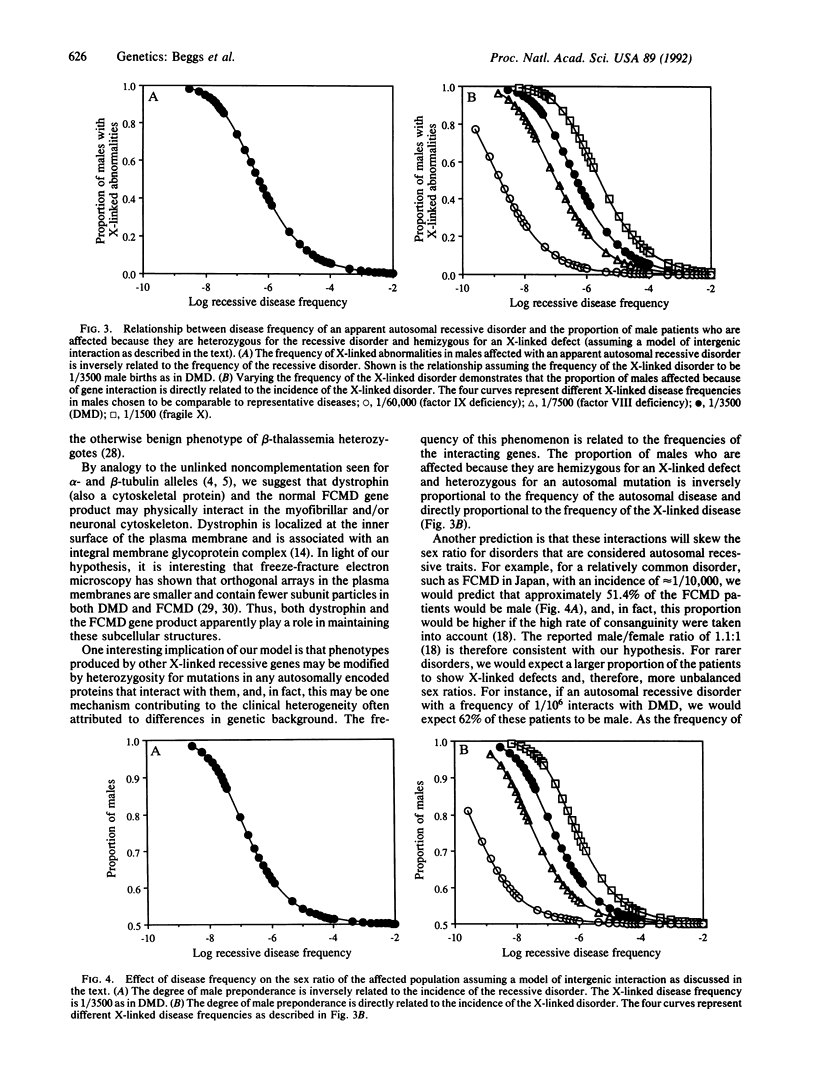
Abstract
Abnormalities of dystrophin, a cytoskeletal protein of muscle and nerve, are generally considered specific for Duchenne and Becker muscular dystrophy. However, several patients have recently been identified with dystrophin deficiency who, before dystrophin testing, were considered to have Fukuyama congenital muscular dystrophy (FCMD) on the basis of clinical findings. Epidemiologic data suggest that only 1/3500 males with autosomal recessive FCMD should have abnormal dystrophin. To explain the observation of 3/23 FCMD males with abnormal dystrophin, we propose that dystrophin and the FCMD gene product interact and that the earlier onset and greater severity of these patients' phenotype (relative to Duchenne muscular dystrophy) are due to their being heterozygous for the FCMD mutation in addition to being hemizygous for Duchenne muscular dystrophy, a genotype that is predicted to occur in 1/175,000 Japanese males. This model may help explain the genetic basis for some of the clinical and pathological variability seen among patients with FCMD, and it has potential implications for understanding the inheritance of other autosomal recessive disorders in general. For example, sex ratios for rare autosomal recessive disorders caused by mutations in proteins that interact with X chromosome-linked gene products may display predictable deviation from 1:1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Hoffman E. P., Kunkel L. M., Ishiura S., Tsukahara T., Ishihara T., Sunohara N., Nonaka I., Ozawa E., Sugita H. Dystrophin diagnosis: comparison of dystrophin abnormalities by immunofluorescence and immunoblot analyses. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7154–7158. doi: 10.1073/pnas.86.18.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa E., Ishihara T., Nonaka I., Sugita H., Arahata K. Immunocytochemical analysis of dystrophin in congenital muscular dystrophy. J Neurol Sci. 1991 Sep;105(1):79–87. doi: 10.1016/0022-510x(91)90122-n. [DOI] [PubMed] [Google Scholar]
- Beggs A. H., Koenig M., Boyce F. M., Kunkel L. M. Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum Genet. 1990 Nov;86(1):45–48. doi: 10.1007/BF00205170. [DOI] [PubMed] [Google Scholar]
- Den Dunnen J. T., Grootscholten P. M., Bakker E., Blonden L. A., Ginjaar H. B., Wapenaar M. C., van Paassen H. M., van Broeckhoven C., Pearson P. L., van Ommen G. J. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am J Hum Genet. 1989 Dec;45(6):835–847. [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V., Crome L. The central nervous system in Duchenne muscular dystrophy. Brain. 1969;92(4):805–808. doi: 10.1093/brain/92.4.805. [DOI] [PubMed] [Google Scholar]
- Emery A. E. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991;1(1):19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Fukuyama Y., Osawa M., Suzuki H. Congenital progressive muscular dystrophy of the Fukuyama type - clinical, genetic and pathological considerations. Brain Dev. 1981;3(1):1–29. doi: 10.1016/s0387-7604(81)80002-2. [DOI] [PubMed] [Google Scholar]
- Hammonds R. G., Jr Protein sequence of DMD gene is related to actin-binding domain of alpha-actinin. Cell. 1987 Oct 9;51(1):1–1. doi: 10.1016/0092-8674(87)90002-x. [DOI] [PubMed] [Google Scholar]
- Hays T. S., Deuring R., Robertson B., Prout M., Fuller M. T. Interacting proteins identified by genetic interactions: a missense mutation in alpha-tubulin fails to complement alleles of the testis-specific beta-tubulin gene of Drosophila melanogaster. Mol Cell Biol. 1989 Mar;9(3):875–884. doi: 10.1128/mcb.9.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Fischbeck K. H., Brown R. H., Johnson M., Medori R., Loike J. D., Harris J. B., Waterston R., Brooke M., Specht L. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988 May 26;318(21):1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989 Jan;2(1):1019–1029. doi: 10.1016/0896-6273(89)90226-2. [DOI] [PubMed] [Google Scholar]
