Abstract
Patient: Male, 53
Final Diagnosis: Acute heart failure • primary AL amyloidosis • hyper IgE-emia
Symptoms: Progressive breathlessness
Medication: Angiotensin-converting enzyme inhibitors and beta blockers
Clinical Procedure: Skin and endomyocardial biopsy
Specialty: Cardiology
Objective:
Rare co-existence of disease or pathology
Background:
Considering the increased prevalence of heart failure with preserved ejection fraction (HFpEF) as a result of the aging population, the pathophysiology of HFpEF needs to be examined. Furthermore, many comorbidity profiles in patients with HFpEF have been reported. Hypertrophic cardiomyopathy is a well-known specific etiology of HFpEF. Cardiac amyloidosis, which mimics infiltrative and hypertrophic cardiomyopathy, resulting from intensive amyloid deposition, is easily overlooked.
Case Report:
A 53-year-old man with a 2-week history of persistent breathlessness was referred to our hospital. Upon admission, transthoracic echocardiography showed concentric mild left ventricular (LV) hypertrophy without a characteristic granular sparkling appearance or pericardial effusion, preserved ejection fraction, and bi-atrial enlargement with normal ventricular chambers. Doppler-derived LV diastolic filling demonstrated a prominent restrictive pattern indicating LV stiffness and elevated LV filling pressure. Blood tests revealed severe elevation of B-type natriuretic peptide and marked elevation of immunoglobulin E without eosinophilia. He was diagnosed with primary amyloid light-chain (AL) amyloidosis via skin and endomyocardial biopsy.
Conclusions:
We encountered a rare case of hypertrophic cardiomyopathy with HFpEF and identified a Doppler-derived restrictive filling pattern suggestive of early-stage heart failure in infiltrative cardiomyopathies. We suggest that infiltrative cardiomyopathies, such as cardiac amyloidosis, should be considered if hypertrophic cardiomyopathy is observed in a patient with HFpEF.
MeSH Keywords: Amyloidosis; Cardiomyopathy, Hypertrophic; Heart Failure, Diastolic; Immunoglobulin E
Background
In daily clinical situations, physicians often encounter pathologies of unknown etiology that are initially unpredictable, and there is a tendency to consider these conditions in an integrated and complicated fashion. Amyloid light-chain (AL) amyloidosis is a rare disease with an estimated prevalence of 5–12 cases per million population per year [1,2]. Hyper-immunoglobulin E (IgE) syndrome is a rare immunodeficiency disease characterized by refractory skin abscesses, lung infections, skeletal abnormalities, and hyper-IgE-emia [3]. Moreover, hyper-IgE syndrome commonly occurs in an autosomal dominant inheritance pattern. Indeed, our patient had experienced chronic inflammatory and recurrent refractory skin abscesses, although he had no family history of hyper-IgE syndrome and none of the characteristic skeletal abnormalities. It was difficult to interpret this systemic amyloidosis as a consequence of hyper-IgE syndrome. Therefore, we concluded that the systemic amyloidosis and hyper-IgE-emia of this patient were independent. Here, we report a rare case of early-stage heart failure with preserved ejection fraction (HFpEF) of the left ventricle that was finally diagnosed as primary AL amyloidosis based on tissue biopsy results.
Case Report
A 53-year-old man came to our hospital with signs and symptoms of acute heart failure after a 2-week history of progressive breathlessness. He had a history of recurrent skin abscesses and atopic dermatitis and regularly visited a dermatologist in our hospital. On arriving at our hospital, his extremities were warm and dry. According to the New York Heart Association criteria, he had class III congestive heart failure (CHF). An electrocardiogram revealed diffuse nonspecific T-wave changes, low voltage (<5 mm) in the extremity leads and poor R-wave progression in the anterior chest leads. Multiple sporadic ventricular premature beats were seen (Figure 1). Chest x-ray film confirmed right pleural effusion, and mild cardiomegaly but no pulmonary congestion (Figure 2). Blood tests showed severely elevated B-type natriuretic peptide (901 pg/mL) and markedly raised IgE (12 000 IU/mL) without eosinophilia (eosinophil count of 1.62×108/L). Biochemical analysis revealed no significant findings: blood urea nitrogen of 14.3 mg/dL [reference value (RV): 7.00–22.00], creatinine of 0.90 mg/dL (RV: 0.60–1.00), C-reactive protein (CRP) of 0.2 mg/dL (RV: 0.00–0.50), serum amyloid A (SAA) of 7.0 μg/mL (RV: 0–8.0), and troponin T of 0.07 ng/mL (RV: 0–0.1). Immunology testing revealed negative perinuclear anti-neutrophil cytoplasmic antibodies and no elevation of myeloperoxidase antibodies. The distribution of albumin and globulin in the serum was normal. Serum protein immuno-electrophoresis did not reveal M-protein, and urinalysis revealed no Bence-Jones protein. Transthoracic echocardiography (Figure 3) showed concentric mild left ventricular (LV) hypertrophy (12 mm) without the characteristic granular sparkling appearance and pericardial effusion, preserved ejection fraction (60%), and bi-atrial enlargement with normal ventricular chambers. Doppler-derived LV diastolic filling demonstrated a restrictive pattern with a trans-mitral early filling wave deceleration time of 160 ms and an elevated E/A ratio of 2.8. A markedly elevation of E/e’ ratio of 27.3 indicated elevated LV filling pressure. On day 1 of hospitalization, we prescribed an angiotensin-converting enzyme (ACE) inhibitor and low-dose diuretics.
