescence images of FM4-64- and Fluo-4 AM-labeled sperm.
Progesterone Triggers a Transitory [Ca2+]i Increase That Promotes AR in Mouse Sperm
The levels of [Ca2+]i and AR were examined in sperm loaded concomitantly with FM4-64 and Fluo-4AM attached to laminin-coated slides and recorded for ∼30 min after progesterone or ionomycin stimulation (Fig. 5E). Figure 6A depicts representative images of an experiment where progesterone induced AR only in one sperm (as judged by FM4-64 staining) despite several cells displaying an [Ca2+]i response (at t = 75 and 350 sec). In contrast to progesterone, ionomycin increased [Ca2+]i in most sperm (t = 70 sec). However, only one sperm stimulated by ionomycin underwent AR at t = 650 sec and it was not until t = 1600 sec that several sperm initiated the AR (Fig. 6B). Figure 6C illustrates with higher magnification that the [Ca2+]i increase promoted by progesterone (t = 75 sec) preceded the increase in FM4-64 fluorescence (t = 85 sec). When FM4-64 staining became evident, a gradual decrease in the Fluo-4 AM fluorescence was observed, while FM4-64 fluorescence remained unchanged (Fig. 6, C and D). On the other hand, stimulation with ionomycin revealed that a sustained [Ca2+]i increase preceded the initiation of the AR for a longer period of time. Interestingly, ionomycin did not promote the loss of Fluo-4 AM fluorescence in the entire head as observed with progesterone (Fig. 6, E and F). Sperm that underwent AR after progesterone addition began losing Fluo-4 AM fluorescence 14 sec after AR initiation. In contrast, experiments using ionomycin showed that only a local loss of Fluo-4 AM fluorescence was noticeable until 50 sec after AR initiation. Only a small area resembling the acrosome was depleted after AR (see arrow in Fig. 6E). Traces corresponding to this experiment are shown in Figure 6F.
As indicated above, mouse sperm displayed five different patterns of [Ca2+]i increase in the head in response to progesterone (Fig. 2B). However, the specific [Ca2+]i increase pattern that was associated with the initiation of the AR in all the cells analyzed was of the immediate transitory pattern (Fig. 6D; n = 8 mice, 21 reacted cells). Further analysis revealed that most sperm underwent AR in response to progesterone during the initial 5–10 min after stimulation (75% ± 11.1%) (Fig. 6G). In contrast, most sperm underwent AR in response to ionomycin only after 20 min of its addition. Progesterone induced AR in fewer cells, but AR begun sooner after the [Ca2+]i increase. In contrast, ionomycin triggered the AR in more sperm, but the AR started later after the [Ca2+]i increase.
The dynamics of [Ca2+]i and AR were further examined in sperm either stimulated with progesterone or ionomycin. The parameters analyzed were: F4 (delay for Fluo-4 fluorescence increase), FM (delay for FM4-64 fluorescence increase), DF4 (delay for Fluo-4 AM fluorescence decrease), and EF4 (delay for complete loss of Fluo-4 AM fluorescence in the head). Figure 6H summarizes the data obtained from experiments using progesterone and ionomycin stimulation. The results of the above analysis indicate that the time relationship between the increases in [Ca2+]i and AR is very different depending on the agonist applied. On average, ionomycin increased [Ca2+]i earlier (2.5 ± 0.6 sec) than progesterone (155 ± 54 sec), but notably, most of the cells underwent AR before with progesterone (172 ± 76 sec) than with ionomycin (1117 ± 68 sec) (Fig. 6, G and H).
