Abstract
Objective
To determine whether amniotic fluid concentration of prostaglandins (PGs) increases in patients with intra-amniotic inflammation and/or proven amniotic fluid infection in preterm PROM, and whether the amniotic fluid PG concentration can predict impending delivery.
Methods
Amniotic fluid PGF2a concentrations were determined by ELISA in 140 singleton pregnancies with preterm PROM (≤35 weeks). Amniotic fluid was cultured for aerobic and anaerobic bacteria, as well as genital mycoplasmas. Intra-amniotic inflammation was defined as an elevated amniotic fluid MMP-8 concentration (>23 ng/mL). Non-parametric tests and survival techniques were used for the statistics.
Results
1) Patients with intra-amniotic inflammation and a negative amniotic fluid culture had a significantly higher median amniotic fluid PGF2a concentration than those without intra-amniotic inflammation and a negative amniotic fluid culture (median, 206 vs. 64 pg/mL; p<0.001); 2) however, there was no difference in the median amniotic fluid PGF2a concentration between patients with intra-amniotic inflammation with a negative culture and those with culture-proven amniotic fluid infection (median, 206 vs. 314 pg/mL, p=0.4); 3) amniotic fluid PGF2a ≥170 pg/mL had a sensitivity of 63% and a specificity of 89% in the identification of intra-amniotic inflammation and/or amniotic fluid infection; 4) patients with an elevated amniotic fluid PGF2a (≥170 pg/mL) had a significantly shorter interval-to-delivery than those with low amniotic fluid PGF2a (median, 72 vs. 155 hours; p<0.001); and 5) an elevated amniotic fluid PGF2a concentration was a significant predictor of the duration of pregnancy after adjusting for gestational age and amniotic fluid inflammation/infection (OR, 2.3; 95% CI, 1.4–4.0; p<0.005).
Conclusions
The amniotic fluid PGF2a concentration increased in patients with intra-amniotic inflammation regardless of amniotic fluid culture results. Moreover, an elevated AF PGF2a concentration was an independent predictor of impending delivery in preterm PROM.
Keywords: Amniotic fluid infection, intra-amniotic inflammation, prostaglandin, preterm PROM, parturition
Introduction
Prostaglandins (PGs) are the central mediators in human parturition [5, 6, 9, 22, 25]. There is substantial evidence that PGs are involved in the mechanisms of human parturition associated with culture-proven intra-amniotic infection [11, 13, 19, 26, 27]. These infections are present in one third of patients with preterm PROM [28]. Microbial invasion of the amniotic cavity can be recognized by the innate immune system in gestational tissue and can lead to an inflammatory response which includes PG production and induction of labor [1, 7, 8, 10].
Intra-amniotic inflammation is found in approximately 40% of patients with preterm premature rupture of membranes (PROM) and is a risk factor for impending preterm delivery, adverse pregnancy and neonatal outcome, regardless of the presence or absence of culture-proven amniotic fluid (AF) infection [31].
There is a paucity of information regarding the concentration of PGs in the AF of patients exposed to intra-amniotic inflammation in the absence of culture-proven AF infection. The purpose of this study was to examine if AF PGs change in patients with intra-amniotic inflammation without culture-proven AF infection and if AF PG concentrations are a risk factor for impending delivery in preterm PROM.
Materials and Methods
Study design
The concentrations of PGF2a were examined in AF obtained from 140 pregnant women admitted to the participating institution with the diagnosis of preterm PROM and who met the following criteria: (1) singleton pregnancy; (2) preterm pregnancy (gestational age ≤35 weeks); and (3) AF obtained by transabdominal amniocentesis or collected at the time of cesarean delivery. Patients were divided into 3 groups according to the presence or absence of intra-amniotic inflammation and AF culture results; Group 1: patients without intra-amniotic inflammation and a negative AF culture (N=81); Group 2: women with intra-amniotic inflammation and a negative AF culture (N=31); Group 3: patients with a positive AF culture (N=28).
Retrieval of AF was performed after obtaining written informed consent. The Institutional Review Board of the participating institution approved the collection and use of these samples and clinical information for research purposes.