Figure 1.
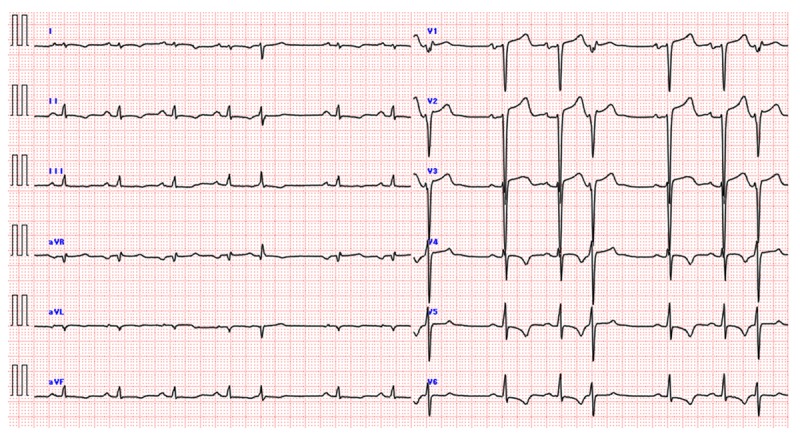
An electrocardiogram showed a low QRS voltage (<5 mm) in the extremity leads, and multiple sporadic ventricular premature beats were detected.
Figure 2.
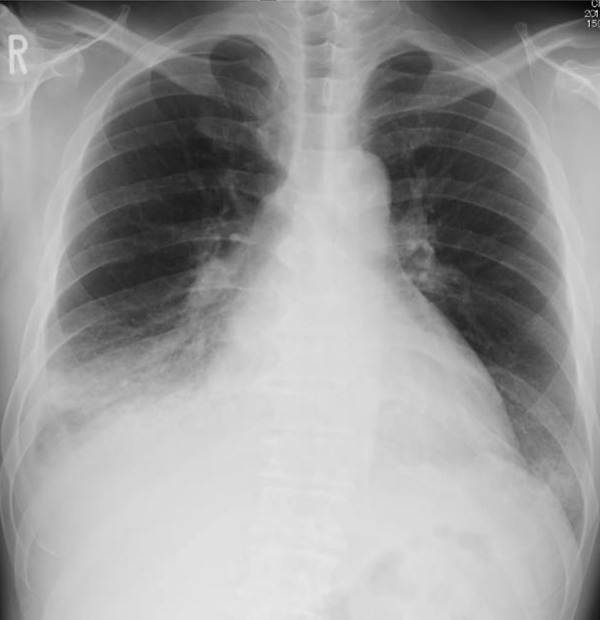
Antero-posterior chest X-ray showed right pleural effusion without pulmonary congestion. The second right angle was enlarged and the angle in the bronchial bifurcation was wide open. These findings implied biatrial enlargement.
Figure 3.