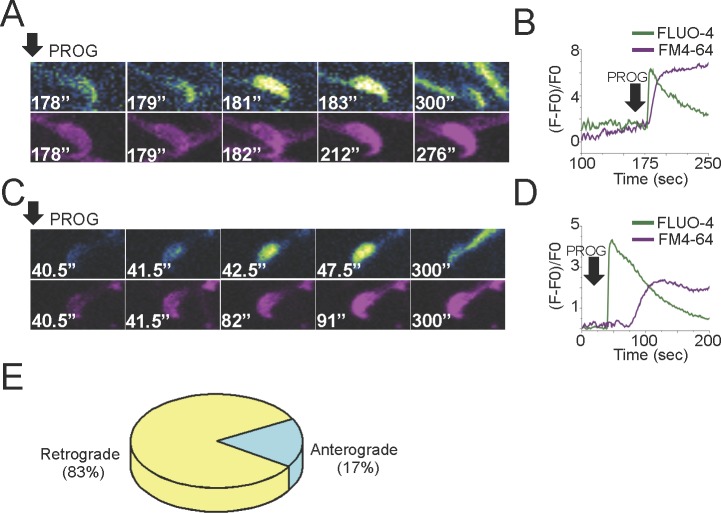
An Anterior-Posterior [Ca2+]i Increase Promoted by Progesterone Induces the AR in Mouse Sperm
Previous experiments were performed at approximately an image every 5 sec, where cellular photodamage was minimal. However, to spatially resolve the [Ca2+]i increase that leads to the initiation of the AR, a higher time resolution was needed. Thus, experiments were performed using an image splitter to simultaneously record Fluo-4 AM and FM4-64 fluorescence and enhance the speed of image acquisition to two images per second while minimizing sperm damage.
We analyzed the directionality of the propagation of the increase in [Ca2+]i in those sperm undergoing AR. The anterograde (from the tail to the head) and retrograde (from the head to the tail) definitions used in Figure 7 are the same as those used in Figure 3. However, although we can clearly observe the directionality of the calcium wave propagation, the number of images acquired per second in these experiments was the minimum to reduce cellular damage, so we could not do the detailed regional analysis as displayed in Figure 3. An example of a sperm where the progesterone [Ca2+]i increase propagated from the posterior area of the sperm head to the anterior zone is depicted in Figure 7C. The corresponding fluorescence traces of the experiment are illustrated in Figure 7D. This direction of the [Ca2+]i increase occurs in 17% of the cells undergoing AR. On the other hand, in 83% of the cells undergoing AR, progesterone initiated the [Ca2+]i increase in the anterior head zone (Fig. 7A). The corresponding fluorescence traces of the experiment are illustrated in Figure 7B. These results are summarized in Figure 7E.
FIG. 7.
Simultaneous [Ca2+]i and AR recordings. A) Fluo-4 AM and FM4-64 images of a representative sperm undergoing AR in which progesterone increases the [Ca2+]i in the posterior area of the sperm head and propagates to the anterior zone. The corresponding Fluo-4 AM and FM4-64 fluorescence traces are shown in B. C) Fluo-4 AM and FM4-64 images of a representative example of a cell in which progesterone initiated an [Ca2+]i increase in the anterior head zone. The corresponding Fluo-4 AM and FM4-64 fluorescence traces are shown in D. E) Percentage of sperm that underwent AR with anterograde or retrograde propagation (n = 4 mice, 17 reacted cells).
DISCUSSION
A common and fundamental feature of physiological and pharmacological AR stimulants such as progesterone or ZP is that they provoke intracellular multicomponent Ca2+ rises [24, 36–38]. However, the spatiotemporal relationship between the [Ca2+]i rise and the progesterone-induced AR had not been fully established before in mouse spermatozoa. In this paper, we demonstrate for the first time that a specific pattern of [Ca2+]i increase originating in the sperm head efficiently triggers the AR induced by progesterone.
Progesterone has been implicated not only in AR but also in several sperm functions such as capacitation, hyperactivation, and chemotaxis [39–41]. The concentration of progesterone that is necessary for these events varies according to the species, but they are usually in the micromolar range, except for chemotaxis that is in the picomolar range [21, 42]. The specific function stimulated by progesterone is likely related to its concentration, the distribution of its receptors, and the spatiotemporal pattern of [Ca2+]i changes that occurs as a result of its actions. Human follicular fluid has been reported to contain ∼5–10 μM free progesterone [43]; however, its effective concentration could be five-fold higher considering the corticosteroid-binding globulin contained in the follicular fluid [44].
