Abstract
Ischemic heart disease (IHD) is a leading cause of death worldwide, and regenerative therapies through exogenous stem cell delivery hold promising potential. One limitation of such therapies is the vulnerability of stem cells to the oxidative environment associated with IHD. Accordingly, manipulation of stem cell mitochondrial metabolism may be an effective strategy to improve survival of stem cells under oxidative stress. MitoNEET is a redox-sensitive, mitochondrial target of thiazolidinediones (TZDs), and influences cellular oxidative capacity. Pharmacological targeting of mitoNEET with the novel TZD, mitoNEET Ligand-1 (NL-1), improved cardiac stem cell (CSC) survival compared to vehicle (0.1 % DMSO) during in vitro oxidative stress (H2O2). 10 μM NL-1 also reduced CSC maximal oxygen consumption rate (OCR) compared to vehicle. Following treatment with dexamethasone, CSC maximal OCR increased compared to baseline, but NL-1 prevented this effect. Smooth muscle α-actin expression increased significantly in CSC following differentiation compared to baseline, irrespective of NL-1 treatment. When CSCs were treated with glucose oxidase for 7 days, NL-1 significantly improved cell survival compared to vehicle (trypan blue exclusion). NL-1 treatment of cells isolated from mitoNEET knockout mice did not increase CSC survival with H2O2 treatment. Following intramyocardial injection of CSCs into Zucker obese fatty rats, NL-1 significantly improved CSC survival after 24 h, but not after 10 days. These data suggest that pharmacological targeting of mitoNEET with TZDs may acutely protect stem cells following transplantation into an oxidative environment. Continued treatment or manipulation of mitochondrial metabolism may be necessary to produce long-term benefits related to stem cell therapies.
Keywords: Stem cells, Thiazolidinediones, Oxidative stress, MitoNEET, Ischemic heart disease
Introduction
Cardiovascular disease is the leading cause of death worldwide. Ischemic heart disease (IHD) accounted for 7.4 million deaths across the globe in 2012, and represents the majority of cardiovascular diseases [20]. Although many interventions and treatments are in place to ameliorate the morbidity and mortality of IHD, chronic heart failure is the outcome for many patients. This unfortunate consequence underscores the rationale that prophylactic treatments to prevent the consequences of IHD, e.g., stimulation of coronary collateral growth, would have enormous benefit.
Regenerative therapies through exogenous stem cell administration hold promising potential [1, 2, 4, 22, 25, 31], but may be limited by excessive cell death after transplantation into an oxidative environment [17]. Since mitochondria are a major source of reactive oxygen species (ROS) in the cell, reductions in mitochondrial metabolism may reduce both the oxidative burden and cell death under oxidative stress. Thiazolidinediones (TZD) are a class of insulin-sensitizing agents that have been shown to attenuate mitochondrial oxygen consumption [7, 14], which may reduce ROS generation and aid cell survival in a highly oxidative environment.
MitoNEET is a redox-sensitive protein on the outer mitochondrial membrane that has been identified as a mitochondrial target of TZDs [6]. Past studies have demonstrated a role for mitoNEET in the regulation of ROS production [35], insulin sensitivity [12], adiponectin expression [12], and myocardial oxidative metabolism [30]. To manipulate the activity of this protein and its effects on mitochondrial metabolism we both designed and used a novel TZD, mitoNEET Ligand-1 (NL-1) [9]. The molecular structure of NL-1 was modified from rosiglitazone to decrease the affinity of NL-1 for PPARγ and render it specific for mitoNEET. The thiazolidinedione moiety has been identified as important for the binding of these compounds to mitoNEET, and thus it is unchanged between NL-1 and rosiglitazone or pioglitazone [9].
Because of the potential for pharmacological targeting of mitoNEET to influence the intracellular redox state, the present studies were conducted to test the impact of NL-1 on cardiac stem cell (CSC) survival under oxidative stress in vitro, as well as to elucidate the effects of this compound on CSC differentiation secondary to changes in mitochondrial metabolism. In addition, the impact of NL-1 treatment on CSC survival in an in vivo oxidative environment was examined via intramyocardial delivery into Zucker obese fatty rats.
Methods
Animals
All animals were Lean and Obese Zucker rats, 4–6 months in age. Animal research protocols were approved by the Institutional Animal Care and Use Committee at North-eastern Ohio Medical University and abided by UP Public Health Service animal welfare policy.
Cardiac stem cells
C-kit+ cardiac stem cells (CSCs), as described in Beltrami et al. [3], were kindly provided as a gift from the group of Drs. Piero Anversa and Annarosa Leri. CSCs were cultured according to the protocols utilized by the original authors. Growth medium consisted of Ham's F-12K medium containing 10 % FBS (HyClone), 5 % horse serum (Gibco), 0.2 mM l-glutathione (Sigma), 5 mU/mL human erythropoietin (Sigma), 50 μg/mL porcine gelatin (Sigma), 10 ng/mL leukemia inhibitor factor (Millipore), 10 ng/mL basic human FGF (Peprotech), 1 % penicillin–streptomycin.
MitoNEET knockout cell isolations
MitoNEET knockout mice were obtained from Taconic Laboratories. Hearts were removed from 10- to 12-week-old, male mitoNEET knockout or wild-type (129/SvEv-C57BL/6) mice, and transferred to 15 mL tubes with sterile PBS. Under a laminar flow cabinet, hearts were transferred to 100-mm dishes containing sterile, ice-cold PBS (without calcium or magnesium), blood was effluxed, and all surrounding connective, vascular, and adipose tissue was dissected away and discarded. Hearts were then transferred to 1 mL of type 2 collagenase [50 mg/mL (Worthington Biochemicals)] in PBS containing 0.1 mM calcium chloride, and minced into approximately, 5-mm fragments, followed by partial trituration with a transfer pipette. Cardiac tissue then underwent two 15-min periods of digestion at 37 °C in 5 mL of collagenase solution, with removal and neutralization of the supernatant with 10 mL complete medium after the first 15-min period. Following removal of the supernatant after the second digestion, 10 mL additional medium was added to the suspension, followed by centrifugation at 10 RCF to remove cardiomyocytes. The supernatant was then filtered through a 40-μm strainer and centrifuged again at 300 RCF. Following removal of the supernatant, the resulting pellet was resuspended in complete medium containing 100 μg/mL Primocin (Invivogen).