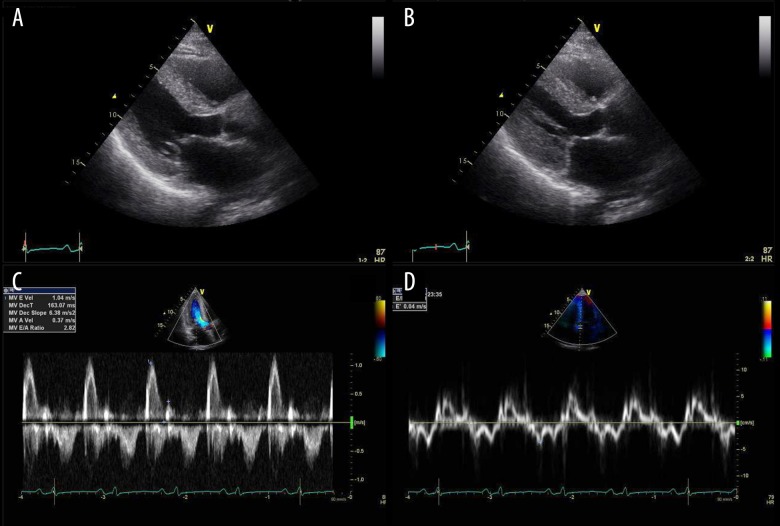
Transthoracic echocardiogram. (A) Parasternal long-axis view in the diastole showing mild concentric left ventricular (LV) hypertrophy (12 mm) without increased myocardial echogenicity (so-called granular sparkling pattern) and normal ventricular chamber size. (B) Parasternal long-axis view in the systole showing normal LV ejection fraction (LVEF) at 60. (C) Doppler-derived LV diastolic filling demonstrated a restrictive pattern with a trans-mitral early filling wave deceleration time of 160 ms and an elevated E/A ratio of 2.8. (D) Tissue Doppler velocities recorded at the septal and lateral sides of the mitral annulus showing reduced systolic velocities (s’) and markedly reduced early and late diastolic velocities with the elevated LV filling pressures (E/e’ rate of 27.3).
On day 3 of hospitalization, after initiating ACE inhibitor and diuretic therapy, the patient’s symptoms resolved. The dermatologists performed a biopsy of a blue macula of the forehead skin. On day 4, we introduced a low-dose β-blocker and performed an endomyocardial biopsy (EMB), obtaining 3 fragments of the right ventricular septum because diagnostic confirmation of cardiac amyloidosis requires the demonstration of amyloid deposits. In addition, we performed right heart catheterization (RHC), and coronary angiography to exclude obstructive coronary artery disease. The data obtained from the RHC indicated subset type IV according to the Forrester classification. The right ventricular pressure curve did not show a dip-and-plateau configuration.
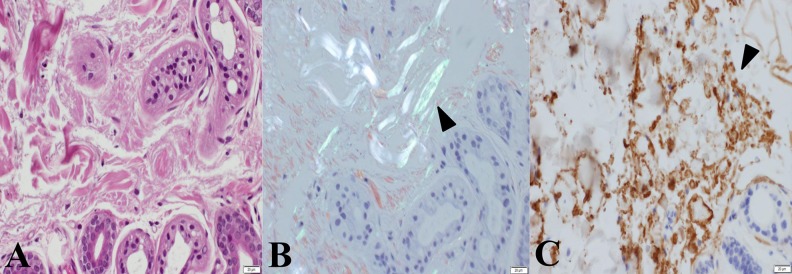
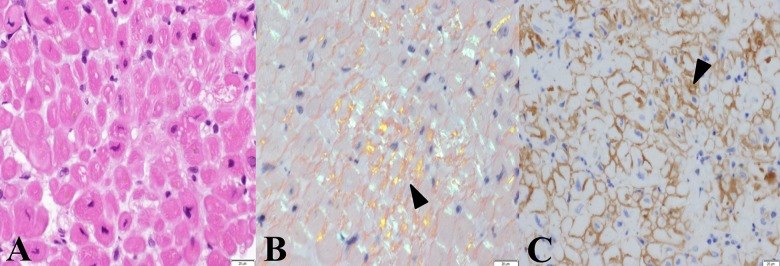
Skin biopsy revealed hyperkeratosis in the epidermis and mild inflammatory changes throughout the dermis with an infiltration of lymphocytes. The specimen exhibited apple-green birefringence with polarized light after Congo red staining (Figure 4). Histological examination of the myocardial specimen showed no signs suggesting myocarditis, eosinophilic granulomas, or cardiomyopathy with iron deposition, but Congo red staining revealed amyloid deposits (Figure 5). In addition, a strongly positive immunohistochemical reaction to immunoglobulin λ-chain in the myocardial interstitium led us to diagnose this patient’s systemic amyloidosis as AL amyloidosis.
Figure 4.
