Abstract
The risks associated with in vivo and ex vivo use of Campath-1H and -1G in a cohort of 206 stem cell transplant recipients for cytomegalovirus (HCMV) DNAemia have been quantified. DNAemia showed a biphasic incidence pattern with an inflexion at day 60. The first phase had a linear risk rate for HCMV DNAemia of 0.3 % day−1 whilst the second phase had a substantially lower risk rate of 0.058 % day−1. In multivariable analyses, risk factors for early DNAemia were HCMV serostatus, radiotherapy based conditioning and CD34 stem cell dose, with the use of in vivo Campath-1H having the most significant risk (Hazards Ratio = 3.68 (95% CI 2.02-6.72; p<0.001). Ex vivo use of Campath was not associated with an increased risk for HCMV DNAemia. Patients receiving either in vivo Campath-1H or -1G experienced HCMV DNAemia earlier (27 and 33 days respectively) compared to patients receiving no Campath (time to DNAemia, 51 days; p = 0.0006). Multivariable analysis of risk factors for HCMV DNAemia occurring beyond 100 days after transplant were older age, acute GVHD > grade II and a lower CD34 stem cell dose whereas Campath-1H use was not associated with late HCMV DNAemia.
Keywords: CMV Disease, immune response, replication kinetics
Introduction
Human cytomegalovirus (HCMV) remains an important pathogen in patients undergoing stem cell transplantation. In contrast to the situation in solid organ transplant recipients where prophylaxis with valganciclovir is widely deployed with beneficial effects, prophylaxis for stem cell transplant recipients (1) does not reduce overall mortality due to increased bacterial and fungal infections secondary to ganciclovir induced myelosuppression. Furthermore, prophylactic administration of ganciclovir has been shown to delay the recovery of HCMV specific immune responses (2) so contributing to the increased risk of late HCMV disease. Consequently, the preferred strategy to manage HCMV infection and disease has been through pre-emptive therapy based on viral monitoring either by antigenaemia, qualitative and more recently, quantitative real time PCR methods (3). Pre-emptive therapy has the advantage of preventing direct effects of HCMV such as pneumonitis and gastrointestinal tract disease while minimising exposure to myelosuppressive and nephrotoxic drugs while still allowing limited replication to provide immune priming within the reconstituting immune system (reviewed in (4)). Recent data has illustrated that the quality of the T-cell response against HCMV is a major factor in the control of HCMV replication after SCT (5-8).
Despite the use of HCMV surveillance and pre-emptive therapy, late HCMV infection (>100 days) remains a problem (9-11). Risk factors for late HCMV infection include HCMV antigenaemia before three months, lymphopaenia (less than 100 cells/ul), undetectable HCMV specific T cell responses, and GVHD. The incidence of late disease among HCMV seropositive patients has been reported to be up to 17.8 % with an associated mortality of 46 % (9;10). GVHD following allogeneic transplantation is a significant cause of mortality and impaired quality of life. The risk of GVHD can be reduced by depleting T-cells from the graft with Campath-1, a monoclonal antibody against CD52, an antigen found on T cells, B cells, NK cells and monocytes (12). The rat IgM form, Campath-1M, is effective at depleting donor T-cells from the graft and reducing the risk of GVHD when used ex vivo with autologous serum as a source of complement (13). However, this technique has been associated with an increased risk of graft rejection probably mediated by host T-cells (14). To overcome this issue, Campath-1G (the rat IgG form) has been used in vivo to deplete host T-cells prior to infusion of the graft. Campath-1G relies on antibody dependent cell-mediated cytotoxicity (ADCC) in vivo (15) and is effective in reducing acute but not chronic GVHD (16). More recently, a recombinant humanised form of anti-CD52, Campath-1H (Alemtuzumab) has been developed which has superseded the use of Campath-1M and 1G both for ex-vivo and in vivo use (17;18). The use of Campath-1H in the reduced intensity conditioned (RIC) setting has been reported to be associated with an increased frequency of HCMV infection (19;20). However, the relative risks associated with the use of Campath-1H or 1G for early and late HCMV DNAemia in the context of other risk factors has not been fully quantified. The present study addresses this issue.
Patients and Methods
Patients
All patients receiving an allogeneic stem cell transplant between 1st of January 1995 and 31st of December 2000 were retrospectively identified. Patient characteristics including age (children were defined as age ≤ 16 years), gender, indication for transplant, disease status at the time of transplant, donor type, stem cell source, degree of HLA match, donor and recipient HCMV serostatus, conditioning regimen, form of T-cell depletion as well as the clinical outcome were collected using the clinical notes and checked against the transplant database. Risk stratification (good prognosis/poor prognosis) was performed on the basis of underlying disease according to standard criteria as detailed in (20). Patients were regarded as being a complete HLA match if they were identical at Class I A, B and Class II DR by PCR-based intermediate resolution HLA typing performed by the Anthony Nolan Trust, London.