In vitro cell survival experiments
Rosiglitazone, GW9662, and H2O2 were purchased from Cayman Chemical. NL-1 was synthesized as described by the authors [9]. All drugs were aliquoted and stored at −20 °C. Fresh aliquots were used for each experiment. H2O2 was prepared fresh for each experiment from the originally manufactured 8.8 M stock (Cayman Chemical). To study the impact of mitoNEET on cell survival in response to an oxidative challenge, cells were resuspended in complete growth medium and plated on tissue culture-treated dishes (rat CSC: 5 × 105 cells/35-mm well; mitoNEET knockout/wild-type cardiac cells: 1.25 × 105 cells/35-mm well). Immediately upon plating of the cells, pharmacological agents [mitoNEET Ligand-1 (NL-1; 10 μM in DMSO); rosiglitazone (10 μM in DMSO); GW9662 (PPARγ antagonist; 20 μM in DMSO) or vehicle (0.1 % DMSO)] were added 10 min prior to oxidative stress treatment with H2O2 (CSC: 500 μM; mitoNEET knockout/wild-type cardiac cells: 250 μM) or glucose oxidase (400 ng/mL). The dose of NL-1 was established from an initial dose-response relationship between cell survival and H2O2 (500 μM). Doses greater than 1 mM of NL-1 were associated with cell death and doses of 10 and 100 μM produced a similar efficacious increase in cell survival. Following oxidative stress treatment, the media were collected and centrifuged to avoid discarding any detached cells. After a 24-h recovery period, media were collected again and adherent cells were harvested and analysed via an automated cell counter with trypan blue exclusion (Invitrogen) or via flow cytometry with propidium iodide exclusion.
Automated cell counting
Cells were analysed using the cell countess (Invitrogen), which detects dead cells via trypan blue exclusion. Prior to each analysis, 10 μL of cell suspension was added to 10 μL of trypan blue and allowed to incubate for 2 min. The field of view was adjusted such that the cells were in focus according to manufacturer's directions.
Flow cytometry
Cells were dissociated, washed three times via centrifugation, counted, and resuspended in 200 μL flow cytometry buffer (1 % BSA, 0.2 % EDTA, and 0.04 % sodium azide in PBS) containing 2 μL of a 2 mM propidium iodide (PI) solution. Cell counts were compared between samples after appropriate gating based on PI-positivity.
CSC differentiation
As described by Beltrami et al. [3], CSCs were subjected to a 7-day differentiation protocol consisting of culture in DMEM, 10 % FBS, and 10−8 M dexamethasone (Sigma). Undifferentiated CSCs were dissociated and centrifuged to remove CSC culture medium. 5 × 105 cells were resuspended in differentiation medium, and plated on 100-mm dishes. Drugs were added immediately upon plating the cells. Medium was changed every 3–4 days, along with the addition of fresh treatment reagents. High-glucose DMEM [4.5 g/L (25 mM)] was used to partially simulate the diabetic condition. CSC differentiation experiments were conducted both in the presence and absence of oxidative stress (induced by 400 ng/mL glucose oxidase). After 7 days of culture in differentiation medium, differentiated cells were analysed via the Seahorse XF24 extracellular flux analyzer, or harvested for molecular or cellular studies.
Mitochondrial metabolism
Mitochondrial metabolic function was assessed using a Seahorse XF24 Extracellular Flux Analyzer (North Bellerica, MA, USA). Preparation for the assay was performed according to the XF24 user manual, first edition (2009). CSCs were plated on rat-tail collagen-coated wells (5 μg/cm2) in full growth medium at a concentration of 3 × 104 cells per well, 14 h before running the assay. On the day of the assay, cells were washed 3 × 300 μL with XF24 medium, and each well was brought to a final volume of 675 μL. XF24 assay medium (Seahorse Biosciences) was prepared according to the XF24 user manual, with equal quantities of D-glucose, l-glutamine, and sodium pyruvate as contained in the CSC growth medium (7 mM glucose, 2 mM l-glutamine, and 2 mM sodium pyruvate). As part of the optimization of the assay for CSC, 0.2 % BSA was added to the XF24 medium used during the analysis. Oligomycin (1 μg/mL, Sigma), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; 2 μM, Sigma), and rotenone (1 μM, Sigma) were delivered to the cells in sequential order during the assay. Following the assay protocol, a BCA protein assay was performed and results were normalized to protein concentration. Assays were run with the indicated treatment agents in the assay media.
Real-time PCR
Quantitative PCR was performed using SYBR Green reagents according to the manufacturer's instructions. RNA was isolated from cardiac stem cells using TRIzol (Ambion) following 7 days of culture in differentiation medium. DNAse-treated RNA was used in first-strand cDNA synthesis via reverse-transcription (Applied Biosystems). cDNA was stored at −20 °C until used in analysis. Primers were constructed using online Integrated DNA Technologies software and are listed in Table 1.
Table 1. Real-time PCR primers.
| Gene | RefSeq | Sequence | |
|---|---|---|---|
| Smooth muscle alpha-actin (SMA) | NM_031004.2 | Forward | 5′ AGGGAGTGATGGTTGGAATG 3′ |
| Reverse | 5′ GGTGATGATGCCGTGTTCTA 3′ | ||
| Calponin | NM_031747.1 | Forward | 5′ TCCGCACACTTTAACCGAG 3′ |
| Reverse | 5′ ATCCATGAAGTTGCTCCCG 3′ | ||
| Von willebrand factor (vWF) | NM_001007663.1 | Forward | 5′ ACAAAGAAGGTGACGAGGTG 3′ |
| Reverse | 5′ AGGACACAGAAGTTGGGTATTT 3′ | ||
| VE-cadherin | NM_001107407.1 | Forward | 5′ CAGTGACAGAGGCCAATACTT 3′ |
| Reverse | 5′ GCCTCCACAGTCAGGTTATAC 3′ | ||
| Cardiac myosin heavy chain 6 (Myh6) | NM_017239.2 | Forward | 5′ GCTGACAAATCTGCCTACCT 3′ |
| Reverse | 5′ GACATACTCGTTACCCACCTTC 3′ | ||
| Troponin I | NM_017144.1 | Forward | 5′ TGGACAAAGTGGATGAAGAGAG 3′ |
| Reverse | 5′ TTGCCACGCAGGTCATAG 3′ | ||
| Cardiac alpha actin (Actc1) | NC_005102.3 | Forward | 5′ TCTTCCAGCCCTCTTTCATTG 3′ |
| Reverse | 5′ CAGCAATCCCAGGGTACATG 3′ | ||
| kGFP | Forward | 5′ GAATTCAAGCTTCGCCACCATGGTGAGCAAGG GCGAGGAG 3′ | |
| Reverse | 5′ AATTCTCTAGATTACTTGTACAGCTCGTCCATGCC 3′ |
Cell injections and CSC survival with or without NL-1 treatment
Zucker obese fatty (ZOF) rats each received 2.5 × 105 CSCs, injected directly into the apical portion of the left ventricular myocardium. Prior to injection, CSCs were dissociated, counted, washed twice with PBS, and resuspended in PBS either with or without the addition of 10 μM NL-1. Using a 50-μL Hamilton syringe fitted with a 25-G needle, total volume of 50 μL was delivered in two separate injection sites. Analysis of CSC survival was conducted after either 24 h or 10 days from the time of CSC injection. CSC survival was determined by GFP expression using real-time PCR analysis of genomic DNA from rat heart tissues with cell injection. To account for possible migration of CSCs following injection, genomic DNA was isolated from the entire rat heart.