Amniotic fluid studies
AF was cultured for aerobic and anaerobic bacteria, as well as for genital mycoplasmas (Mycoplasma hominis and Ureaplasma urealyticum). An aliquot of AF was transported to the laboratory and processed for white blood cell (WBC) count and matrix metalloproteinase-8 (MMP-8) determinations. AF MMP-8 concentration was measured with a commercially available enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech, Inc, Bucks, UK). The sensitivity of the test was 0.3 ng/mL. Intra- and inter-assay coefficients of variation were 3.1% and 9.5%, respectively. MMP-8 was used to assess the presence of intra-amniotic inflammation because previous studies indicated that it is a sensitive and specific index of inflammation [3, 23, 31]. Intra-amniotic inflammation was defined as an elevated AF MMP-8 concentration (>23 ng/mL), as previously reported [23, 31]. Intra-amniotic infection/inflammation was defined as a positive AF culture and/or elevated AF MMP-8 concentration (>23ng/ml).
Amniotic fluid prostaglandins
AF was centrifuged and supernatants were stored at −70° until assayed. PGF2a concentrations at 12,000 rpm for 10 minutes and the supernatant was collected and frozen. PGF2a was measured with a commercially available enzyme-linked immunoassay (Assay Design, Inc, Ann Arbor, MI, USA). AF was assayed in duplicate samples. All assays were conducted by the same individual, blinded to the clinical information. The sensitivity of the assay was 7.7 pg/mL. Intra- and inter-assay coefficients of variation were 1.2% and 8.8%. High AF PGF2a concentration was defined when AF PGF2a concentration was ≥170 pg/mL. This was based upon our previous report [18] that AF PGF2a concentration of 170 pg/mL corresponds to the value of 95 percentile of AF PGF2a concentration among pregnant women who were at 15–36 weeks of gestation and not in labor.
Statistical analysis
AF PGs concentrations were compared among groups with Kruskal-Wallis and Mann-Whitney U tests. The amniocentesis-to-delivery interval was compared using the generalized Wilcoxon test for survival analysis. The interval-to-delivery of patients delivered for maternal or fetal indications was treated as a censored observation, with a censoring time equal to the amniocentesis-to-delivery interval. A p-value <0.05 was considered statistically significant.
Results
Table 1 describes the clinical characteristics and pregnancy outcomes of the study population according to the results of AF culture and MMP-8 concentrations. Patients with intra-amniotic inflammation but with a negative AF culture (group 2) had a significantly lower median gestational age at amniocentesis as well as at delivery, a higher median WBC count and AF MMP-8 concentration, and a shorter interval-to-delivery than those with a negative AF culture without intra-amniotic inflammation (group 1). However, there were no significant differences in the clinical characteristics and pregnancy outcomes between cases with intra-amniotic inflammation and a negative AF culture (group 2) and those with proven AF infection (group 3).
Table 1.
Clinical characteristics and pregnancy outcomes of the study population according to the results of AF culture and MMP-8 concentrations
| (−) AF culture Low MMP-8 (group 1; n=81) |
P* | (−) AF culture High MMP-8 (group 2; n=31) |
P† | (+) AF culture (group 3; n=28) |
P‡ | |
|---|---|---|---|---|---|---|
| Maternal age (y) | 30 (21–45) | NS | 31 (23–39) | NS | 29 (24–37) | NS |
| Nulliparity (n) | 40 (49%) | NS | 9 (29%) | NS | 9 (32%) | NS |
| GA at amniocentesis (week)# | 33.3 (20.4–35.0) | <.001 | 29.7 (21.3–34.7) | NS | 29.6 (21.6–34.3) | <.001 |
| GA at delivery (week)# | 34.7 (23.7–41.6) | <.001 | 30.9 (22.3–35.7) | NS | 30.0 (21.9–34.7) | <.001 |
| Amniocentesis-to-delivery interval (hour)§ | 141 (0–3545) | <.005 | 46 (0–1849) | NS | 116 (2–748) | <.005 |
| AF WBC (cells/mm3)# | 1 (0–250) | <.001 | 153 (0- >1000) | NS | 270 (0- >1000) | <.001 |
| AF MMP-8 (ng/mL)# | 1.1 (0.3–22.3) | <.001 | 211.8 (35.0–2874.5) | NS | 119.3 (1.6–5019.5) | <.001 |
Values are medians and ranges.