Histological findings in the skin lesions. Hyperkeratosis in the epidermis and lymphocytes infiltration was observed in the biopsied specimen obtained from the skin. Also, melanin pigmentation was observed in the basal layer of the epidermis and the top layer of the dermis. Deposition of hemosiderin was not found. (A) The deposited materials are observed in the interstitium and around the sweat glands (hematoxylin and eosin, objective lens ×60). (B) A bright greenish color (arrowhead) after staining with Congo red can be seen under polarized light (Congo red, objective lens ×60). (C) A strongly positive immunohistological reaction for immunoglobulin λ-chain can be seen in the interstitium (arrowhead, dark-brown color) (immunoperoxidase reaction for λ-chain, objective lens ×60).
Figure 5.
Histological findings in myocardium from the right ventricle. An infiltration of lymphocytes was observed in the biopsied specimen. There were focal atrophic myocardial changes accompanied by deposition of eosinophilic materials. (A) Deposition of eosinophilic materials can be observed in the myocardial interstitium (hematoxylin and eosin, objective lens ×60). (B) A bright greenish yellow color (arrowhead) after staining with Congo red can be seen under polarized light (Congo red, objective lens ×60). (C) A strongly positive immunohistological reaction for immunoglobulin λ-chain can be seen in the myocardial interstitium (arrowhead, dark-brown color) (immunoperoxidase reaction for λ-chain, objective lens ×60).
The patient generally tolerated the low-dose β-blocker well and was discharged on day 10 with no complications. The patient is currently being followed-up at 6-month intervals as an out-patient, with no exacerbation of the CHF thus far.
Discussion
Amyloidosis develops as a result of deposition of insoluble amyloid fibrils in the extracellular spaces of organs and tissues and clinically varies from mild to severe. Amyloidosis can be divided broadly into primary, hereditary, systemic senile, isolated atrial, reactional or secondary, and dialysis-related types. Initially, we considered this patient’s condition as a disease associated with hyper-IgE syndrome [3]. His IgE level was markedly elevated, and he had experienced recurrent refractory skin abscesses. We considered a scenario whereby the increasing SAA produced by chronic inflammatory disease had developed into AA amyloidosis, also known as secondary amyloidosis. This scenario meant that the hyper-IgE syndrome had progressed to the point where amyloid protein was systemically deposited in the patient’s organs. However, the patient’s SAA was normal and AA amyloidosis involving the heart is unusual owing to the amyloid’s tissue-specific affinity [4–6]. In addition, he had no family history of hyper-IgE syndrome or characteristic skeletal abnormalities.
Upon excluding AA amyloidosis, 2 subtypes of amyloidosis seemed more probable: AL amyloidosis and systemic senile type, which occurs by deposition of wild-type transthyretin amyloid. Ng et al. [7] reported important differences in the prognosis and evolution of these 2 subtypes, with a mean survival after cardiovascular involvement of 11 and 75 months, respectively. Additionally, the insidious course of the disease is the rule for senile amyloidosis, whereas the AL type shows rapid evolution of symptoms and a higher cardiac involvement. We finally diagnosed primary AL amyloidosis by performing special immunological staining of tissues obtained from the EMB and skin biopsy. In the absence of confirmed plasma cell dyscrasia, immunohistochemical techniques are the most useful for classifying the type of amyloidosis [8]. Several studies concluded that cardiac involvement in amyloidosis is the major determinant of mortality in patients with AL amyloidosis, and the prognosis is very poor [9–11]. However, Quevedo et al. [12] insisted that early diagnosis and prompt therapy are crucial to the reversal of AL-related cardiac dysfunction. In support of this idea, we prescribed an ACE inhibitor and β-blocker as conventional therapies for CHF; accordingly, the patient’s CHF condition did not worsen. Amyloid deposition essentially restructures the heart and restricts it from stretching and properly filling with blood, resulting in the so-called restrictive cardiomyopathy (RCM). In time, RCM progresses from diastolic to systolic dysfunction and eventually to heart failure [13]. A report demonstrated the importance of the Doppler-derived restrictive filling pattern in the prognosis of hypertrophic cardiomyopathy [14]. Our patient’s cardiomyopathy may have been in a very early stage because his systolic function was within the normal range and therefore was termed HFpEF. In the early stages of RCM, systolic function may be normal, although deterioration of systolic function is usually observed as the disease progresses [15]. The prognosis of HFpEF is difficult to determine because the definition of HFpEF is ambiguous, and a cut-off for systolic function has not been determined (indeed, there are 2 groups: EF ≥50% and EF >45%). Furthermore, most studies did not include diastolic function in their inclusion criteria [16–20]. The prevalence of HFpEF has been increasing along with the aging population [16,17]. In contrast to HF with reduced EF, there has been little or no progress in developing strategies for treating HFpEF over the last 20 years [18–20]. However, diagnosing and treating comorbidities for patients with HFpEF is very important. HFpEF has been associated with the presence of hypertension, diabetes mellitus, obesity, coronary artery disease, and LV hyper-trophy, which are also common in elderly people but had not previously been linked so strongly with cardiac amyloidosis. A few studies have reported an approximately 10% frequency of cardiac amyloidosis in patients with HFpEF [21,22].