Conditioning and GVHD Prophylaxis
Standard conditioning regimens were used depending on the indication for transplantation. Non-myeloablative transplants were non-total body irradiation (TBI) based, using fludarabine, Campath and either busulfan, melphalan, or BEAM (carmustine, etoposide, cytosine arabinoside and melphalan), depending on the underlying condition. Second transplants were conditioned with a fludarabine, cytosine arabinoside and idarubicin based regimen as described previously (21). Non T-cell depleted transplants, with the exception of syngeneic transplants, received short course methotrexate and ciclosporine for GVHD prophylaxis. In the case of T-cell depleted transplants, GVHD prophylaxis with ciclosporine was only used for HLA mismatched or aplastic anaemia transplants. Ex-vivo T-cell depletion was performed by the addition of 20mg of Campath to the stem cell graft for a minimum of 30 minutes at room temperature prior to infusion into the recipient. Campath-1G was used prior to 15th October 1998, and Campath-1H subsequently. In 7 cases ex-vivo T-cell depletion was performed by positive CD34 selection using the Baxter Isolex system (22). In-vivo T-cell depletion was performed by the administration of Campath (Campath-1G prior to 15th October 1998 and Campath-1H subsequently) to the transplant recipient prior to transplantation. The standard regimen consisted of 20mg Campath-1G or Campath-1H administered intravenously daily for 5 days prior to infusion of the graft, with a dose reduction for paediatric patients (aged 16 or under, 0.2mg/kg/day for 5 days). Radiotherapy when used in the conditioning regimen (N=152) was TBI at a 7.5 Gray target dose given as a single fraction, with the exception of two patients who received fractioned TBI (six fractions over three days). In addition, eight patients received total lymphoid irradiation (TLI), and five patients with Fanconi anaemia received thoraco-abdominal (TA) irradiation.
Infection Prophylaxis
All patients were nursed in positive pressure HEPA (High efficiency particulate air filter) filtered rooms. Ciprofloxacin, amphotericin suspension, and itraconazole or fluconazole were commenced pre-transplant. Pneumocystis pneumonia prophylaxis consisted of nebulized pentamidine every four weeks until engraftment, then oral trimethoprim/sulfamethoxazole 480 mg twice daily two days per week (dose adjusted for paediatric patients). All HCMV seropositive transplant recipients and seronegative recipients with a HCMV seropositive donor received aciclovir (10mg/kg intravenously three times daily) during their inpatient stay, followed by aciclovir (800mg four times daily) or valaciclovir (2 g four times daily) until 100 days post transplant. Doses were modified in the presence of renal impairment. Otherwise, aciclovir (200mg three times daily) was administered as prophylaxis for Herpes Simplex Virus (HSV) if HSV serology was positive. HCMV seronegative and leucodepleted blood products were given to HCMV seronegative patients (irrespective of donor HCMV serology).
HCMV PCR Monitoring
DNA was extracted from whole blood using the QiaAMP DNA Blood minikit (Qiagen, UK). Qualitative HCMV PCR was performed using an in-house PCR assay (23). The lower limit of sensitivity of this assay is 200 genome copies/ml of whole blood. Stem cell transplant recipients underwent a minimum protocol of HCMV PCR monitoring comprising twice weekly as an inpatient, then weekly for the first month following discharge, and fortnightly thereafter. Monitoring was continued for a minimum of three months. During the period of analysis only qualitative PCR data was available.
HCMV Pre-emptive Therapy
HCMV pre-emptive therapy was commenced after two consecutive HCMV PCR positive results. HCMV DNAemia was treated with either ganciclovir (5mg/kg intravenously twice daily), or foscarnet (90mg/kg intravenously twice daily) or a combination of both at half dose (24). Doses were adjusted in the presence of renal failure according to the manufacturer’s guidelines. G-CSF was administered to counteract the bone marrow suppressive effect of ganciclovir when the absolute neutrophiul count was below 1 × 109 cells/L. Pre-emptive HCMV therapy was continued until two consecutive negative HCMV PCR results were obtained.
Clinical Outcomes
The major endpoints of this study were time to first HCMV DNAemia, late HCMV DNAemia, and HCMV disease. Time to HCMV DNAemia was defined as the time between transplant and the first positive HCMV PCR result. Late HCMV DNAemia was defined as any DNAemia occurring after day 100, irrespective of whether a HCMV DNAemia had occurred prior to 100 days. Other endpoints of the study were overall survival, relapse free survival and time to death. Acute GVHD was graded according to standard criteria (25).
Statistical Analysis
Statistical analysis was performed using SPSS version 12.1. Kaplan Meier survival analysis was used to calculate rates of HCMV DNAemia, acute GVHD rates, overall survival, and relapse free survival. The null hypothesis of the influence of prognostic factors was tested by the log rank score, and p values were two tailed. Univariable risk analysis for HCMV DNAemia was performed using Cox proportional hazards regression models, and multivariable risk analysis was performed using a backwards selection procedure (26). The null hypothesis for regression analysis was tested by the Wald statistic, and the relative risks were estimated as hazard ratios. Median time to HCMV DNAemia was compared using the Mann Whitney U test.
Results
Patients and transplant related outcomes
The characteristics of the 206 patients studied are summarised in Table 1. The majority of the patients analysed were adults (76 %) with 83 patients (40 %) receiving ex vivo T-cell depletion with either Campath-1H or Campath-1G, 96 patients (46 %) receiving in vivo depletion with either Campath-1H or -1G and 22 patients (11 %) receiving only ex vivo depletion with Campath (1H or 1G). A subset of patients received both in vivo and ex vivo depletion with Campath-1H (n=31) or Campath-1G (n=28). Seventy-seven patients received no in vivo or ex vivo T-cell depletion.