AF, amniotic fluid; MMP-8, matrix metalloproteinase-8; Low MMP-8, matrix metalloproteinase-8 <23 ng/mL; High MMP-8, matrix metalloproteinase-8 >23 ng/mL; GA, gestational age; NS, not significant; WBC, white blood cell
Comparison between groups 1 and 2.
Comparison between groups 2 and 3.
Comparison between groups 3 and 1.
P<.05, by Kruskal-Wallis ANOVA test
Compared by generalized Wilcoxon test for survival analysis.
Figure 1 shows that patients with a negative AF culture but with intra-amniotic inflammation (group 2) had a significantly higher median AF PGF2a concentration than those with a negative AF culture, without intra-amniotic inflammation (group 1) (median, 206.2 pg/mL [range, 26.1–38715.2 pg/mL] vs. median, 64.0 pg/mL [range, 7.7–2776.2 pg/mL], p<0.001). However, there was no significant difference in the median AF PGF2a concentration between patients with intra-amniotic inflammation but a negative AF culture (group 2) and those with culture-proven AF infection (group 3) (median, 206.2 pg/mL [range, 26.1- 38715.2 pg/mL] vs. median, 313.7 pg/mL [range, 40.5- 8492.3 pg/mL], p=0.4).
Figure 1.
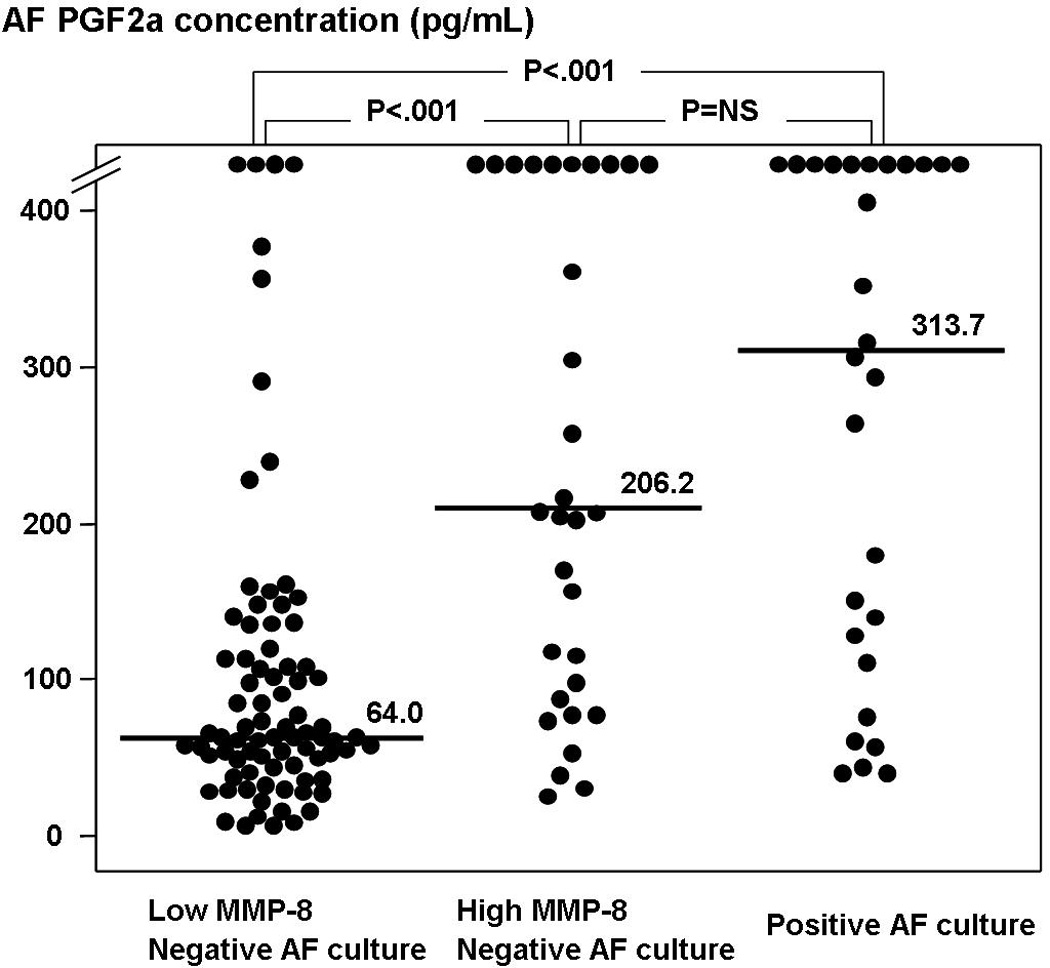
Amniotic fluid (AF) prostaglandin F2a (PGF2a) concentrations according to the results of AF culture and AF MMP-8 concentrations (group 1: median, 64.0 pg/mL [range, 7.7–2776.2 pg/mL]; group 2: median, 206.2 pg/mL [range, 26.1–38715.2 pg/mL]; group 3: median, 313.7 pg/mL [range, 40.5–8492.3 pg/mL]; p<0.001, by Kruskal-Wallis ANOVA test).