Only a subset of mouse sperm responded with [Ca2+]i elevations when stimulated with progesterone. These responses were varied: slow and sustained, fast and either maintained or transitory, and oscillatory (Fig. 2). Among the [Ca2+]i increases induced by progesterone in mouse sperm, 8% displayed an immediate transitory pattern of Ca2+ increase, which to our surprise were the only sperm able to trigger the AR. These numbers are consistent with other reports pointing out that a reduced fraction of the cells undergo exocytosis after stimulation with progesterone [15, 34, 35]. The small percentage of acrosome-reacted sperm may also result from the fact that at any given time during capacitation only a small fraction of sperm have undergone the molecular events necessary to achieve this reaction [45].
In contrast to what was reported in mouse sperm, observations of the progesterone-induced AR and [Ca2+]i changes in human sperm revealed that a second [Ca2+]i increase precedes AR and the loss of Fluo-4 AM fluorescence. The presence of the second [Ca2+]i peak is the signal to initiate the AR in the majority of cells [32]. In humans, AR does not initiate in the same region in every sperm. By using ionomycin, sperms initiate the increase in [Ca2+]i with no preference regarding different sperm head regions in mouse and human sperm [32]. In contrast, AR induced by a more physiological stimulus like progesterone typically starts at the anterior region of the sperm head. A similar conclusion was reached in mouse spermatozoa regarding the starting point of AR promoted by either ZP or Ca2+ ionophore [31]. Similarly to what we observed in mouse sperm, most human sperm that experience [Ca2+]i oscillations after progesterone addition fail to undergo AR.
There is still controversy regarding the origin of the [Ca2+]i increase that leads to AR. Some reports claim that the [Ca2+]i increase induced by ZP initiates in the sperm flagellum [26], while others describe only the Ca2+ dynamics in the sperm head without analyzing those occurring in the flagella [46]. Our studies consistently indicated that only the [Ca2+]i rise originating in the sperm head is able to promote AR in response to progesterone. It has been claimed that Ca2+ is stored in two main organelles: the acrosome itself and in vesicles from the redundant nuclear envelope [27, 29, 47]. The initial and transient Ca2+ elevation activates a Ca2+-sensitive PLCδ that hydrolyzes phospatidylinositol 4,5-bisphosphate to produce diacyl glycerol and inositol 1,4,5-trisphosphate (IP3) [46]. The absence of PLCδ4/ATLII significantly impairs the [Ca2+]i release induced by ZP or progesterone [30]. Both, the acrosome and the redundant nuclear envelope possess IP3 receptors, which are essential for the IP3-induced Ca2+ release. Because specific inhibitors to selectively block Ca2+ release from these reservoirs are still not available, it has not been possible to determine which Ca2+ store(s) is responsible for releasing this cation in the final steps of the AR.
According to our findings, only mouse sperm that display a transitory rise in [Ca2+]i in response to progesterone undergo AR within seconds. Sperm displaying a persistent [Ca2+]i elevation did not undergo exocytosis. This agrees with our observation that sperm stimulated with ionomycin immediately increased their [Ca2+]i in a sustained manner, but interestingly, they did not undergo significant exocytosis until 20 min later. It is remarkable that in this situation, most of the cells underwent AR. The molecular basis of this difference is currently unknown. However, it is possible that a rapid decrease in [Ca2+]i is important to allow exocytosis in an efficient manner. In the case of progesterone, the [Ca2+]i decrease observed may be caused by a fast uptake into calcium stores present in the sperm head and efflux to the extracellular medium, and not to the loss of the dye during exocytosis caused by plasma membrane vesiculation, because the addition of Ca2+ ionophore to those cells that displayed this transitory pattern and efficiently triggered AR resulted in a new increase in [Ca2+]i (see Fig. 2). In this regard, it was recently shown that the calcium ATPase SPCA1 is critical in refilling the Ca2+ stores in the human sperm head after addition of progesterone [48]. These possibilities need to be further explored in the future.