Table 1. Characteristics of stem cell transplant recipients studied.
| No. of transplants, n | 206 |
|---|---|
| Median age at transplant, yrs (range) | 27 (3-60) |
| Proportion of males (%) | 138 (67 %) |
| Indication for transplant | |
| Acute Myeloblastic Leukaemia | 61 (30 %) |
| Acute Lymphoblastic Leukaemia | 52 (25 %) |
| Chronic Myeloid Leukaemia | 30 (15 %) |
| Myelodysplastic Syndrome | 16 (8 %) |
| Non-Hodgkin’s Lymphoma | 12 (6 %) |
| Other Malignant Disordera | 12 (6 %) |
| Other Non-malignant Disorderb | 23 (11 %) |
| Risk Group | |
| Good prognosis | 86 (42 %) |
| Poor prognosis | 120 (58 %) |
| Donor Type | |
| Sibling | 130 (63 %) |
| Non-sibling related donor | 8 (4 %) |
| Unrelated donor | 68 (33 %) |
| HLA Match c | |
| 6 Antigen match | 183 (89 %) |
| Less than 6 antigen match | 21 (10 %) |
| Source of Graft d | |
| Bone marrow | 141 (68 %) |
| Peripheral blood stem cell | 64 (31 %) |
| Median CD34 dose ×106/kg recipient weighte (range) | 2.6 (0.2-18.4) |
| Type of transplant | |
| Reduced intensity conditioning | 12 (6 %) |
| Myeloablative | 194 (94 %) |
| Pre-transplant conditioning | |
| Non radiotherapy based conditioning | 41 (20 %) |
| Radiotherapy based conditioning | 165 (80 %) |
| Campath In Vivo Usef | |
| No Campath in vivo | 105 (51 %) |
| Campath-IG in vivo | 44 (21 %) |
| Campath-IH in vivo | 52 (25 %) |
| Form of Ex vivo T-Cell Depletion | |
| No ex vivo T-Cell Depletion | 116 (56 %) |
| Campath-IG | 43 (21 %) |
| Campath-IH | 40 (19 %) |
| CD34 selection | 7 (3 %) |
| HCMV serology (recipient/donor)g | |
| Neg/Neg | 56 (26 %) |
| Neg/Pos | 20 (10 %) |
| Pos/Neg | 36 (17 %) |
| Pos/Pos | 94 (46 %) |
Idiopathic myelofibrosis, multiple myeloma, sarcoma,
Aplastic anaemia, Fanconi anaemia, thalassaemia major, metachromic leucodystrophy
HLA tissue typing data unavailable in 2 recipient/donor pairs
source of graft unavailable for 1 patient
CD34 dose unavailable in 17 patients
In-vivo Campath use data unavailable in 5 patients
HCMV serology unavailable in 3 recipient/donor pairs
Forty-one patients experienced acute GVHD of grade II or greater, with an actuarial incidence of 24 ± 3.3 % when analysed with death as a competing variable. The incidence of grade II, III and IV acute GVHD was 16.2 ± 2.9 % (n = 27), 4.3 ± 1.6 % (n = 7) and 4.1 ± 1.5 % (n = 7) respectively. After a median follow-up of 528 days, overall survival was 79 ± 2.9 % at day 100, 57.1 ± 3.6 % at 1 year, and 38.2 ± 4.1 % at 3 years. By univariable Cox regression analysis, neither HCMV serostatus of the recipient and/or donor, nor the presence of HCMV DNAemia had a statistically significant effect on the overall survival when the entire cohort was analysed, and by subgroup analysis for HLA-identical sibling transplants and matched unrelated transplants. Overall survival for patients transplanted for malignant conditions (n=183) was 77.5 ± 3.1 % at day 100, 52.8 ± 3.8 % at one year and 32.3 ± 4.2 % at 3 years, with a progression free survival of 70.9 ± 3.4 % at day 100, 40.8 ± 3.7 % at one year and 26.1 ± 3.7 % at 3 years. Neither HCMV serostatus of the recipient and/or donor or HCMV DNAemia had a statistically significant impact on overall survival nor progression free survival in this subgroup.
There were five confirmed cases of HCMV disease in the cohort, including three cases of HCMV colitis, one case of HCMV hepatitis, and one case of HCMV pneumonitis. There were an additional three cases of probable HCMV disease that did not fulfil the current working group definitions of HCMV disease (27). There was one confirmed death due to HCMV disease, in a patient with myelodysplastic syndrome and primary graft failure who developed fatal HCMV pneumonitis. The actuarial incidence of confirmed HCMV disease for the entire cohort of patients as determined by Kaplan Meier survival analysis was 2.6 ± 1.2 %. Due to the small number of patients experiencing HCMV disease, no single factor was identified as significantly increasing the risk of HCMV disease by Cox regression analysis (data not shown).
Incidence and timing of HCMV DNAemia
Median follow-up for HCMV PCR monitoring was 129 days. Sixty-six patients experienced HCMV DNAemia, with a median time to HCMV DNAemia of 39 days post transplant (range 0 to 206 days).
Kaplan Meier survival analysis was performed with respect to time to first HCMV DNAemia post transplant on all 150 patients at risk of HCMV DNAemia based on a positive donor and/or recipient HCMV serology.