Table 2 describes the clinical characteristics and pregnancy outcomes of the patients according to the AF PGF2a concentrations. There was no significant difference in the gestational age at amniocentesis between groups. However, patients with a high AF PGF2a concentration (≥170 pg/mL) had a significantly lower median gestational age at delivery, a shorter interval-to delivery, and a higher frequency of preterm delivery within 36 weeks than those patients with a low AF PGF2a concentration (<170 pg/mL). Patients with a high AF PGF2a concentration had a higher median WBC count and AF MMP-8 concentration, a higher rate of a positive AF culture, intra-amniotic infection/inflammation and funisitis compared with those with a low AF PGF2a concentration.
Table 2.
Clinical characteristics and pregnancy outcomes of the patients according to the concentration of AF PGF2a
| Low PGF2a (n=94) |
High PGF2a (n=46) |
P | |
|---|---|---|---|
| Maternal age (y) | 30 (23–45) | 30 (21–38) | NS |
| Nulliparity (n) | 42 (45%) | 16 (35%) | NS |
| GA at amniocentesis (week)* | 32.6 (20.4–35.0) | 30.5 (21.6–34.7) | NS |
| GA at delivery (week)* | 34.3 (23.7–41.6) | 31.5 (21.9–35.7) | <.001 |
| Amniocentesis-to-delivery interval (hour)† | 155 (0–3545) | 72 (0–1849) | <.001 |
| within 48 hours | 14 (15%) | 20 (44%) | <.001 |
| within 7 days | 52 (55%) | 37 (80%) | <.005 |
| Preterm delivery <36 weeks | 80 (85%) | 46 (100%) | <.005 |
| Positive AF culture | 10 (11%) | 18 (39%) | <.001 |
| Intraamniotic infection/inflammation ‡ | 22 (23%) | 37 (80%) | <.001 |
| AF WBC (cells/mm3)* | 1 (0–621) | 397 (0- >1000) | <.001 |
| AF MMP-8 (ng/mL)* | 1.8 (0.3–260.5) | 267.0 (0.3–5019.5) | <.001 |
| Clinical chorioamnionitis | 4 (4%) | 4 (9%) | NS |
| Histologic chorioamnionitis (n/N) | 39/72 (54%) | 24/36 (67%) | NS |
| Funisitis (n/N) | 21/74 (28%) | 20/36 (56%) | <.01 |
Values are medians and ranges.
AF, amniotic fluid; MMP-8, matrix metalloproteinase-8; Low PGF2a, PGF2a <170 pg/mL; High PGF2a, PGF2a ≥170 pg/mL; GA, gestational age; NS, not significant; WBC, white blood cell
P<.05, by Kruskal-Wallis ANOVA test
Compared by generalized Wilcoxon test for survival analysis.
Intraamniotic infection/inflammation was defined as a positive AF culture and/or elevated AF MMP-8 concentration (>23ng/ml).