Hearts were excised from the animal, effluxed of blood, snap frozen in liquid nitrogen, and stored at −80 °C until further use. Tissue was minced in lysis buffer (50 mM Tris–Cl, pH 8.0; 100 mM EDTA; 100 mM NaCl; 1 % SDS) into ∼ 1- to 2-mm fragments. Proteinase K was added to a final concentration of 1.2 mg/mL. Complete digestion was achieved through incubation at 55 °C overnight, with mixing. The next day, DNA was extracted with phenol:chloroform:isoamyl alcohol (25:24:1; Fischer Scientific), followed by ethanol precipitation, two washes with 70 % EtOH, and resuspension in Tris–EDTA solution. The resulting A260/280 ratios of DNA were above 1.9 and A260/230 ratios were above 2.0 for each sample. DNA was diluted to 50 ng/μL and used as the template DNA for realtime PCR. Real-time PCR was performed using a GFP primer, to determine relative amplification of the GFP gene between samples from ZOF rat hearts that received CSCs either with or without NL-1 treatment. Resulting Ct values were normalized to those for CSCs without NL-1 treatment. NL-1 was not used in the whole animal because of its very short half-life [21], and our goal to confine the effects of the drugs to the cells we were administering.
Statistics
Data and the groups were analysed using one- and two-way ANOVAs using GraphPad Prism software version 6 followed by multiple comparison testing. A modality of the software package was employed to use the most appropriate test based on the distribution, sample size, variation, etc. One-way ANOVA with the named post hoc tests were used for the following analyses: Bonferroni's multiple comparisons test for NL-1 treatment of CSCs under oxidative stress (Fig. 1); Tukey's multiple comparisons test for oxygen consumption rate of CSCs (Figs. 2, 3); and Sidak's multiple comparisons test for CSC differentiation and survival (Figs. 4, 5). Two-way ANOVA with the named multiple comparison test were used for the following analyses: Tukey's multiple comparisons test for CSC Differentiation and Survival (Figs. 4a, 7) and Sidak's multiple comparisons test for oxidative treatment of MitoNEET null cells (Fig. 6). In the text, data are reported as mean ± SD. P values less than 0.05 were considered significant.
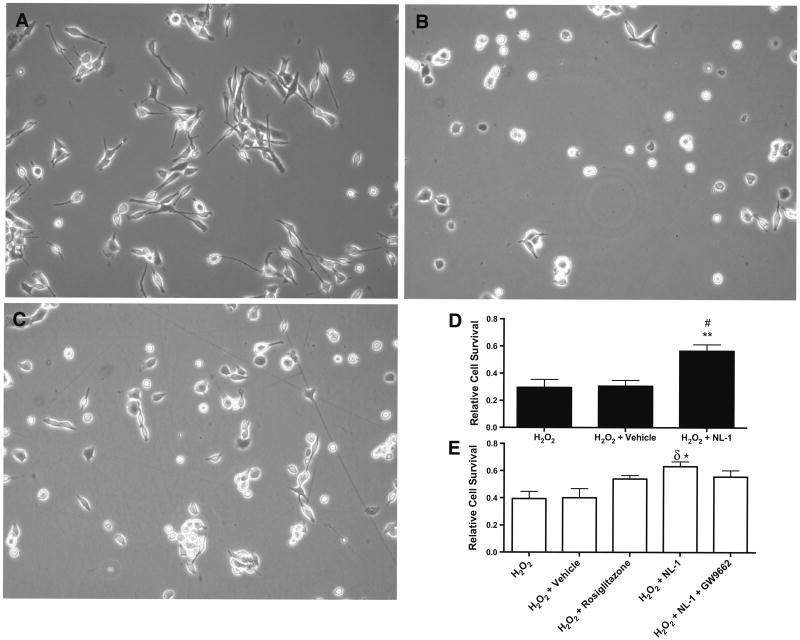
Fig. 1.
NL-1 treatment increases CSC survival under oxidative stress. a Morphology of CSC under phase microscopy. b CSC immediately following 4-h treatment with 500 μM H2O2. c CSC immediately following 4-h treatment with 500 μM H2O2 + 10 μM NL-1. d, e Represent CSC survival determined by an automated cell counter with trypan blue exclusion (d) and flow cytometry with propidium iodide exclusion (e). Relative cell survival was normalized to non-treated CSC cultured in parallel with treated groups. *p < 0.05, **p < 0.01 vs. H2O2. #p < 0.01, δp < 0.05 vs. H2O2 + vehicle. a-d N = 6, e N = 3
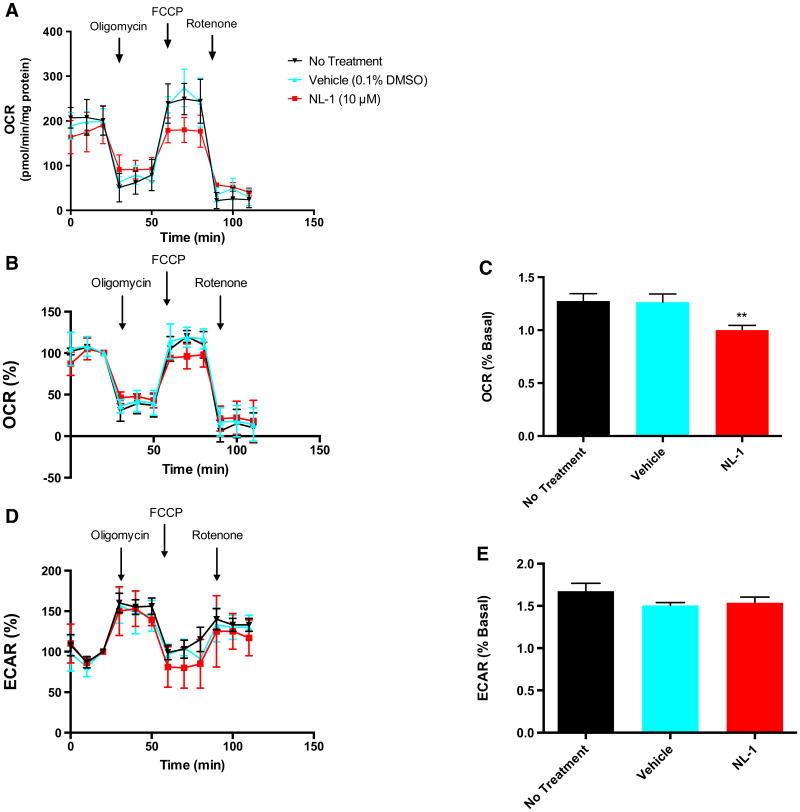
Fig. 2.