Overall, the cumulative DNAemia rate was 15.3 ± 3.0 % by 1 month (30 days), 40.1 ± 4.2 % by 2 months (60 days), 42.4 ± 4.2 % by 3 months (100 days), 48.7 ± 4.4 % by 6 months (180 days) and 50.7 ± 4.4 % by 1 year (365 days) after transplant (Figure 1). Kaplan Meier analysis of the incidence of HCMV DNAemia stratified by donor (D) and recipient (R) HCMV serostatus is summarised in Figure 2. The cumulative DNAemia rate at 6 months for the R+D− group was 56.3 ± 9.1 %, for R+D+ was 55.5 ± 5.5 % and for the R−D+ group was 16.9 ± 9 %. The difference in the cumulative HCMV DNAemia rates between the R−D+ group, and the R+D− and R+ D+ groups was statistically significant (p = 0.009 for R+D− group and p = 0.007 for R+D+ group when compared with the R−D+ group, Log Rank Score). There was no statistically significant difference in the DNAemia rates between the R+D− group and the R+D+ group. No HCMV DNAemia was detected in the R−D− group, although one patient in this group developed HCMV colitis in the absence of HCMV DNAemia.
Figure 1. Kaplan Meier estimate of the cumulative incidence of HCMV DNAemia in recipient and/or donor HCMV seropositive patients (n=147).
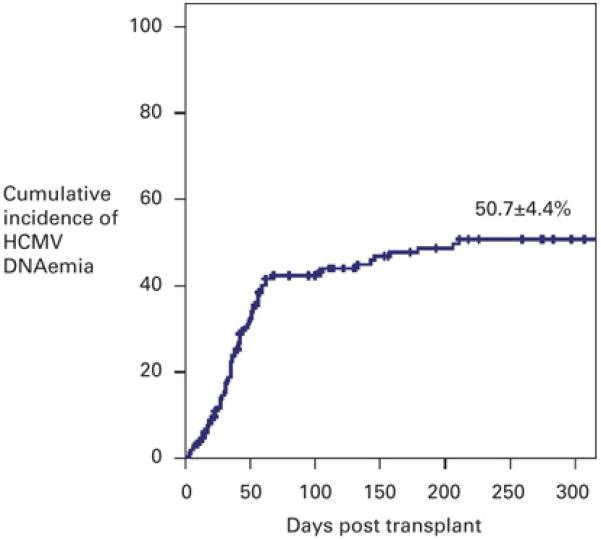
The cumulative incidence of DNAemia was 15.3 ± 3.0 % at 30 days, 40.1 ± 4.2 % at 60 days, 42.4 ± 4.2 % at 100 days, 48.7 ± 4.4 % at 180 days and 50.7 ± 4.4 % at 360 days. The cross bars indicate censored events.
Figure 2. Kaplan Meier estimate of the cumulative incidence of HCMV DNAemia stratified according to HCMV serology.
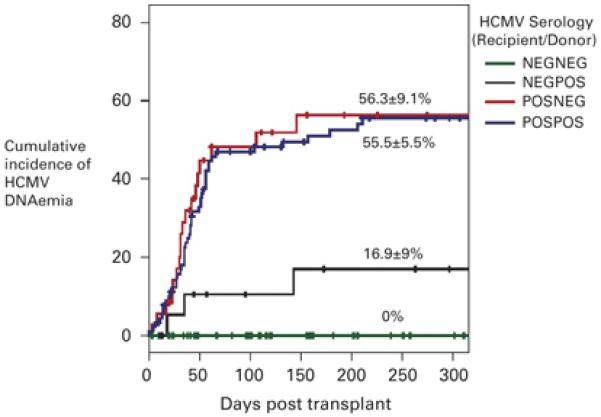
The R−D− group (n=56, green line) did not experience any episodes of DNAemia. The cumulative incidence of DNAemia of the R−D+ group (n=20, black line) was 16.9 ± 9 %, the R+D− group (n=36, red line) was 56.3 ± 9.1 % and the R+D+ group (n=91, blue line) was 55.5 ± 5.5 %. The difference in the incidence of HCMV DNAemia between the R−D+ group, and the R+D− and R+ D+ groups was statistically significant (p = 0.009 for R+D− group and p = 0.007 for R+D+ group when compared with the R−D+ group, Log Rank Score). There was no statistically significant difference in DNAemia rates between the R+D− group and the R+D+ group (p = 0.71, Log Rank Score). The cross bars indicate censored events.
Risk Factors for HCMV DNAemia
Consistent with the results of the cumulative incidence of HCMV DNAemia by Kaplan Meier analysis, univariable Cox regression analysis showed that R+D+ serostatus (RH 4.28, p = 0.02) and R+D− serostatus (RH 4.73, p = 0.02) were associated with the greatest relative hazards for HCMV DNAemia. Among the other factors examined, the use of Campath-1H both in vivo (RH 2.15, p = 0.006) and ex vivo (RH 2.11, p = 0.009) were identified as significant risks for HCMV DNAemia.
Following multivariable Cox regression analysis using a proportional hazards model (Table 2), R+D+ (adjusted RH 8.1, p = 0.004) and R+D− (adjusted RH 5.91, p = 0.02) serostatus remain the greatest risk factors for HCMV DNAemia. The use of Campath-1H in vivo (adjusted RH 3.68, p < 0.001) but not Campath-1G in vivo (adjusted RH 1.76, p = 0.15) also remained independently associated with a significantly increased risk of HCMV DNAemia. Ex-vivo T-cell depletion by any method was no longer identified as increasing the relative hazard of HCMV DNAemia. The only other factors which increased the relative hazard of HCMV DNAemia independently was use of radiotherapy based conditioning (adjusted RH 2.3, p = 0.03) and the CD34 stem cell dose (adjusted RH 0.87 per 1×106/kg CD34 cells, p = 0.04), while age was no longer significant.
Table 2. Multivariable Cox Regression analysis of the risk factors for HCMV DNAemia.
