Abstract
Background
Denervation-induced skeletal muscle atrophy results in significant biochemical and physiological changes potentially leading to devastating outcomes including increased mortality. Effective treatments for skeletal muscle diseases are currently not available. Muscle-specific miRNAs, such as miR-206, play an important role in the regulation of muscle regeneration.
The aim of the present study was to examine the beneficial effects of miR-206 treatment during the early changes in skeletal muscle atrophy, and to study the underlying signaling pathways in a rat skeletal muscle atrophy model.
Material/Methods
The rat denervation-induced skeletal muscle atrophy model was established. miRNA-206 was overexpressed with or without TGF-β1 inhibitor in the rats. The mRNA and protein expression of HDAC4, TGF-β1, and Smad3 was determined by real-time PCR and western blot. The gastrocnemius muscle cross-sectional area and relative muscle mass were measured. MyoD1, TGF-β1, and Pax7 were determined by immunohistochemical staining.
Results
After sciatic nerve surgical transection, basic muscle characteristics, such as relative muscle weight, deteriorated continuously during a 2-week period. Injection of miR-206 (30 μg/rat) attenuated morphological and physiological deterioration of muscle characteristics, prevented fibrosis effectively, and inhibited the expression of TGF-β1 and HDAC4 as assessed 2 weeks after denervation. Moreover, miR-206 treatment increased the number of differentiating (MyoD1+/Pax7+) satellite cells, thereby protecting denervated muscles from atrophy. Interestingly, the ability of miR-206 to govern HDAC4 expression and to attenuate muscle atrophy was weakened after pharmacological blockage of the TGF-β1/Smad3 axis.
Conclusions
TGF-β1/Smad3 signaling pathway is one of the crucial signaling pathways by which miR-206 counteracts skeletal muscle atrophy by affecting proliferation and differentiation of satellite cells. miR-206 may be a potential target for development of a new strategy for treatment of patients with early denervation-induced skeletal muscle atrophy.
MeSH Keywords: Cell Differentiation, Histone Deacetylases, MicroRNAs, Muscle Denervation, Muscular Atrophy, Transforming Growth Factor beta1, Smad3 Protein
Background
Muscle atrophy is a common skeletal muscle disease with a potential devastating outcome that can be caused by muscle denervation. Muscle denervation can result from trauma, diabetic neuropathy, degenerative disc disease, alcoholic neuropathy, pernicious anemia, amyotrophic lateral sclerosis, spinal muscular atrophy, Charcot-Marie-Tooth disease, polio infection, and others. Denervation-induced skeletal muscle atrophy results in serious biochemical and physiological changes in the muscle, including loss of muscle mass, formation of fibrotic tissue, and reduction in the number of satellite cells, the skeletal muscle precursor cells[1–3]. Denervation-induced skeletal muscle atrophy is progressive, but reverses with timely reinnervation of the skeletal muscle. Therefore, functional recovery is usually poor following peripheral nerve injury when reinnervation is delayed[4,5]. However, there is still limited knowledge about the underlying signaling mechanisms leading to denervation-induced skeletal muscle atrophy, and effective treatment methods for muscle atrophy are currently lacking.
MicroRNAs (miRNAs) are short noncoding RNAs 20 to 22 nucleotides long that are highly conserved between species and that can regulate various cellular processes. Muscle-specific miRNAs, such as miR-206, have been well investigated in skeletal muscle[6,7]. For instance, miR-206 promotes the differentiation of skeletal muscle by inhibition of histone deacetylase 4 (HDAC4), which controls muscle differentiation[8–10]. HDAC4 is required for TGF-β1-induced myofibroblastic differentiation. Interestingly, the TGF-β1/small mothers against decapentaplegic homolog 3 (Smad3) axis has been found to be involved in tissue development, repair, and regeneration. Winbanks et al. demonstrated that TGF-β1 controls myogenic differentiation through increased HDAC4 expression[11]. TGF-β via the TGF-β1-signaling protein Smad3 regulates HDAC4 [12,13]. Based on this circumstantial evidence, we hypothesized that miR-206 might protect against denervation-induced muscle atrophy via the TGF-β/Smad3 signaling pathway to regulate HDAC4, and in turn regulate muscle cell differentiation and regeneration.