NL-1 reduces maximal oxygen consumption rate of CSCs. a Absolute oxygen consumption rate (OCR) of CSCs with or without NL-1 treatment represented as pmol O2/min/mg protein. b OCR of under same conditions as A represented as percent baseline. Baseline is set at 100 %, and is defined as the OCR (or ECAR) at the time point immediately preceding oligomycin administration. c Maximal OCR as measured during the first three time points following FCCP administration. d Extracellular acidification rate (ECAR) of CSCs with or without NL-1 treatment. e Maximal ECAR as measured during the first three time points following oligomycin administration. **p < 0.01 vs. both no treatment and vehicle. N = 3
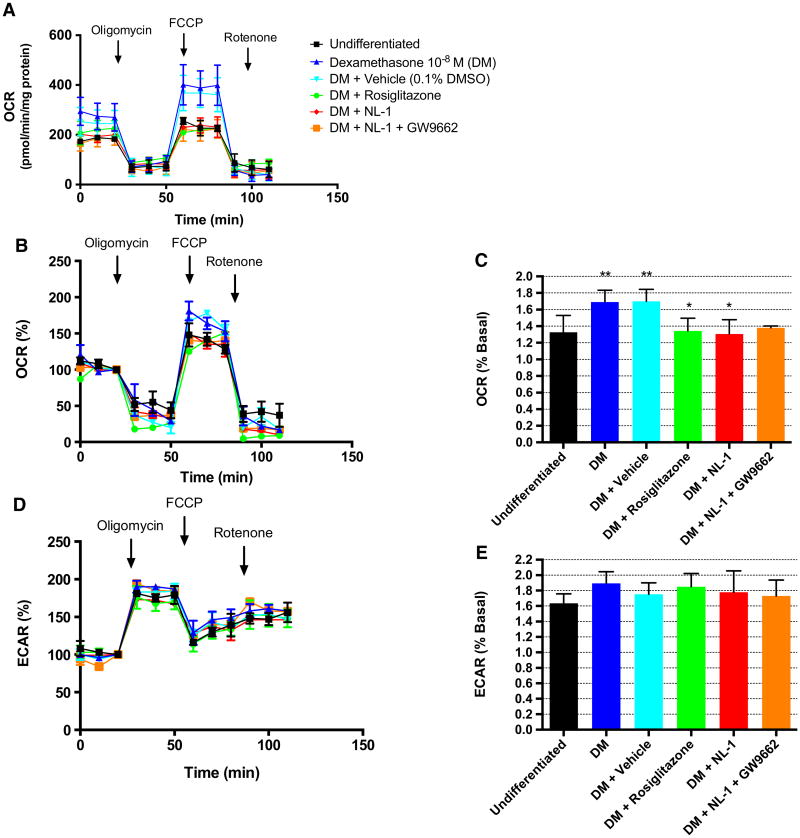
Fig. 3.
NL-1 reduces maximal ocr of differentiated CSCs. a Absolute oxygen consumption rate (OCR) of CSCs following a 7-day differentiation protocol under the specified treatment conditions, represented as pmol O2/min/mg protein. b OCR and d extracellular acidification rate (ECAR) of CSC represented as percent baseline (baseline is defined as OCR or ECAR at time point immediately preceding FCCP or oligomycin administration, respectively). c Maximal OCR as measured during the first three time points after FCCP administration and represented as percent baseline. e Maximal ECAR as measured during the first three time points following oligomycin administration and represented as percent baseline. **p < 0.01 vs. undifferentiated. #p < 0.01 vs. both DM and DM + vehicle. *p < 0.05 vs. both DM and DM + vehicle. N = 4. DM dexamethasone-treated CSCs
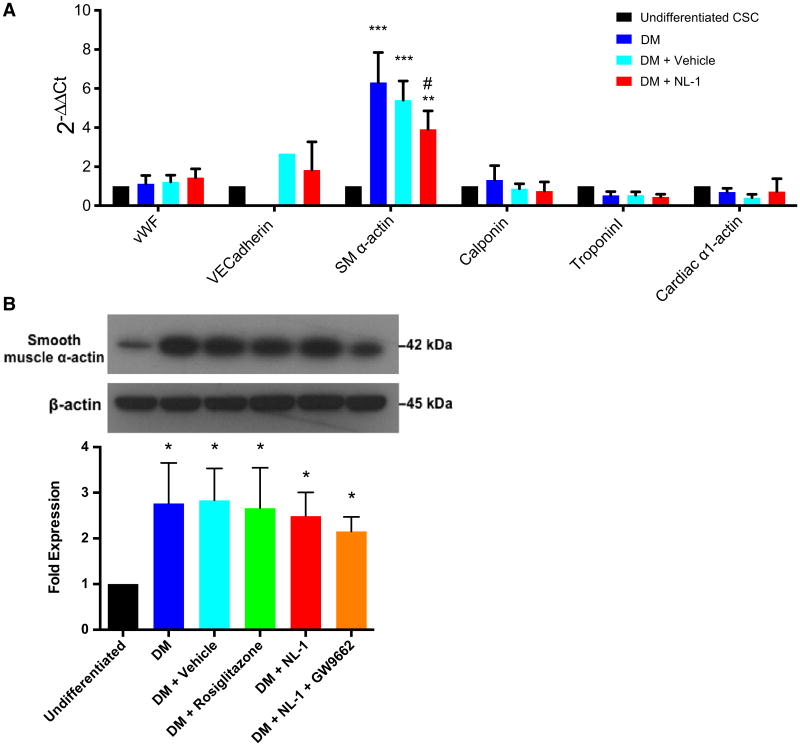
Fig. 4.
NL-1 treatment does not appear to influence CSC differentiation. a Quantitative PCR and b western blot analyses showing relative expression of SM α-actin normalized to undifferentiated CSCs. *p < 0.05, **p < 0.01, ***p < 0.001 vs. undifferentiated CSCs. #p < 0.01 vs. DM. N = 3. DM dexamethasone-treated CSCs
Fig. 5.
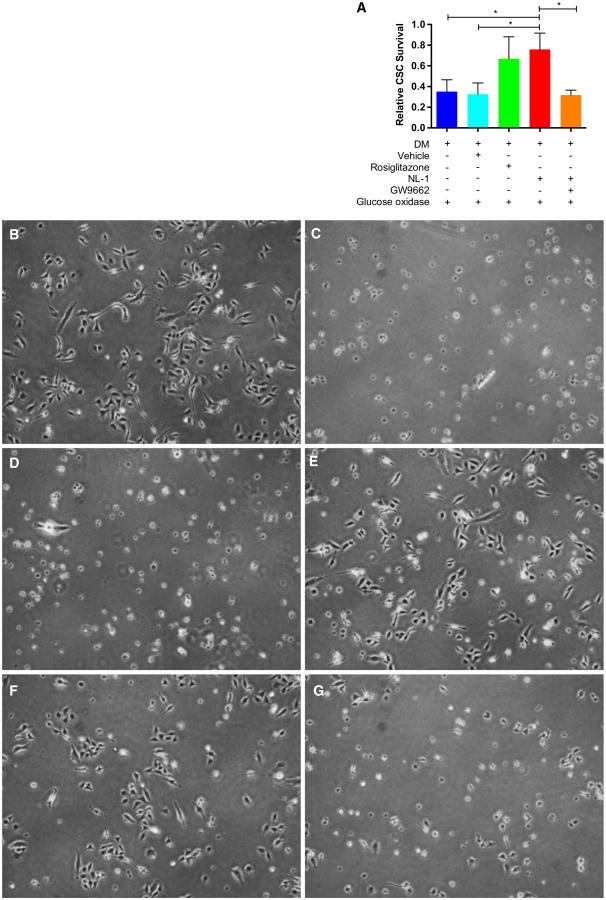
NL-1 treatment increases CSC Survival during differentiation under chronic oxidative stress. a Cardiac stem cell (CSC) survival determined via automated cell counting (trypan blue exclusion) following 7 days' culture in differentiation medium [10−8 M dexamethasone (DM)] in the presence of 800 ng/mL of glucose oxidase. Data are presented relative to percent of viable cells receiving no glucose oxidase treatment. N = 3 separate experiments. *p < 0.05. b–g Phase-contrast microscopy of CSCs following 4 days of culture in differentiation medium under the following treatment conditions: b dexamethasone (DM) only (no glucose oxidase); c DM + glucose oxidase (GO) (800 ng/mL); d DM + DMSO (0.1 %) + GO; e DM + NL-1 (10 μM) + GO; f DM + rosiglitazone (10 μM) + GO; g DM + NL-1 + GW9662 (20 μM) + GO
Fig. 7.