N/A = not applicable
| Relative Hazard |
95% CI | p value | ||
|---|---|---|---|---|
| Campath In Vivo Use | No Campath | 1.00 | N/A | N/A |
| Campath-1G | 1.76 | 0.81-3.84 | 0.15 | |
| Campath-1H | 3.68 | 2.02-6.72 | <0.001 | |
| Pre-transplant Conditioning | Radiotherapy based conditioning | 2.30 | 1.07-4.91 | 0.03 |
| CD34 dose | per 106/kg recipient weight | 0.87 | 0.77-0.99 | 0.04 |
| HCMV serology | NegPos | 1.00 | 0.02 | |
| (Recipient/Donor) | PosNeg | 5.91 | 1.34-26.06 | 0.02 |
| PosPos | 8.10 | 1.93-33.91 | 0.004 |
Effect of In Vivo Campath Use on Time to HCMV DNAemia
Kaplan Meier survival curves for HCMV DNAemia stratified by use of Campath in vivo were plotted for all patients at risk of HCMV DNAemia, (Figure 3). The cumulative HCMV DNAemia rate for patients receiving Campath-1H in vivo was 64.7 ± 8.6 %, for patients receiving Campath-1G in vivo was 41.6 ± 10.4 % and for patients receiving no Campath-1 in vivo was 42.8 ± 5.8 %. The difference in the cumulative incidence of HCMV DNAemia between the Campath-1H group and the non-Campath group was highly significant (p = 0.0024, Log Rank Score), but the difference between the Campath-1G group and the Campath-1H group did not reach statistical significance. Although the group receiving no Campath in vivo had a lower incidence of DNAemia prior to day 100 (cumulative incidence of 32.2 ± 5.3 %), the overall cumulative incidence was comparable to the Campath-1G group.
Figure 3. Kaplan Meier estimate of the cumulative incidence of HCMV DNAemia according to Campath in vivo use.
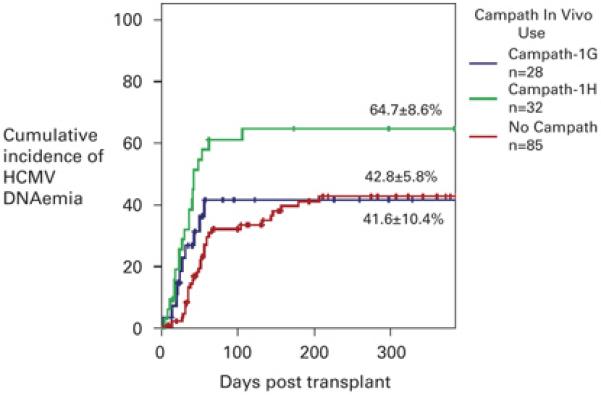
The cumulative incidence of HCMV DNAemia in the group receiving no Campath in vivo (red line, n=85) was 42.8 ± 5.8 %, for the group receiving Campath-1G in vivo (blue line, n=28) was 41.6 ± 10.4 %, and for the group receiving Campath-1H (green line, n=32) was 64.7 ± 8.6 %. The difference in the cumulative incidence of DNAemia between the Campath-1H group and the no Campath group was significant (p = 0.002, Log Rank Score), while the difference between the group receiving Campath-1G and no Campath group (p = 0.71, Log Rank Score), and Campath-1G and Campath-1H (p = 0.13, Log Rank Score) was not significant. The cross bars indicate censored data.
When the cumulative incidence of DNAemia between R+D− and R+D+ patients was compared in a subgroup analysis according to Campath in vivo use, none of the groups showed a statistically significant difference (p = 0.13 for no Campath group, p = 0.61 for Campath-1G in vivo group and p = 0.24 for Campath-1H in vivo group, Log Rank Score).
HCMV DNAemia occurred significantly earlier in patients receiving either Campath-1G in vivo, with a median time to DNAemia of 27 days, or Campath-1H in vivo with a median time to DNAemia of 33 days, when compared to patients receiving no Campath in vivo (median time to DNAemia of 51 days (p = 0.007 for Campath-1G vs. no Campath and p = 0.006 for Campath-1H vs. no Campath, Mann Whitney U Test; Figure 4)). The difference in the time to HCMV DNAemia between the Campath-1G and the Campath-1H group was not statistically significant (p = 0.97, Mann Whitney U Test).
Figure 4. Time to first HCMV load >200 genomes/ml blood according to Campath in vivo use.
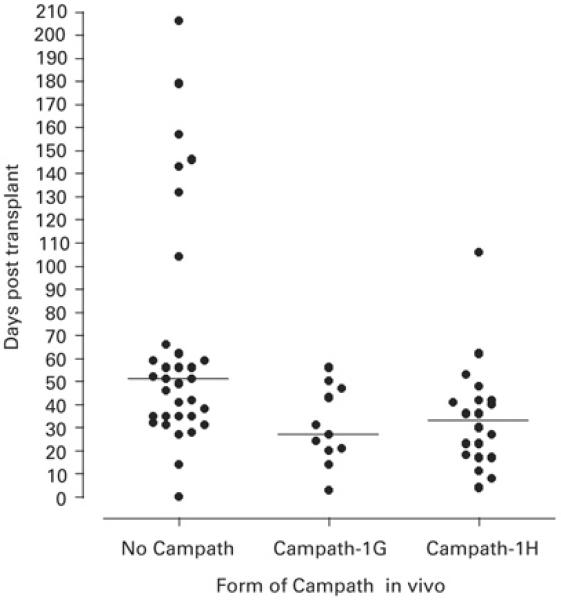
The horizontal bar indicates the median value of each dataset. The median time to HCMV DNAamia in the group not receiving Campath in vivo (n=33) was 51 days (range 0 to 206 days), for the group receiving Campath-1G (n=11) was 27 days (range 3 to 56 days), and for the group receiving Campath-1H (n=20) was 33 days (range 4 to 106 days). Significant differences were apparent in time to first HCMV DNAemia between the Campath-1G group and the non Campath group (p = 0.007, Mann Whitney U Test), and between the Campath-1H group and the non Campath group (p = 0.006, Mann Whitney U Test). The difference between the Campath-1G and Campath-1H groups was not significant (p = 0.97, Mann Whitney U Test).