The identity of the receptor involved in the progesterone-induced AR is still unknown. The effect of progesterone in sperm does not seem to involve the classical regulation by nuclear receptors. Some nongenomic progesterone receptors have been reported to be present in mammalian sperm membrane, including CatSper (see the references in [22, 23, 49]). Early observations indicated the involvement of a unique human sperm steroid receptor/Cl− channel complex in the progesterone-initiated AR [50]. In other studies, it was proposed that a gamma-aminobutyric acid-like receptor participates in this process in mouse spermatozoa [51]. Along this idea, it was also put forward that a rapid Cl− efflux is necessary for the progesterone-mediated human AR [52]. With the available new tools, it is expected that the mechanisms by which progesterone triggers the AR will be unraveled in the near future.
In summary, progesterone produces different Ca2+ responses in mouse sperm, but only a specific transitory increase in [Ca2+]i promotes acrosomal exocytosis. These results emphasize the importance of studying the different aspects of sperm physiology in single cells using live imaging methods.
ACKNOWLEDGMENT
We would like to thank Nicolás Gilio, Jorge Torres, Jose Luis de la Vega-Beltran, Paulina Torres-Rodríguez, Yoloxochitl Sánchez, Takuya Nishigaki Shimizu, Nicolás Jiménez, and Claudia Rivera-Cerecedo for their advice and technical assistance. Also, we are grateful to Ana Maria Escalante, Francisco Pérez (IFC-UNAM), and Omar Arriaga (IBT-UNAM) for expert computing assistance.
Footnotes
This work was supported by the National Institutes of Health (R01TW008662 to M.G.B. and HD038082 to A.D.), Agencia Nacional de Promoción Cientifica y Tecnológica (PICT 2013-1175 to M.G.B.), CONICET (PIP 740 to M.G.B.), Dirección General de Asuntos del Personal Académico (DGAPA-UNAM, grants IN222413 and IN211616 to A.H.C.), and Consejo Nacional de Ciencia y Tecnología (CONACYT, grants 260866 240305). We also want to thank PLISSER fellowship and Rene Baron Foundation.
These authors contributed equally to this work.
REFERENCES
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B Biol Sci. 1951;4:581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Foster JA, Gerton GL. The role of the acrosomal matrix in fertilization. Int J Dev Biol. 2008;52:511–522. doi: 10.1387/ijdb.072532mb. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Ijiri TW, Cao W, Merdiushev T, Aghajanian HK, Gerton GL. Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev. 2012;79:4–18. doi: 10.1002/mrd.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–657. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- Sosnik J, Miranda PV, Spiridonov NA, Yoon S-Y, Fissore RA, Johnson GR, Visconti PE. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci. 2009;122:2741–2749. doi: 10.1242/jcs.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Hirohashi N, Gerton GL. Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biol Reprod. 2014;90:112. doi: 10.1095/biolreprod.114.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life. 2007;59:286–292. doi: 10.1080/15216540701222872. [DOI] [PubMed] [Google Scholar]
- Cherr GN, Lambert H, Meizel S, Katz DF. In vitro studies of the golden hamster sperm acrosome reaction: completion on the zona pellucida and induction by homologous soluble zonae pellucidae. Dev Biol. 1986;114:119–131. doi: 10.1016/0012-1606(86)90388-x. [DOI] [PubMed] [Google Scholar]
- Florman HM, Storey BT. Mouse gamete interactions: the zona pellucida is the site of the acrosome reaction leading to fertilization in vitro. Dev Biol. 1982;91:121–130. doi: 10.1016/0012-1606(82)90015-x. [DOI] [PubMed] [Google Scholar]
- Storey BT, Lee MA, Muller C, Ward CR, Wirtshafter DG. Binding of mouse spermatozoa to the zonae pellucidae of mouse eggs in cumulus: evidence that the acrosomes remain substantially intact. Biol Reprod. 1984;31:1119–1128. doi: 10.1095/biolreprod31.5.1119. [DOI] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci U S A. 2011;108:20008–20011. doi: 10.1073/pnas.1116965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman RA, Andria ML, Jones AD, Meizel S. Steroid induced exocytosis: the human sperm acrosome reaction. Biochem Biophys Res Commun. 1989;160:828–833. doi: 10.1016/0006-291x(89)92508-4. [DOI] [PubMed] [Google Scholar]