Table 3 describes the relationship between AF PGF2a concentrations and the presence or absence of intra-amniotic infection/inflammation. Forty-two percent (59/140) of patients had intra-amniotic infection/inflammation defined as a positive AF culture and/or elevated AF MMP-8 concentration (>23 ng/mL). Sixty-three percent (37/59) of patients with intra-amniotic infection/inflammation had an elevated AF PGF2a concentration (≥170 pg/mL), whereas those without intra-amniotic infection/inflammation had only 11% (9/81) (p<0.001). An AF PGF2a concentration of ≥170 pg/mL had a sensitivity of 63% and a specificity of 89% in the identification of intra-amniotic infection/inflammation.
Table 3.
AF PGF2a concentrations according to the presence or absence of intraamniotic infection/inflammation
| Intraamniotic infection/inflammation (−) |
Intraamniotic infection/inflammation (+) |
Total | |
|---|---|---|---|
| Low PGF2a | 72 (89%) | 22 (37%) | 94 |
| High PGF2a | 9 (11%) | 37 (63%) | 46 |
| Total | 81 | 59 | 140 |
P<.001, by Chi-square test; Intraamniotic infection/inflammation was defined as a positive AF culture and/or elevated AF MMP-8 concentration (>23ng/ml).
AF, amniotic fluid; Low PGF2a, PGF2a <170 pg/mL; High PGF2a, PGF2a ≥170 pg/mL
Figure 2 illustrates interval-to-delivery according to the concentration of AF PGF2a among patients with and without intra-amniotic infection/inflammation, respectively. Among patients with intra-amniotic infection/inflammation, those with a high AF PGF2a had a significantly shorter amniocentesis-to-delivery interval than those with a low AF PGF2a (median, 137 hours [range, 1–1587 hours] vs. median, 75 hours [range, 0–1849 hours], p<0.05). Moreover, among patients without intra-amniotic infection/inflammation, those with a high AF PGF2a had a significantly shorter amniocentesis-to-delivery interval than those with a low AF PGF2a (median, 159 hours [range, 0–3545 hours] vs. median, 66 hours [range, 2–141 hours], p<0.001). A high AF PGF2a concentration (≥170 pg/mL) was associated with a shorter amniocentesis-to-delivery than those with lower AF PGF2a concentrations after adjusting for gestational age and the presence of AF inflammation/infection (OR, 2.3; 95% CI, 1.4–4.0; p<0.005).
Figure 2.
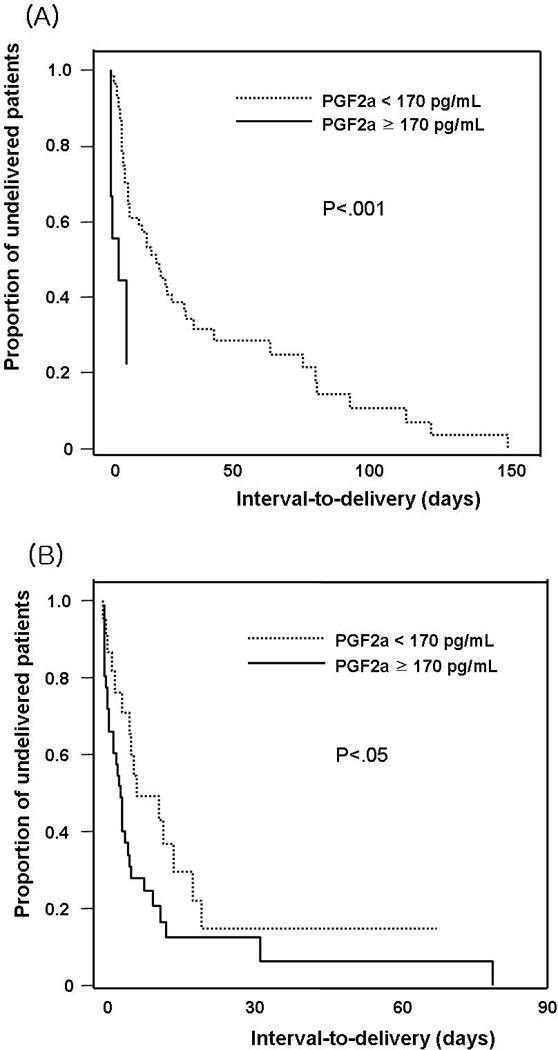
Survival analysis of the interval-to-delivery according to the concentration of AF PGF2a; (A) among patients without intra-amniotic infection/inflammation: median, 159 hours [range, 0–3545 hours] vs. median, 66 hours [range, 2–141 hours], p<0.001; (B) among patients with intra-amniotic infection/inflammation: median, 137 hours [range, 1–1587 hours] vs. median, 75 hours [range, 0–1849 hours], p<0.05.