Late HCMV DNAemia
One hundred and seventeen patients with follow-up beyond 100 days were identified and included in the analysis of risk factors for late HCMV DNAemia. Twenty patients experienced HCMV DNAemia beyond 100 days. The Kaplan Meier estimate of the incidence of late HCMV DNAemia was 19.5 ± 5.4 %. There was no statistically significant difference in the incidence of late HCMV DNAemia according to HCMV serostatus. Univariable Cox regression analysis of the risk factors (for late HCMV DNAemia showed that DNAemia before day 100 (RH 2.5, p = 0.05) was the only significant factor predictive of the occurrence of late DNAemia. However, following multivariable Cox regression analysis, increasing age (adjusted RH 1.04 per year p = 0.04) and an acute GVHD score of II or greater (adjusted RH 4.0, p = 0.01) were both statistically significant predictors of an increased risk of late HCMV DNAemia, while a higher CD34 stem cell dose was associated with a reduced risk of late DNAemia (RH 0.8 per 1 × 106 CD34 cells/kg recipient weight, p = 0.05). These data are summarised in Table 3.
Table 3. Multivariable Cox Regression analysis of the risk factors for late (>100 days) HCMV DNAemia.
| Risk | Relative Hazard |
95% CI | Significance (p value) |
|---|---|---|---|
| Age (per year) | 1.04 | 1.00-1.09 | 0.04 |
| Acute GVHD grade II or higher | 3.96 | 1.39-11.30 | 0.01 |
| Radiotherapy based conditioning | 6.06 | 0.60-61.71 | 0.13 |
| Reduced intensity SCT | 10.11 | 0.87-117.54 | 0.07 |
| CD34 dose per 106/kg recipient weight | 0.78 | 0.61-0.99 | 0.05 |
Discussion
This study has quantified the risk for HCMV DNAemia associcated with different forms, and clinical deployment of Campath in the context of other risk factors. Consistent with previous studies of stem cell transplant recipients, HCMV seropositivity represented the most significant risk factor for HCMV DNAemia. However, donor HCMV was not found to be protective against HCMV DNAemia in this study consistent with previous data from our centre (28). Nevertheless, one of the most important risk factors for developing DNAemia was the use of Campath-1H in vivo. While this observation is consistent with the findings of Chakrabarti et al. (19) we further showed that the time to HCMV DNAemia in recipients of Campath-1H or Campath-1G in vivo compared to patients receiving no Campath in vivo was significantly shorter (by approximately 21 days; p = 0.0006) although there was no difference in time to DNAemia between the Campath-1H and Campath-1G groups (33 days and 27 days respectively). There was also a trend towards a higher incidence of HCMV DNAemia with Campath-1H compared to Campath-1G probably reflecting the difference in half lives of the two forms of Campath. Following the administration of 20 mg of Campath-1H for five days (−8 to −4), the antibody remains detectable for 28 days after transplant (29) whereas Campath-1G has a significantly shorter half life, of approximately 24 hours (30), and when used in vivo, effectively depletes circulating host dendritic cells without delaying donor dendritic cell reconstitution (31). The mechanisms by which Campath-1H increases the risk of HCMV DNAemia and shifts it to an earlier time point awaits more detailed immunological investigations of NK cells and HCMV specific CD4, CD8 T-cells in the critical 3-month post-transplant period.
In contrast to the in vivo use of Campath, the ex vivo use of Campath to T-cell deplete the graft did not significantly increase the risk of HCMV DNAemia. Compared to T-cell depletion by positive selection, ex vivo T-cell depletion by Campath results in the maintenance of a proportion of T-cells and NK cells (22). The use of Campath “in the bag”, particularly the lower dose of 10mg, has been shown to decrease the incidence of graft versus host disease without compromising immune recovery (32;33). Thus the lower incidence of DNAemia in this group likely reflects the improved ability of the reconstituting immune system to respond to HCMV.
The cumulative incidence of HCMV DNAemia over the period of follow-up followed a biphasic curve with a linear increase in incidence over the first 60 days at a rate of 0.3 % day −1 and a second slower rate of increase (0.058 % day −1) from day 60 to day 200. While this may be partially influenced by the frequency of PCR monitoring this is unlikely to account entirely for this inflexion since many patients continued to have HCMV PCR monitoring performed more frequently than the protocol due to other clinic visits. Many studies have used day 100 as the cut-off for defining late HCMV infection whereas our data indicate that the late DNAemia rate may begin as early as day 60. Nevertheless using the conventional cut-off at day 100, recipient age, an acute GVHD score of II or greater and a low CD34 stem cell dose were identified as the most significant predictors of late HCMV DNAemia. In contrast to the effects of Campath on the risk of early DNAemia it was not a risk factor for DNAemia after 100 days. This observation is consistent with the data on the aforementioned clearance rates of Campath from the body.