Conclusions
Although the heart is a common organ for amyloid deposition, cardiac amyloidosis may be an under-appreciated cause of hypertrophic cardiomyopathy. We anticipate new specific tools for diagnosing infiltrative cardiomyopathy in the early stage of HFpEF and suggest aggressive intervention to prevent the progression of the CHF.
Acknowledgments
We thank Dr. Nao Nishitani (dermatologist), Dr. Minako Terai (dermatologist), Dr. Masahiro Ayata (pathologist), and all staff in our hospital, including nurses, echo-sonographers, pathological technicians, mechanical engineers, radiation technicians, and volunteers.
Footnotes
Statement
The present study had not been supported for any grant or funders.
Conflict of interest
None declared.
References:
- 1.Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: An 8-year study. Ann Intern Med. 2004;140:85–93. doi: 10.7326/0003-4819-140-2-200401200-00008. [DOI] [PubMed] [Google Scholar]
- 2.Getz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. Am J Hematol. 2005;79:319–28. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 3.Grimbacher B, Holland SM, Gallin JI, et al. Hyper-IgE syndrome with recurrent infections – an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 4.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–60. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 5.Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356:2361–71. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 6.Banypersad SM, Moon JC, Whelan C, et al. Update in cardiac amyloidosis: A eeview. J Am Heart Assoc. 2012;(2):e000364. doi: 10.1161/JAHA.111.000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng B, Connors LH, Davidoff R, et al. Senile systemic amyloidosis presenting with heart failure: A comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–29. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 8.Benson MD, Breall J, Cummings OW, et al. Biochemical characterization of amyloid by endomyocardial biopsy. Amyloid. 2009;16:9–14. doi: 10.1080/13506120802676914. [DOI] [PubMed] [Google Scholar]
- 9.Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: Updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106:528–40. doi: 10.1016/j.acvd.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor P, Thenappen T, Singh E, et al. Cardiac amyloidosis: A practical approach to diagnosis and management. Am J Med. 2011;124:1006–15. doi: 10.1016/j.amjmed.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Yuda S, Hayashi T, Yasui K, et al. Pericardial effusion and multiple organ involvement are independent predictors of mortality in patients with systemic light chain amyloidosis. Intern Med. 2015;54:1833–40. doi: 10.2169/internalmedicine.54.3500. [DOI] [PubMed] [Google Scholar]
- 12.Quevedo K, Teleb M, Saad HH, et al. Cardiac amyloidosis-induced heart failure. Med Sci Case Rep. 2015;2:5–9. [Google Scholar]
- 13.Gupta T, Garg J, Sharma M, et al. A rare concurrence: Nonischemic cardiomyopathy and multiple myeloma without amyloidosis. Am J Med. 2014;127:1063–66. doi: 10.1016/j.amjmed.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Biagini E, Spirito P, Rocchi G, et al. Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:1727–31. doi: 10.1016/j.amjcard.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 15.Ammash NM, Seward JB, Bailey KR, et al. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490–96. doi: 10.1161/01.cir.101.21.2490. [DOI] [PubMed] [Google Scholar]
- 16.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–59. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 18.Komajda M, Lam CS. Heart failure with preserved ejection fraction: A clinical dilemma. Eur Heart J. 2014;35:1022–32. doi: 10.1093/eurheartj/ehu067. [DOI] [PubMed] [Google Scholar]
- 19.Butler J, Fonarow GC, Zile MR, et al. Developing therapies for heart failure with preserved ejection fraction: Current state and future directions. JACC Heart Fail. 2014;2:97–112. doi: 10.1016/j.jchf.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senni M, Paulus WJ, Gavazzi A, et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797–815. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–94. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 22.Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–22. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]