Comment
Principal findings of this study
1) Intra-amniotic inflammation in preterm PROM was associated with an increased AF PGF2a concentration, regardless of the presence or absence of culture-proven AF infection; 2) an elevated AF PGF2a concentration (≥170 pg/mL) was present in 63% of cases with intra-amniotic infection/inflammation and in 11% of those without intra-amniotic infection/inflammation; 3) a high AF PGF2a concentration (≥170 pg/mL) was an independent risk factor for impending delivery, not only among patients with intra-amniotic infection/inflammation, but also among cases without intra-amniotic infection/inflammation in preterm PROM.
Amniotic fluid prostaglandin, intra-amniotic infection and preterm birth
Substantial evidence indicates that PGs play a role in preterm birth associated with intra-amniotic infection [11, 13, 19, 26, 27, 29, 30]. Microbial products such as endotoxin or microorganisms can induce an immune response in fetal membranes [7, 16, 21, 24] and decidua [1, 21], and stimulate PG production. Indeed, there is evidence that patients with AF infection had significantly higher concentrations of AF PG compared with those with sterile AF [26, 27, 29, 30]. Our data, obtained from a larger cohort, is consistent with the previous study and extends the observations by exploring the relationship between intra-amniotic inflammation with negative AF cultures. Such study has not been reported before.
An increase of amniotic fluid PG in the absence of culture-proven amniotic fluid infection
A novel finding of this study is that patients with intra-amniotic inflammation, even in the absence of proven AF infection, had a higher concentration of AF PGF2a than those without intra-amniotic infection/inflammation. Previous studies in vitro demonstrated that PG production was induced by pro-inflammatory cytokines (e.g., interleukin-1 beta, tumor necrosis factor-alpha) [7, 21]. Previous studies showed that the intensity of the intra-amniotic inflammatory process (measured by AF WBC count and AF MMP-8 concentration) was not different between patients with intra-amniotic inflammation and a negative culture and patients with culture-proven AF infection [17, 31]. Therefore, an increase of AF PG concentration in preterm PROM with intra-amniotic inflammation, even in the absence of a positive AF culture, is not an unexpected finding. Our data is the first evidence of an increase in PG AF concentrations in sterile AF in humans.
Why are amniotic fluid prostaglandin concentrations elevated in patients with intra-amniotic inflammation without culture-proven amniotic fluid infection?
One possibility is that infection is present but microorganisms escaped detection by traditional microbiological methods. Studies using molecular microbiology techniques indicate that culture methodology underestimates the frequency of infection of the amniotic cavity [20, 34]. Some cases with intra-amniotic inflammation and a negative AF culture may have an extra-amniotic intrauterine infection. Decidual colonization of microorganisms can induce an immune reaction and PG production from decidual cells [1, 2, 21].
Non-infection related causes of amniotic fluid prostaglandin production
The possibility that AF PG concentrations can be elevated by non-infection-related mechanisms must also be considered. Activation of the hypothalamic pituitary-adrenal (HPA) axis of the fetus leads to an increase in fetal cortisol secretion and ultimately an increase in PGF2a production [5, 9, 14, 22, 33]. The fetus would activate the HPA axis in response to stress caused by inflammation or other pathologic processes [5, 33]. Moreover, CRH, produced in the placenta in response to maternal [12] and fetal [5, 33] stress, stimulates PG production from the fetal membranes. Even in normal term parturition, an abrupt increase of AF PGF2a concentrations (25 fold) occurs before the onset of spontaneous labor [18].