Although the frequency of acute GVHD was low, probably due to the greater use of T-cell depletion in our cohort, acute GVHD remained a risk for both early and late HCMV DNAemia. Yanada et al. (34) found that acute GVHD (grades II-IV) was the only significant factor to predict HCMV DNAemia on multivariate analysis. Allogeneic reactions are also known to stimulate HCMV from a latent state into the replicative state (35), and the use of steroids to treat GVHD, further suppresses immune function, allowing viral replication rate to increase, and resulting in an increased incidence of HCMV DNAemia. The increased frequency of late HCMV DNAemia in patients with acute GVHD is likely to be due to the use of increased immunosuppression. The other risk factors for late HCMV DNAemia (increasing age of the recipient and a lower CD34 stem cell dose) may reflect impaired immune recovery. In matched sibling allogeneic transplant recipients using ATG for conditioning, bone marrow as a source of stem cells is associated with an increased risk of HCMV DNAemia (36). The Seattle group have shown similar findings in the non-T cell depleted setting (37), as well as delayed CD4 and CD8 recovery. Thus, prolonged monitoring for HCMV DNAemia should be performed in selected patients, including RIC transplants, older transplant recipients, patients with GHVD, and patients with stem cell grafts with a low CD34 count.
In conclusion, the use of Campath in vivo is a significant risk factor for the incidence and more rapid temporal appearance of HCMV DNAemia in stem cell transplant recipients but has no effect on the incidence of late HCMV DNAemia.
Acknowledgements
This work was supported in part by a Wellcome Trust programme grant and a UK Medical research Council Centre Grant.
Reference List
- (1).Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–71. [PubMed] [Google Scholar]
- (2).Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–9. [PubMed] [Google Scholar]
- (3).Gerna G, Lilleri D, Caldera D, Furione M, Zenone Bragotti L, Alessandrino EP. Validation of a DNAemia cutoff for preemptive therapy of cytomegalovirus infection in adult hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41:873–9. doi: 10.1038/sj.bmt.1705986. [DOI] [PubMed] [Google Scholar]
- (4).Singh N. Antiviral drugs for cytomegalovirus in transplant recipients: advantages of preemptive therapy. Rev Med Virol. 2006;16:281–7. doi: 10.1002/rmv.513. [DOI] [PubMed] [Google Scholar]
- (5).Avetisyan G, Larsson K, Aschan J, Nilsson C, Hassan M, Ljungman P. Impact on the cytomegalovirus (CMV) viral load by CMV-specific T-cell immunity in recipients of allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:687–92. doi: 10.1038/sj.bmt.1705507. [DOI] [PubMed] [Google Scholar]
- (6).Morita-Hoshi Y, Heike Y, Kawakami M, Sugita T, Miura O, Kim SW, et al. Functional analysis of cytomegalovirus-specific T lymphocytes compared to tetramer assay in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:515–21. doi: 10.1038/sj.bmt.1705932. [DOI] [PubMed] [Google Scholar]
- (7).Widmann T, Sester U, Gartner BC, Schubert J, Pfreundschuh M, Kohler H, et al. Levels of CMV specific CD4 T cells are dynamic and correlate with CMV viremia after allogeneic stem cell transplantation. PLoS ONE. 2008;3:e3634. doi: 10.1371/journal.pone.0003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lilleri D, Fornara C, Chiesa A, Caldera D, Alessandrino EP, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in adult allogeneic hematopoietic stem cell transplant recipients and immune control of viral infection. Haematologica. 2008;93:248–56. doi: 10.3324/haematol.11912. [DOI] [PubMed] [Google Scholar]
- (9).Peggs KS, Preiser W, Kottaridis PD, McKeag N, Brink NS, Tedder RS, et al. Extended routine polymerase chain reaction surveillance and pre-emptive antiviral therapy for cytomegalovirus after allogeneic transplantation. Br J Haematol. 2000;111:782–90. [PubMed] [Google Scholar]
- (10).Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–14. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- (11).Nakamae H, Kirby KA, Sandmaier BM, Norasatthada L, Maloney DG, Maris MB, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hale G, Bright S, Chumbley G, Hoang T, Metcalf D, Munro AJ, et al. Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood. 1983;62:873–82. [PubMed] [Google Scholar]
- (13).Waldmann H, Polliak A, Hale G, Or R, Cividalli G, Weiss L, et al. Elimination of graft-versus-host disease by in-vitro depletion of alloreactive lymphocytes with a monoclonal rat anti-human lymphocyte antibody (CAMPATH-1) Lancet. 1984;2:483–6. doi: 10.1016/s0140-6736(84)92564-9. [DOI] [PubMed] [Google Scholar]
- (14).Bunjes D. T cell depletion of allogeneic stem cell grafts with anti-CD 52 monoclonal antibodies: the Ulm experience from 1983-1999. Transfus Sci. 2000;23:151–62. doi: 10.1016/s0955-3886(00)00079-5. [DOI] [PubMed] [Google Scholar]
- (15).Hale G, Zhang MJ, Bunjes D, Prentice HG, Spence D, Horowitz MM, et al. Improving the outcome of bone marrow transplantation by using CD52 monoclonal antibodies to prevent graft-versus-host disease and graft rejection. Blood. 1998;92:4581–90. [PubMed] [Google Scholar]