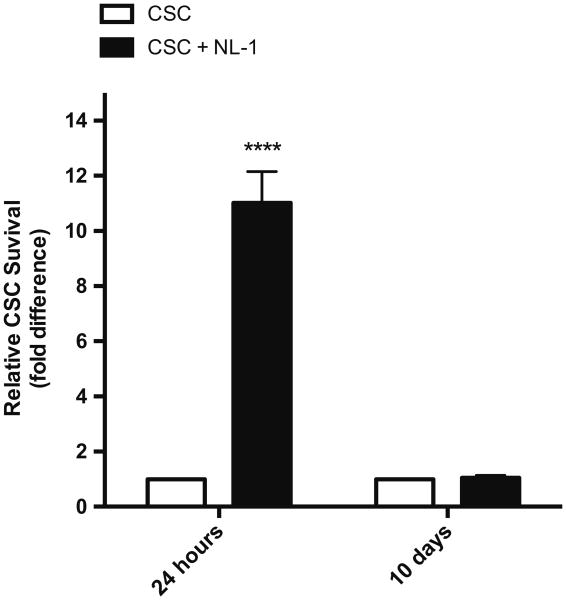
NL-1 treatment improves short-term, but not long-term CSC survival under in vivo oxidative stress. Real-time PCR analysis of relative CSC survival following injection into ZOF rat myocardia. Values were normalized to CSC injection without NL-1 treatment. N = 3 separate experiments. ****p < 0.0001
Fig. 6.
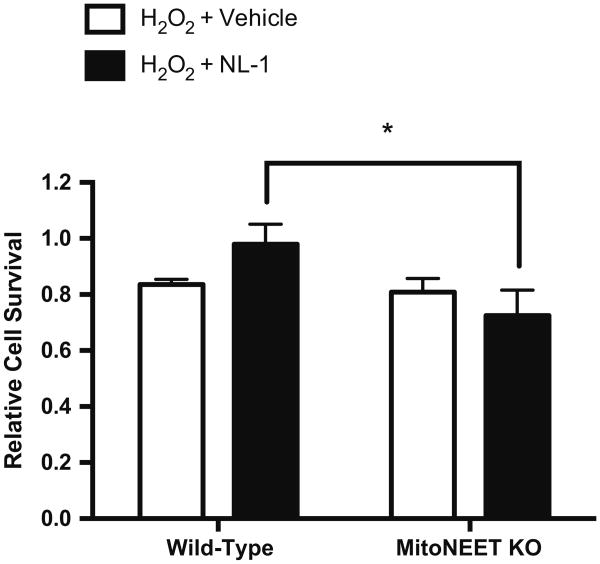
NL-1 treatment of mitoNEET knockout cells. Flow cytometric analysis of cell survival (propidium iodide exclusion) in cardiac cells from mitoNEET knockout or wild-type mice following treatment with 250 μM H2O2 and a 24-h recovery period. N = 3. *p < 0.05
Results
NL-1 treatment increases CSC survival under oxidative stress
When the CSCs were incubated for 10 min with 10 μM NL-1 prior to H2O2 administration, phase-contrast microscopy demonstrated that the H2O2 + NL-1 group exhibited less cytopathologic change compared with the H2O2 groups (Fig. 1b, c), and relative cell survival was higher when analysed with both automated cell counting (H2O2: 0.295 ± 0.120; H2O2 + vehicle: 0.306 ± 0.090; H2O2 + NL-1: 0.565 ± 0.120) and flow cytometry (H2O2: 0.394 ± 0.092; H2O2 + vehicle: 0.401 ± 0.120; H2O2 + NL-1: 0.632 ± 0.070 (Fig. 1d, e). NL-1 treatment in the presence of the PPARγ antagonist, GW9662, trended toward an increase in CSC survival compared with vehicle, but this difference was not significant (H2O2 + vehicle: 0.306 ± 0.090, H2O2 + NL-1 + GW9662: 0.556 ± 0.093). Similarly, rosiglitazone treatment appeared to slightly improve CSC survival, without achieving statistical significance compared to vehicle treatment (H2O2 + rosiglitazone: 0.540 ± 0.052).
NL-1 reduces maximal oxygen consumption rate of CSCs
The effect of NL-1 on CSC mitochondrial metabolism appeared to be a reduction of the maximal oxygen consumption rate (Fig. 2a–c). Following FCCP treatment, NL-1 significantly decreased both absolute (Fig. 2a) and normalized oxygen consumption rates (OCR) compared with non-treated or vehicle-treated cells (no treatment: 1.276 ± 0.118; vehicle: 1.263 ± 0.133; NL-1: 1.001 ± 0.077) (Fig. 2b). Conversely, NL-1 treatment had no significant effect on maximal extracellular acidification rate (ECAR) (no treatment: 1.673 ± 0.162; vehicle: 1.506 ± 0.057; NL-1: 1.538 ± 0.092) (Fig. 2e), as measured following oligomycin (Fig. 2d). This observation suggests that NL-1 may have minimal to no impact on the shift of cellular metabolism towards anaerobic glycolysis.
NL-1 reduces maximal OCR of differentiated CSCs
With the observed effect of NL-1 on mitochondrial metabolism, possible influence of this drug on CSC differentiation was explored. The first experiments included analyses of mitochondrial metabolism. After 7 days of culture, dexamethasone (DM)-treated and DM + vehicle-treated CSCs showed a significant increase in maximal OCR compared to non-treated (undifferentiated) CSCs (Fig. 3a, c). NL-1 had a similar effect on maximal OCR as seen with the undifferentiated cells. In this case, DM + NL-1 treatment reduced maximal OCR compared to DM or DM + vehicle, and there was no difference between the OCR of DM + NL-1 and undifferentiated CSCs during uncoupling (undifferentiated: 1.325 ± 0.204; DM: 1.691 ± 0.144; DM + vehicle: 1.699 ± 0.145; DM + NL-1: 1.305 ± 0.173) (Fig. 3c). Rosiglitazone treatment produced a similar effect on maximal OCR as NL-1 (DM + rosiglitazone: 1.342 ± 0.154). NL-1 had no significant effects on anaerobic metabolism (ECAR) (Fig. 3d, e).