- Roldan ER, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 1994;266:1578–1581. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- Oren-Benaroya R, Orvieto R, Gakamsky A, Pinchasov M, Eisenbach M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum Reprod. 2008;23:2339–2345. doi: 10.1093/humrep/den265. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- Sagare-Patil V, Galvankar M, Satiya M, Bhandari B, Gupta SK, Modi D. Differential concentration and time dependent effects of progesterone on kinase activity, hyperactivation and acrosome reaction in human spermatozoa. Int J Androl. 2012;35:633–644. doi: 10.1111/j.1365-2605.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- Servin-Vences MR, Tatsu Y, Ando H, Guerrero A, Yumoto N, Darszon A, Nishigaki T. A caged progesterone analog alters intracellular Ca2+ and flagellar bending in human sperm. Reproduction. 2012;144:101–109. doi: 10.1530/REP-11-0268. [DOI] [PubMed] [Google Scholar]
- Gatica LV, Guidobaldi HA, Montesinos MM, Teves ME, Moreno AI, Uñates DR, Molina RI, Giojalas LC. Picomolar gradients of progesterone select functional human sperm even in subfertile samples. Mol Hum Reprod. 2013;19:559–569. doi: 10.1093/molehr/gat037. [DOI] [PubMed] [Google Scholar]
- Guidobaldi HA, Teves ME, Uñates DR, Anastasía A, Giojalas LC. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. Plos One. 2008;3:e3040. doi: 10.1371/journal.pone.0003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Treviño CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev. 2011;91:1305–1355. doi: 10.1152/physrev.00028.2010. [DOI] [PubMed] [Google Scholar]
- Chung J-J, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun. 2011;2:153. doi: 10.1038/ncomms1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Ren D. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol Reprod. 2009;80:1092–1098. doi: 10.1095/biolreprod.108.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas G, Michaut M, Treviño CL, Tomes CN, Yunes R, Darszon A, Mayorga LS. The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. J Biol Chem. 2002;277:49326–49331. doi: 10.1074/jbc.M208587200. [DOI] [PubMed] [Google Scholar]
- Herrick SB, Schweissinger DL, Kim S-W, Bayan KR, Mann S, Cardullo RA. The acrosomal vesicle of mouse sperm is a calcium store. J Cell Physiol. 2005;202:663–671. doi: 10.1002/jcp.20172. [DOI] [PubMed] [Google Scholar]
- Ho H-C, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod. 2003;68:1590–1596. doi: 10.1095/biolreprod.102.011320. [DOI] [PubMed] [Google Scholar]
- Fukami K, Yoshida M, Inoue T, Kurokawa M, Fissore RA, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cdelta4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J Cell Biol. 2003;161:79–88. doi: 10.1083/jcb.200210057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Rodriguez-Miranda E, Storey BT, Gerton GL. Acrosomal exocytosis of mouse sperm progresses in a consistent direction in response to zona pellucida. J Cell Physiol. 2009;220:611–620. doi: 10.1002/jcp.21781. [DOI] [PubMed] [Google Scholar]
- Sánchez-Cárdenas C, Servín-Vences MR, José O, Treviño CL, Hernández-Cruz A, Darszon A. Acrosome reaction and Ca2+ imaging in single human spermatozoa: new regulatory roles of [Ca2+]i. Biol Reprod. 2014;91:67. doi: 10.1095/biolreprod.114.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasuwa H, Muro Y, Ikawa M, Kato N, Tsujimoto Y, Okabe M. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp Anim Jpn Assoc Lab Anim Sci. 2010;59:105–107. doi: 10.1538/expanim.59.105. [DOI] [PubMed] [Google Scholar]
- Kobori H, Miyazaki S, Kuwabara Y. Characterization of intracellular Ca(2+) increase in response to progesterone and cyclic nucleotides in mouse spermatozoa. Biol Reprod. 2000;63:113–120. doi: 10.1095/biolreprod63.1.113. [DOI] [PubMed] [Google Scholar]