- (16).Willemze R, Richel DJ, Falkenburg JH, Hale G, Waldmann H, Zwaan FE, et al. In vivo use of Campath-1G to prevent graft-versus-host disease and graft rejection after bone marrow transplantation. Bone Marrow Transplant. 1992;9(4):255–61. [PubMed] [Google Scholar]
- (17).Williams RJ, Clarke E, Blair A, Evely R, Hale G, Waldmann H, et al. Impact on T-cell depletion and CD34+ cell recovery using humanised CD52 monoclonal antibody (CAMPATH-1H) in BM and PSBC collections; comparison with CAMPATH-1M and CAMPATH-1G. Cytotherapy. 2000;2:5–14. doi: 10.1080/146532400539008. [DOI] [PubMed] [Google Scholar]
- (18).Phillips J, Drumm A, Harrison P, Bird P, Bhamra K, Berrie E, et al. Manufacture and quality control of CAMPATH-1 antibodies for clinical trials. Cytotherapy. 2001;3:233–42. doi: 10.1080/146532401753174061. [DOI] [PubMed] [Google Scholar]
- (19).Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O’Gorman P, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–63. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- (20).Junghanss C, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99:1978–85. doi: 10.1182/blood.v99.6.1978. [DOI] [PubMed] [Google Scholar]
- (21).Pawson R, Potter MN, Theocharous P, Lawler M, Garg M, Yin JA, et al. Treatment of relapse after allogeneic bone marrow transplantation with reduced intensity conditioning (FLAG +/− Ida) and second allogeneic stem cell transplant. Br J Haematol. 2001;115:622–9. doi: 10.1046/j.1365-2141.2001.03150.x. [DOI] [PubMed] [Google Scholar]
- (22).Dreger P, Viehmann K, Steinmann J, Eckstein V, Muller-Ruchholtz W, Loffler H, et al. G-CSF-mobilized peripheral blood progenitor cells for allogeneic transplantation: comparison of T cell depletion strategies using different CD34+ selection systems or CAMPATH-1. Exp Hematol. 1995;23:147–54. [PubMed] [Google Scholar]
- (23).Kidd IM, Fox JC, Pillay D, Charman H, Griffiths PD, Emery VC. Provision of prognostic information in immunocompromised patients by routine application of the polymerase chain reaction for cytomegalovirus. Transplantation. 1993;56:867–71. doi: 10.1097/00007890-199310000-00018. [DOI] [PubMed] [Google Scholar]
- (24).Mattes FM, Hainsworth EG, Geretti AM, Nebbia G, Prentice G, Potter M, Burroughs AK, Sweny P, Hassan-Walker AF, Okwuadi S, Sabin C, Amooty G, Brown VS, Grace SC, Emery VC, Griffiths PD. A randomised control trial comparing ganciclovir to ganciclovir plus foscarnet (each at half dose) for preemptive therapy of cytomegalovirus infection in transplant recipints. J Infect Dis. 2004;189:1355–61. doi: 10.1086/383040. [DOI] [PubMed] [Google Scholar]
- (25).Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- (26).Cox DR. Regression models and life tables (with discussion) J R Stat B. 1972;34:187–220. [Google Scholar]
- (27).Ljungman P, Griffiths PD, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–7. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- (28).Grob JP, Grundy JE, Prentice HG, Griffiths PD, Hoffbrand AV, Hughes MD, et al. Immune donors can protect marrow-transplant recipients from severe cytomegalovirus infections. Lancet. 1987;1:774–6. doi: 10.1016/s0140-6736(87)92800-5. [DOI] [PubMed] [Google Scholar]
- (29).Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102:404–6. doi: 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- (30).Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G. Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy. 2001;3:261–7. doi: 10.1080/146532401317070899. [DOI] [PubMed] [Google Scholar]
- (31).Klangsinsirikul P, Carter GI, Byrne JL, Hale G, Russell NH. Campath-1G causes rapid depletion of circulating host dendritic cells (DCs) before allogeneic transplantation but does not delay donor DC reconstitution. Blood. 2002;99:2586–91. doi: 10.1182/blood.v99.7.2586. [DOI] [PubMed] [Google Scholar]
- (32).Chakrabarti S, MacDonald D, Hale G, Holder K, Turner V, Czarnecka H, et al. T-cell depletion with Campath-1H “in the bag” for matched related allogeneic peripheral blood stem cell transplantation is associated with reduced graft-versus-host disease, rapid immune constitution and improved survival. Br J Haematol. 2003;121:109–18. doi: 10.1046/j.1365-2141.2003.04228.x. [DOI] [PubMed] [Google Scholar]
- (33).Novitzky N, Thomas V, Hale G, Waldmann H. Campath-1 Abs ‘in the bag’ for hematological malignancies: the Cape Town experience. Cytotherapy. 2004;6:172–81. doi: 10.1080/14653240310004520. [DOI] [PubMed] [Google Scholar]
- (34).Yanada M, Yamamoto K, Emi N, Naoe T, Suzuki R, Taji H, et al. Cytomegalovirus antigenemia and outcome of patients treated with pre-emptive ganciclovir: retrospective analysis of 241 consecutive patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:801–7. doi: 10.1038/sj.bmt.1704232. [DOI] [PubMed] [Google Scholar]
- (35).Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–26. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- (36).Mohty M, Jacot W, Faucher C, Bay JO, Zandotti C, Collet L, et al. Infectious complications following allogeneic HLA-identical sibling transplantation with antithymocyte globulin-based reduced intensity preparative regimen. Leukemia. 2003;17:2168–77. doi: 10.1038/sj.leu.2403105. [DOI] [PubMed] [Google Scholar]
- (37).Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–7. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]