NL-1 treatment does not appear to influence CSC differentiation
To further determine the impact of NL-1 on CSC differentiation, expressions of genes and proteins specific to differentiated cell types (cardiomyocytes, endothelial cells, and smooth muscle cells) were examined. These particular cell types were chosen because of previous evidence that c-kit+ CSCs are precursors for these three lineages [3, 28]. However, real-time PCR and western blotting revealed that, among those markers examined in the present study, these particular CSCs primarily differentiate into smooth muscle as indicated by the expression of smooth muscle α-actin (Fig. 4a, b). Real-time PCR showed a significant upregulation of this gene following the 7-day differentiation protocol in DM, DM + vehicle, DM + NL-1 compared to undifferentiated CSCs (DM: 6.303 ± 1.534; DM + vehicle: 5.410 ± 0.979; DM + NL-1: 3.912 ± 0.945) (Fig. 4a).
Interestingly, smooth muscle α-actin cDNA was significantly reduced in DM + NL-1 compared to DM, suggesting a possible inhibitory effect of NL-1 on CSC differentiation. Although western blot data showed a significant increase in smooth muscle α-actin expression in differentiated vs. undifferentiated CSCs, no significant differences were observed among the differentiated CSC treatment groups (DM: 2.766 ± 0.886; DM + vehicle: 2.832 ± 0.699; DM + rosiglitazone: 2.663 ± 0.881; DM + NL-1: 2.488 ± 0.517; DM + NL-1 + GW9662: 2.151 ± 0.318) (Fig. 4b).
NL-1 treatment increases CSC survival during differentiation under chronic oxidative stress
To assess how NL-1 may impact cell survival during differentiation under oxidative stress, glucose oxidase (an enzyme that oxidizes glucose to yield H2O2 and d-glucono-δ-lactone) was used to produce a chronic exposure to oxidative stress in vitro (compared to the short-term H2O2 treatments described above). With glucose oxidase treatment, both NL-1 and rosiglitazone significantly improved CSC survival compared to dexamethasone treatment alone (DM) or dexamethasone plus vehicle (0.1 % DMSO) treatment (DM + Vehicle) (DM: 0.351 ± 0.114; DM + vehicle: 0.325 ± 0.111; DM + rosiglitazone: 0.669 ± 0.212; DM + NL-1: 0.760 ± 0.158) (Fig. 5a). Interestingly, the protective effect of NL-1 was diminished in the presence of compound GW9662, a PPARγ antagonist (DM + NL-1 + GW9662: 0.318 ± 0.047) (Fig. 5a). Phase-contrast microscopic images of CSCs under these treatment conditions reveal preservation of cell morphology with either rosiglitazone or NL-1 treatment during glucose oxidase exposure (Fig. 5b–g).
NL-1 treatment of mitoNEET knockout cells
The novelty of NL-1 as a selective ligand for mitoNEET calls for experimental evidence of the specificity of this compound. Therefore, a small population of cardiac cells was isolated from mitoNEET knockout and wild-type mice, treated with 250 μM H2O2 for 4 h, and assessed for cell survival (flow cytometry/propidium iodide exclusion). In these experiments, a significant difference was noted between mitoNEET knockout and wild-type cells treated with NL-1 (knockout: 0.725 ± 0.053; wild-type: 0.980 ± 0.050; Fig. 6). However, there was no significant difference between cells treated with vehicle (0.1 % DMSO) and cells treated with NL-1 for either the knockout or the wild-type cells; it might be speculated that this difference could be partly due to the different cell type used in these experiments (i.e., predominantly committed cells as opposed to stem cells).
NL-1 treatment improves short-term, but not long-term CSC survival under in vivo oxidative stress
Cardiac stem cells injected in the presence of 10 μM NL-1 demonstrated a robust relative increase in survival at 24 h following injection (fold difference: 11.02 ± 1.14 compared to CSCs injected without NL-1) (Fig. 7). However, this difference was lost at 10-days following injection (fold difference: 1.05 ± 0.088). Therefore, this novel drug may have an acutely protective effect on stem cells in the oxidative environment characteristic of the metabolic syndrome.
Discussion
In summary, the primary findings of these studies are: (1) NL-1 treatment can increase CSC survival during exposure to high levels of ROS in vitro, possibly secondary to a reduction in cellular respiration; (2) NL-1 can preserve stem cell viability during differentiation under continuous exposure to oxidative stress elicited by glucose oxidase; and (3) NL-1 exerted a short-term protective effect on CSCs when delivered into an oxidative in vivo environment.
Specific mechanisms through which TZDs exert these effects are still being investigated. The reduction in maximal OCR suggests a possible mechanism by which NL-1 may exert its protective effect on CSC during oxidative stress; a decrease in oxygen consumption may occur due to fewer electrons being passed through the electron transport chain, which in turn diminishes the rate of production of ROS. Thus, NL-1 may be cytoprotective due to a general slowing of electron transport chain activity.
Additionally, since iron is rate-limiting for electron transfer through the mitochondrial electron transport chain (ETC), one potential mechanism is through the control of iron levels in the mitochondria via mitoNEET. MitoNEET has demonstrated redox-sensitive properties owing to its 2Fe-2S clusters, and has demonstrated the transfer of iron to apo-acceptor proteins located in both the mitochondria and the cytoplasm [15, 35, 36]. Pioglitazone was found to inhibit Fe–S transfer from mitoNEET into the mitochondria [36]. In support of this proposed mechanism, there was a reduction in maximal OCR with NL-1 and rosiglitazone treatment, and this observation is supported by results from other studies [13, 14].
Importantly, recent findings suggest that TZD-induced inhibition of mitochondrial respiration may be mediated through inactivation and disassembly of NADH dehydrogenase (complex I) of the ETC [8]. Pioglitazone was shown to bind directly to complex I and subsequently cause its disassembly and a reduction in its specific activity. Such an effect could be responsible for the reduced maximal OCR and improved stem cell survival with H2O2 or glucose oxidase treatment observed in the present study.
Direct association between pioglitazone and complex I calls into question whether TZDs target multiple proteins in the mitochondria. Indeed, pioglitazone bound directly to several distinct subunits on complex I—including NDUFA6, NDUFA9, and NDUFB6. Another possibility is that mitoNEET associates directly with complex I, and may be identified as a complex I subunit during binding studies, though there are no current data to support this conjecture. Interestingly, [H3]-pioglitazone bound to a complex I subunit at 17 kDa, which is the same molecular weight as mitoNEET [6]. Therefore, the possibility of [H3]-pioglitazone associating with mitoNEET was not ruled out.