- Homyk T., Jr, Emerson C. P., Jr Functional interactions between unlinked muscle genes within haploinsufficient regions of the Drosophila genome. Genetics. 1988 May;119(1):105–121. doi: 10.1093/genetics/119.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki Y., Sakamoto Y., Nishitani Y. [Peculiar type of congenital muscular dystrophy (Fukuyama type) (author's transl)]. Rinsho Shinkeigaku. 1980 Nov;20(11):897–903. [PubMed] [Google Scholar]
- Johnson W. G. Metabolic interference and the + - heterozygote. a hypothetical form of simple inheritance which is neither dominant nor recessive. Am J Hum Genet. 1980 May;32(3):374–386. [PMC free article] [PubMed] [Google Scholar]
- KUGELBERG E., WELANDER L. Heredofamilial juvenile muscular atrophy simulating muscular dystrophy. AMA Arch Neurol Psychiatry. 1956 May;75(5):500–509. doi: 10.1001/archneurpsyc.1956.02330230050005. [DOI] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Kulozik A. E., Thein S. L., Wainscoat J. S., Gale R., Kay L. A., Wood J. K., Weatherall D. J., Huehns E. R. Thalassaemia intermedia: interaction of the triple alpha-globin gene arrangement and heterozygous beta-thalassaemia. Br J Haematol. 1987 May;66(1):109–112. doi: 10.1111/j.1365-2141.1987.tb06898.x. [DOI] [PubMed] [Google Scholar]
- Kusch M., Edgar R. S. Genetic studies of unusual loci that affect body shape of the nematode Caenorhabditis elegans and may code for cuticle structural proteins. Genetics. 1986 Jul;113(3):621–639. doi: 10.1093/genetics/113.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov H. G., Byers T. J., Watkins S. C., Kunkel L. M. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature. 1990 Dec 20;348(6303):725–728. doi: 10.1038/348725a0. [DOI] [PubMed] [Google Scholar]
- Moore K. J., Seperack P. K., Strobel M. C., Swing D. A., Copeland N. G., Jenkins N. A. Dilute suppressor dsu acts semidominantly to suppress the coat color phenotype of a deletion mutation, dl20J, of the murine dilute locus. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8131–8135. doi: 10.1073/pnas.85.21.8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T., Aberfeld D. C., Grob D. Chronic proximal spinal muscular atrophy. J Neurol Sci. 1970 Nov;11(5):401–423. doi: 10.1016/0022-510x(70)90001-8. [DOI] [PubMed] [Google Scholar]
- Regan C. L., Fuller M. T. Interacting genes that affect microtubule function: the nc2 allele of the haywire locus fails to complement mutations in the testis-specific beta-tubulin gene of Drosophila. Genes Dev. 1988 Jan;2(1):82–92. doi: 10.1101/gad.2.1.82. [DOI] [PubMed] [Google Scholar]
- Rutledge B. J., Mortin M. A., Schwarz E., Thierry-Mieg D., Meselson M. Genetic interactions of modifier genes and modifiable alleles in Drosophila melanogaster. Genetics. 1988 Jun;119(2):391–397. doi: 10.1093/genetics/119.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Botstein D. Unlinked noncomplementation: isolation of new conditional-lethal mutations in each of the tubulin genes of Saccharomyces cerevisiae. Genetics. 1988 Jun;119(2):249–260. doi: 10.1093/genetics/119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainscoat J. S., Kanavakis E., Wood W. G., Letsky E. A., Huehns E. R., Marsh G. W., Higgs D. R., Clegg J. B., Weatherall D. J. Thalassaemia intermedia in Cyprus: the interaction of alpha and beta thalassaemia. Br J Haematol. 1983 Mar;53(3):411–416. doi: 10.1111/j.1365-2141.1983.tb02041.x. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Kumagai T., Jimi T. Small size of orthogonal array in muscle plasma membrane of Fukuyama type congenital muscular dystrophy. Acta Neuropathol. 1986;72(2):130–133. doi: 10.1007/BF00685974. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Okayasu H., Shibuya S., Kumagai T. Duchenne dystrophy: reduced density of orthogonal array subunit particles in muscle plasma membrane. Neurology. 1984 Oct;34(10):1313–1317. doi: 10.1212/wnl.34.10.1313. [DOI] [PubMed] [Google Scholar]