Our study indicates that 11% of patients without intra-amniotic infection/inflammation, had a high level of AF PGF2a concentration (Table 3), and these patients had a shorter interval-to-delivery than those who had a lower concentration of AF PGF2a (Figure 2). These findings suggest that AF PGF2a can be elevated in cases with neither infection nor inflammation.
Interval to delivery
PGs can induce increased uterine contractility and cervical ripening in animals and humans. Therefore, PGs have been proposed to be part of the common pathway of parturition, regardless of the cause (physiologic parturition or pathologic parturition). Our data indicates that a high AF PGF2a concentration is a risk factor for impending delivery in patients without intra-amniotic infection/inflammation as well as in those with intra-amniotic infection/inflammation in preterm PROM. Moreover, a high AF PGF2a was a significant predictor of the duration of pregnancy after adjusting for gestational age. We propose that once AF PGF2a increased, regardless of cause or gestational age, the ruptured fetal membranes should be easy to allow PGF2a to reach the decidua and myometrium and to initiate a preterm labor. Further studies are required to determine if the interval-to-delivery in patients with intact membranes is longer than that in patients with ruptured membranes among cases with increased AF PG concentration. Such studies are now in progress to examine this question.
Clinical implication
The major implication of our study is that the assessment of AF PGF2a concentration has prognostic value in preterm PROM. Currently, studies of AF in preterm PROM have been limited to the determination of AF inflammatory markers, infection and fetal lung maturity [4, 15, 32]. We propose that the determination of AF PGF2a concentration could have prognostic value in patients presenting with preterm PROM.
Acknowledgments
This work was supported in part by Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (No. R01-2006-000-10607-0), and in part by the Intramural Division of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
This study was presented at the 28th Annual Clinical Meeting of the Society for Maternal–Fetal Medicine, Dallas, TX, Jan. 28-Feb. 2, 2008.
References
- 1.Aaltonen R, Heikkinen J, Vahlberg T, Jensen JS, Alanen A. Local inflammatory response in choriodecidua induced by Ureaplasma urealyticum. BJOG. 2007;114(11):1432–1435. doi: 10.1111/j.1471-0528.2007.01410.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–612. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 3.Angus SR, Segel SY, Hsu CD, Locksmith GJ, Clark P, Sammel MD, et al. Amniotic fluid matrix metalloproteinase-8 indicates intra-amniotic infection. Am J Obstet Gynecol. 2001;185:1232–1238. doi: 10.1067/mob.2001.118654. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol. 1999;11(6):541–547. doi: 10.1097/00001703-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Challis JR, Bloomfield FH, Bocking AD, Casciani V, Chisaka H, Connor K, et al. Fetal signals and parturition. J Obstet Gynaecol Res. 2005 Dec;31(6):492–499. doi: 10.1111/j.1447-0756.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Collins PL, Goldfien A, Roberts JM. Exposure of human amnion to amniotic fluid obtained before labor causes a decrease in chorion/decidual prostaglandin release. J Clin Endocrinol Metab. 1992 May;74(5):1198–1205. doi: 10.1210/jcem.74.5.1569168. [DOI] [PubMed] [Google Scholar]
- 7.Furuta I, Yamada H, Sagawa T, Fujimoto S. Effects of inflammatory cytokines on prostaglandin E(2) production from human amnion cells cultured in serum-free condition. Gynecol Obstet Invest. 2000;49(2):93–97. doi: 10.1159/000010222. [DOI] [PubMed] [Google Scholar]
- 8.Giannoulias D, Haluska GJ, Gravett MG, Sadowsky DW, Challis JR, Novy MJ. Localization of prostaglandin H synthase, prostaglandin dehydrogenase, corticotropin releasing hormone and glucocorticoid receptor in rhesus monkey fetal membranes with labor and in the presence of infection. Placenta. 2005;26(4):289–297. doi: 10.1016/j.placenta.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Gibb W. The role of prostaglandins in human parturition. Ann Med. 1998;30(3):235–241. doi: 10.3109/07853899809005850. [DOI] [PubMed] [Google Scholar]
- 10.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intra-amniotic infection. Infect Dis Clin North Am. 1997;11(1):135–176. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 11.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81(6):941–948. [PubMed] [Google Scholar]