The positive effect of TZDs on CSC survival with exposure to oxidative agents is consistent with previous studies using other stem cell types [6, 10, 33, 34]. Rosiglitazone treatment protected neuroblasts against apoptosis via upregulation of Bcl-2/Bcl-xl and maintenance of mitochondrial membrane potential under increased H2O2 production induced by hypoxia-reperfusion [33]. In addition, both rosiglitazone and pioglitazone have demonstrated protection of EPCs against apoptosis under oxidative stress [10, 27, 34]. Also of note is that rosiglitazone and pioglitazone restored functional ability of endothelial progenitor cells from type II diabetics to participate in re-endothelialization [5, 23, 26]. This effect appeared to be mediated through reduction in NADPH-oxidase-produced superoxide and an increase in NO bioavailability in the EPCs [26]. However, p47phox siRNA did not produce as great of a benefit as PEG-SOD (isoform not specified), thus potentially suggesting an impact of rosiglitazone on mitochondrial superoxide production. Further data also implicate NO as a mechanism by which rosiglitazone rescues EPC dysfunction associated with diabetes, showing Akt/eNOS activation with in vitro rosiglitazone treatment at a dose of only 10 nM (in contrast to 10 μM used the present experiments) [18]. These findings have relevance for coronary collateral growth as well, since NO appears to be required for collaterogenesis to occur [19]. We also emphasize that interventions that are associated with decreasing mitochondrial function also appear to confer resistance to ischemic injury [11, 32], which is a similar paradigm to our thoughts about using NL-1 to increase stem cell survival. We would be remiss in not mentioning that PPARγ antagonists can have broad effects on critical cell signaling pathways, e.g., Akt activity [16], which could affect the actions of NL-1 in a manner independent of mitoNEET.
Delineating PPARγ-dependent and -independent functions of TZDs appears to be somewhat of a challenge in research. The cytoprotective role of TZDs under oxidative stress appears to be independent of PPARγ signaling [10, 34]. Conversely, most of the data regarding the impact of TZDs on improvement in EPC function, particularly the studies involving in vivo interventions, have been documented as dependent on PPARγ activation and secondary rise in adiponectin expression [26, 29, 33]. However, as shown in Kusminski et al. [12], tissue-specific mitoNEET overexpression led to a rise in serum adiponectin levels, indicating potential cross talk between TZD actions on both mitochondrial proteins as well as PPARγ. In the present study, PPARc antagonism with GW9662 appeared to partially reverse the effects of NL-1 treatment. These results were not expected, since the aromatic chains that increase affinity of the glitazones for PPARγ are absent in NL-1. Thus, it is suspected that either the NL-1 has a higher affinity for PPARc than predicted, or that there may be independent effects of GW9662 treatment that adversely impact CSC survival.
Since NL-1 treatment was not associated with an increase in cell survival of the mitoNEET knockout cells, whereas there appeared to be a modest protective effect on wild-type cells in the same experiments, it can be inferred that NL-1 does interact with its intended target. Since the majority of cells used in this particular experiment were not stem cells and were different from the CSCs used in the other experiments, the tolerance or sensitivity to oxidative stress might be different. Even though the overall effect was small in the wild-type cells, further purification of stem cells or optimization of treatment conditions is required to determine these relationships more clearly.
The present study supports an effect of TZDs on CSC mitochondrial metabolism, but little to no evidence is provided for an effect of this drug on CSC differentiation. However, differentiation may be indirectly influenced through an increase in PGC-1α expression with NL-1 and rosiglitazone treatment compared to undifferentiated CSC. Yet, expression of proteins involved in oxidative phosphorylation did not increase significantly, contrary to what would be expected with increased PGC-1α levels. The lack of a difference in expressions of differentiation markers (i.e., smooth muscle α-actin) between TZD-treated CSCs also counts against the notion of a strong relationship between TZDs and CSC differentiation.
Consistent with in vitro studies, NL-1 treatment appeared to improve CSC survival 24 h after intramyocardial delivery into Zucker obese fatty (ZOF) rats—animal models of the metabolic syndrome, characterized by relatively high systemic levels of ROS compared to their lean littermates [24]. However, the positive effect of NL-1 on CSC survival was not sustained following 10 days after injection, suggesting an acutely protective effect of this drug. Optimization of treatment regimens, including repeated exposure to NL-1, or combination of NL-1 treatment with other cytoprotective strategies may translate into enhanced efficacy of stem cell therapies in vascular disease.
In conclusion, the novel thiazolidinedione, NL-1, has the potential to improve CSC survival under highly oxidative conditions, and heightens the appeal for targeting mitochondrial metabolism as a strategy to improve stem cell therapies in cardiovascular disease.
Acknowledgments
This work was supported by NIH/NHLBI (RHL100828Z and R01 83366-01) to W. M. C.; NIH/NHLBI (1R15HL115540-01) and AHA (14BGIA18770028) to L. Y.; as well as the Stark Community Foundation, Canton, OH, the DiYorio Foundation, Youngstown, OH, and the Schermer Trust Foundation, Youngstown, OH to W. J. G. and R. T. C.
Footnotes
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Suzanna J. Logan, Department of Integrative Medical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA
Liya Yin, Department of Integrative Medical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA.
Werner J. Geldenhuys, Department of Pharmaceutical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA
Molly K. Enrick, Department of Integrative Medical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA
Kelly M. Stevanov, Department of Integrative Medical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA
Richard T. Carroll, Department of Pharmaceutical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA
Vahagn A. Ohanyan, Department of Integrative Medical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA
Christopher L. Kolz, Department of Integrative Medical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA
William M. Chilian, Email: wchilian@neomed.edu, Department of Integrative Medical Sciences, Northeast Ohio Medical University, 4209 State Rt. 44, Rootstown, OH 44272, USA.