- Hirohashi N, Spina FAL, Romarowski A, Buffone MG. Redistribution of the intra-acrosomal EGFP before acrosomal exocytosis in mouse spermatozoa. Reprod Camb Engl. 2015;149:657–663. doi: 10.1530/REP-15-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meizel S, Turner KO, Nuccitelli R. Progesterone triggers a wave of increased free calcium during the human sperm acrosome reaction. Dev Biol. 1997;182:67–75. doi: 10.1006/dbio.1997.8477. [DOI] [PubMed] [Google Scholar]
- Marín-Briggiler CI, Gonzalez-Echeverría F, Buffone M, Calamera JC, Tezón JG, Vazquez-Levin MH. Calcium requirements for human sperm function in vitro. Fertil Steril. 2003;79:1396–1403. doi: 10.1016/s0015-0282(03)00267-x. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Kirichok Y, Ren D, Navarro B, Chung J-J, Clapham DE. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol. 2012;74:453–475. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboni B, Mattioli M, Seren E. Influence of progesterone on boar sperm capacitation. J Endocrinol. 1995;144:13–18. doi: 10.1677/joe.0.1440013. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Ho HC. Hyperactivation of mammalian sperm. Cell Mol Biol. 2003;49:351–356. [PubMed] [Google Scholar]
- Baldi E, Luconi M, Muratori M, Marchiani S, Tamburrino L, Forti G. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions. Mol Cell Endocrinol. 2009;308:39–46. doi: 10.1016/j.mce.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Oren-Benaroya R, Orvieto R, Gakamsky A, Pinchasov M, Eisenbach M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum Reprod. 2008;23:2339–2345. doi: 10.1093/humrep/den265. [DOI] [PubMed] [Google Scholar]
- Westergaard LG, Erb K, Laursen SB, Rasmussen PE, Rex S, Westergaard CG, Andersen CY. Concentrations of gonadotrophins and steroids in pre-ovulatory follicular fluid and serum in relation to stimulation protocol and outcome of assisted reproduction treatment. Reprod Biomed Online. 2004;8:516–523. doi: 10.1016/s1472-6483(10)61097-8. [DOI] [PubMed] [Google Scholar]
- Miska W, Fehl P, Henkel R. Biochemical and immunological characterization of the acrosome reaction-inducing substance (ARIS) of hFF. Biochem Biophys Res Commun. 1994;199:125–129. doi: 10.1006/bbrc.1994.1203. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Wertheimer EV, Visconti PE, Krapf D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim Biophys Acta. 2014;1842:2610–2620. doi: 10.1016/j.bbadis.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N, Takenawa T. Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science. 2001;292:920–923. doi: 10.1126/science.1059042. [DOI] [PubMed] [Google Scholar]
- Correia J, Michelangeli F, Publicover S. Regulation and roles of Ca2+ stores in human sperm. Reproduction. 2015;150:R65–R76. doi: 10.1530/REP-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Wootton L, Michelangeli F, Lefièvre L, Barratt C, Publicover S. Secretory pathway Ca(2+)-ATPase (SPCA1) Ca(2)+ pumps, not SERCAs, regulate complex [Ca(2+)](i) signals in human spermatozoa. J Cell Sci. 2005;118:1673–1685. doi: 10.1242/jcs.02297. [DOI] [PubMed] [Google Scholar]
- Thomas P, Tubbs C, Garry VF. Progestin functions in vertebrate gametes mediated by membrane progestin receptors (mPRs): identification of mPRalpha on human sperm and its association with sperm motility. Steroids. 2009;74:614–621. doi: 10.1016/j.steroids.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Wistrom CA, Meizel S. Evidence suggesting involvement of a unique human sperm steroid receptor/Cl- channel complex in the progesterone-initiated acrosome reaction. Dev Biol. 1993;159:679–690. doi: 10.1006/dbio.1993.1274. [DOI] [PubMed] [Google Scholar]
- Shi QX, Roldan ER. Evidence that a GABAA-like receptor is involved in progesterone-induced acrosomal exocytosis in mouse spermatozoa. Biol Reprod. 1995;52:373–381. doi: 10.1095/biolreprod52.2.373. [DOI] [PubMed] [Google Scholar]
- Turner KO, Meizel S. Progesterone-mediated efflux of cytosolic chloride during the human sperm acrosome reaction. Biochem Biophys Res Commun. 1995;213:774–780. doi: 10.1006/bbrc.1995.2197. [DOI] [PubMed] [Google Scholar]