- 12.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks' gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180(1 Pt 3):S257–S263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CD, Meaddough E, Aversa K, Hong SF, Lee IS, Bahodo-Singh RO, et al. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am J Perinatol. 1998;15(12):683–687. doi: 10.1055/s-2007-999302. [DOI] [PubMed] [Google Scholar]
- 14.Jones SA, Challis JR. Effects of corticotropin-releasing hormone and adrenocorticotropin on prostaglandin output by human placenta and fetal membranes. Gynecol Obstet Invest. 1990;29(3):165–168. doi: 10.1159/000293368. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy KA, Clark SL. Premature rupture of the membranes: management controversies. Clin Perinatol. 1992;19(2):385–397. [PubMed] [Google Scholar]
- 16.Lamont RF, Anthony F, Myatt L, Booth L, Furr PM, Taylor-Robinson D. Production of prostaglandin E2 by human amnion in vitro in response to addition of media conditioned by microorganisms associated with chorioamnionitis and preterm labor. Am J Obstet Gynecol. 1990;162(3):819–825. doi: 10.1016/0002-9378(90)91017-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intra-amniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197(3) doi: 10.1016/j.ajog.2007.07.006. 294.e1–6. [DOI] [PubMed] [Google Scholar]
- 18.Lee SE, Romero R, Park IS, Seong HS, Park CW, Yoon BH. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Maternal Fetal Neonatal Med. 2007;21(1):1–6. doi: 10.1080/14767050701830514. [DOI] [PubMed] [Google Scholar]
- 19.Mazor M, Wiznitzer A, Maymon E, Leiberman JR, Cohen A. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci. 1990;26(8):425–428. [PubMed] [Google Scholar]
- 20.Miralles R, Hodge R, McParland PC, Field DJ, Bell SC, Taylor DJ, et al. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res. 2005;57(4):570–577. doi: 10.1203/01.PDR.0000155944.48195.97. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell MD, Romero RJ, Avila C, Foster JT, Edwin SS. Prostaglandin production by amnion and decidual cells in response to bacterial products. Prostaglandins Leukot Essent Fatty Acids. 1991;42(3):167–169. doi: 10.1016/0952-3278(91)90152-u. [DOI] [PubMed] [Google Scholar]
- 22.Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17(5):717–730. doi: 10.1016/s1521-6934(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 23.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–1161. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 24.Reisenberger K, Egarter C, Knöfler M, Schiebel I, Gregor H, Hirschl AM, et al. Cytokine and prostaglandin production by amnion cells in response to the addition of different bacteria. Am J Obstet Gynecol. 1998;178(1 Pt 1):50–53. doi: 10.1016/s0002-9378(98)70625-8. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Baumann P, Gonzalez R, Gomez R, Rittenhouse L, Behnke E, et al. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am J Obstet Gynecol. 1994;171(6):1613–1620. doi: 10.1016/0002-9378(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet. 1986 Jun 14;1(8494):1380. doi: 10.1016/s0140-6736(86)91685-5. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157(6):1461–1467. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intra-amniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159(3):661–666. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1988;34(3):141–145. doi: 10.1016/0952-3278(88)90137-8. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Wu YK, Sirtori M, Oyarzun E, Mazor M, Hobbins JC, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins. 1989;37(1):149–161. doi: 10.1016/0090-6980(89)90038-5. [DOI] [PubMed] [Google Scholar]
- 31.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 32.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ, Escoto DT, Mirochnick MH. Qualitative amniotic fluid volume versus amniocentesis in predicting infection in preterm premature rupture of the membranes. Obstet Gynecol. 1986;67(4):579–583. [PubMed] [Google Scholar]
- 33.Whittle WL, Patel FA, Alfaidy N, Holloway AC, Fraser M, Gyomorey S, et al. Glucocorticoid regulation of human and ovine parturition: the relationship between fetal hypothalamic-pituitary-adrenal axis activation and intrauterine prostaglandin production. Biol Reprod. 2001;64(4):1019–1032. doi: 10.1095/biolreprod64.4.1019. [DOI] [PubMed] [Google Scholar]
- 34.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–1137. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]