References
- 1.Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, Seifried E, Schachinger V, Dimmeler S, Zeiher AM. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD registry. Circ Res. 2007;100:1234–1241. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 2.Assmus B, Leistner DM, Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Sedding D, Yu J, Corti R, Mathey DG, Barth C, Mayer-Wehrstein C, Burck I, Sueselbeck T, Dill T, Hamm CW, Tonn T, Dimmeler S, Zeiher AM. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35:1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- 3.Beltrami A, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/S0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Chao-Hung W, Ming-Kuo T, Subodh V, Li-Tang K, Ning IY, Hsieh IC, Shin-Yi W, Agnes H, Wen-Jin C. Pioglitazone increases the numbers and improves the functional capacity of endothelial progenitor cells in patients with diabetes mellitus. Am Heart J. 2006 doi: 10.1016/j.ahj.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Colca J, McDonald W, Waldon D, Leone J, Lull J, Bannow C, Lund E, Mathews W. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286:60. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- 7.Düfer M, Noack K, Edalat A, Krippeit-Drews P, Drews G. Glitazones exert multiple effects on β-cell stimulus-secretion coupling. Mol Pharmacol. 2013;83:51–60. doi: 10.1124/mol.112.081638. [DOI] [PubMed] [Google Scholar]
- 8.García-Ruiz I, Solís-Muñoz P, Fernández-Moreira D, Muñoz-Yagüe T, Solís-Herruzo J. Pioglitazone leads to an inactivation and disassembly of complex I of the mitochondrial respiratory chain. BMC Biol. 2013;11:88. doi: 10.1186/1741-7007-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geldenhuys W, Funk M, Barnes K, Carroll R. Structure-based design of a thiazolidinedione which targets the mitochondrial protein mitoNEET. Bioorg Med Chem Lett. 2010;20:819–823. doi: 10.1016/j.bmcl.2009.12.088. [DOI] [PubMed] [Google Scholar]
- 10.Gensch C, Clever Y, Werner C, Hanhoun M, Böhm M, Laufs U. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Heather LC, Cole MA, Tan JJ, Ambrose LJ, Pope S, Abd-Jamil AH, Carter EE, Dodd MS, Yeoh KK, Schofield CJ, Clarke K. Metabolic adaptation to chronic hypoxia in cardiac mitochondria. Basic Res Cardiol. 2012;107:268. doi: 10.1007/s00395-012-0268-2. [DOI] [PubMed] [Google Scholar]
- 12.Kusminski C, Holland W, Sun K, Park J, Spurgin S, Lin Y, Askew G, Simcox J, McClain D, Li C, Scherer P. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamontagne J, Jalbert-Arsenault E, Pepin E, Peyot ML, Ruderman N, Nolan C, Joly E, Madiraju S, Poitout V, Prentki M. Pioglitazone acutely reduces energy metabolism and insulin secretion in rats. Diabetes. 2013 doi: 10.2337/db12-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamontagne J, Pepin E, Peyot ML, Joly E, Ruderman N, Poitout V, Madiraju S, Nolan C, Prentki M. Pioglitazone acutely reduces insulin secretion and causes metabolic deceleration of the pancreatic beta-cell at submaximal glucose concentrations. Endocrinology. 2009;150:3465–3474. doi: 10.1210/en.2008-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landry A, Ding H. Redox control of human mitochondrial outer membrane protein mitoNEET [2Fe-2S] clusters by biological Thiols and hydrogen peroxide. J Biol Chem. 2014;289:4307–4315. doi: 10.1074/jbc.M113.542050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MW, Kim DS, Kim HR, Kim HJ, Yang JM, Ryu S, Noh YH, Lee SH, Son MH, Jung HL, Yoo KH, Koo HH, Sung KW. Cell death is induced by ciglitazone, a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, independently of PPARγ in human glioma cells. Biochem Biophys Res Commun. 2012;417:552–557. doi: 10.1016/j.bbrc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Li TS, Kubo M, Ueda K, Murakami M, Ohshima M, Kobayashi T, Tanaka T, Shirasawa B, Mikamo A, Hamano K. Identification of risk factors related to poor angiogenic potency of bone marrow cells from different patients. Circulation. 2009;120:61. doi: 10.1161/CIRCULATIONAHA.108.837039. [DOI] [PubMed] [Google Scholar]
- 18.Liang C, Ren Y, Tan H, He Z, Jiang Q, Wu J, Zhen Y, Fan M, Wu Z. Rosiglitazone via upregulation of Akt/eNOS pathways attenuates dysfunction of endothelial progenitor cells, induced by advanced glycation end products. Br J Pharmacol. 2009;158:1865–1873. doi: 10.1111/j.1476-5381.2009.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsunaga T, Warltier D, Weihrauch D, Moniz M, Tessmer J, Chilian W. Ischemia-induced coronary collateral growth is dependent on vascular endothelial growth factor and nitric oxide. Circulation. 2000;102:3098–3103. doi: 10.1161/01.CIR.102.25.3098. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. The top 10 causes of death. [Accessed July 26, 2014];Fact Sheets. 2014 http://www.who.int/mediacentre/factsheets/fs310/en/
- 21.Pedada KK, Zhou X, Jogiraju H, Carroll RT, Geldenhuys WJ, Lin L, Anderson DJ. A quantitative LC-MS/MS method for determination of thiazolidinedione mitoNEET ligand NL-1 in mouse serum suitable for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;945–946:141–146. doi: 10.1016/j.jchromb.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 22.Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, Strasser D, Ting AE, Sherman W. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 23.Pistrosch F, Herbrig K, Oelschlaegel U, Richter S, Passauer J, Fischer S, Gross P. PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183:163–167. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 24.Pung Y, Rocic P, Murphy M, Smith R, Hafemeister J, Ohanyan V, Guarini G, Yin L, Chilian W. Resolution of mitochondrial oxidative stress rescues coronary collateral growth in Zucker obese fatty rats. Arterioscler Thromb Vasc Biol. 2012;32:325–334. doi: 10.1161/ATVBAHA.111.241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schutt RC, Trachtenberg BH, Cooke JP, Traverse JH, Henry TD, Pepine CJ, Willerson JT, Perin EC, Ellis SG, Zhao DX, Bhatnagar A, Johnstone BH, Lai D, Resende M, Ebert RF, Wu JC, Sayre SL, Orozco A, Zierold C, Simari RD, Moye L, Cogle CR, Taylor DA. Bone marrow characteristics associated with changes in infarct size after STEMI: a biorepository evaluation from the CCTRN TIME trial. Circ Res. 2015;116:99–107. doi: 10.1161/CIRCRESAHA.116.304710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorrentino S, Bahlmann F, Besler C, Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 27.Spigoni V, Picconi A, Cito M, Ridolfi V, Bonomini S, Casali C, Zavaroni I, Gnudi L, Metra M, Dei Cas A. Pioglitazone improves in vitro viability and function of endothelial progenitor cells from individuals with impaired glucose tolerance. PLoS ONE. 2012 doi: 10.1371/journal.pone.0048283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallini Y, Greene K, Craven M, Spealman A, Breitbach M, Smith J, Fisher P, Steffey M, Hesse M, Doran R, Woods A, Singh B, Yen A, Fleischmann B, Kotlikoff M. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner C, Kamani CH, Gensch C, Bohm M, Laufs U. The peroxisome proliferator activated receptor-agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007 doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 30.Wiley S, Murphy A, Ross S, van der Geer P, Dixon J. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci USA. 2007;104:5318–5323. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wojtovich AP, Brookes PS. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol. 2009;104:121–129. doi: 10.1007/s00395-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu JS, Lin TN, Wu K. Rosiglitazone and PPAR-gamma overexpression protect mitochondrial membrane potential and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family proteins. J Cell Physiol. 2009;220:58–71. doi: 10.1002/jcp.21730. [DOI] [PubMed] [Google Scholar]
- 34.Zhang HF, Wang L, Yuan HJ, Ma YH, Wang YF, Hu ZY, Su Y, Zhao ZG. PPAR-γ agonist pioglitazone prevents apoptosis of endothelial progenitor cells from rat bone marrow. Cell Biol Int. 2013;37:430–435. doi: 10.1002/cbin.10046. [DOI] [PubMed] [Google Scholar]
- 35.Zuris J, Ali S, Yeh H, Nguyen T, Nechushtai R, Paddock M, Jennings P. NADPH inhibits [2Fe-2S] cluster protein transfer from diabetes drug target MitoNEET to an apo-acceptor protein. J Biol Chem. 2012;287:11649–11655. doi: 10.1074/jbc.M111.319731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuris JA, Harir Y, Conlan AR, Shvartsman M, Michaeli D, Tamir S, Paddock ML, Onuchic JN, Mittler R, Cabantchik ZI, Jennings PA, Nechushtai R. Facile transfer of [2Fe-2S] clusters from the diabetes drug target mitoNEET to an apo-acceptor protein. Proc Natl Acad Sci USA. 2011;108:13047–13052. doi: 10.1073/pnas.1109986108. [DOI] [PMC free article] [PubMed] [Google Scholar]