In the present study, we evaluated whether injecting miR-206 in vivo has beneficial effects on muscle differentiation preventing denervation-induced muscle atrophy. Moreover, the involvement of the TGF-β/Smad3 axis and HDAC4 and their effect on satellite cell differentiation was investigated as potential underlying molecular mechanisms of the effect of miR-206 on muscle atrophy.
Material and Methods
Animals
Male Sprague-Dawley (SD) rats (250–300 g, Experimental Animal Center of the Shanxi Medical University, Taiyuan, China) were housed in cages at a constant temperature and given free access to food and water. All studies were conducted according to the guidelines of the Institutional Animal Care and Use of Experimental Animal Center of the Shanxi Medical University and were approved by the Animal Ethics Committee (scxk (jin 0)09-0001).
Animal model of denervation-induced skeletal muscle atrophy
SD rats were randomly assigned into three groups, a denervated (Den) group, a sham-operated (Sham) group, and a control group (n=4 for each time point of each group). During surgery, rats were fixated in the prone position and anesthetized by intraperitoneal injection of 2 mL/kg chloral hydrate (10%). Surgery was performed only on the right lower limb through a dorsolateral skin incision. The sciatic nerve was exposed between the biceps femoris and gluteus muscle, separated from the surrounding connective tissue, and about 1.5 cm were excised. The 2 sciatic nerve ends were turned by 180 degrees and sewn on the muscle membrane with a 10-0 nylon simple suture in order to prevent the nerve from reconnecting. For the sham-operated group, the left sciatic nerve was mildly exposed and mobilized from the surrounding tissue [14,15]. Rats were euthanized at 0 d, 3 d, 7 d, 10 d, and 14 d after surgery.
Then, SD rats which had under gone denervation surgery were randomly assigned to 5 groups (n=4 in each group, Den+ saline+ transfection reagent (TR) treatment; Den+miR-206 groups: denervated plus 15 μg/rat, 30 μg/rat, or 60 μg miR-206/rat; Den+miR-206+SB431542 [Selleck Chemicals, USA] group: denervated plus miR-206 and SB431542 [TGF-β1 inhibitor]) and compared with the control group (n=10). To study the effect of miR-206 (miR-206 was a gift from the Orthopedic Laboratory, Shanxi Medical University) in vivo, animals of the Den+miR-206 group were injected with 3 different doses of miR-206 plasmids. Injections into the gastrocnemius muscles (GMs) were performed on the side of the surgery in anesthetized denervated rats using a 28-gauge syringe as previously described [16,17]. Entranster™-in vivo transfection reagent (Engreen Biosystem, Beijing, China), used to deliver the plasmids. SB431542 at a concentration of 11.2 mg/kg [18,19], was injected intraperitoneally at the same time as the miR-206 (30μg/rat) administration was performed in the Den+miR-206+SB431542 group. In the Den+saline+RT group saline (25 μL) was injected. On day 14, all rats were euthanized, body weight was measured, and the GMs on the surgical side were extracted and weighed. Part of the muscle tissue was immediately preserved in 10% formalin and the rest frozen at −80°C.
Histological examination of GMs
GMs were embedded in paraffin and transverse sections were cut from the mid-belly region. Following hematoxylin and eosin (H&E) and Masson’s trichrome staining, photographs were taken from each section with a digital camera (Olympus, Tokyo, Japan) attached to a light microscope (Olympus). For evaluation, at least 10 cross-sectional areas from each group were selected randomly and analyzed. The muscle fibers were viewed by Adobe Photoshop software (Adobe Systems, Mountain View, CA, USA), noncircular muscle fibers were excluded, and images were analyzed using Image J (NIH, Bethesda, MD, USA).
Immunohistochemical analysis of GMs
Muscle sections were dewaxed and antigen retrieval was achieved by high temperature and pressure. Immunohistochemical staining was performed using the following antibodies: Pax7 antibody (Santa Cruz, CA, USA; 1:300), MyoD1 antibody (Santa Cruz, CA, USA; 1:300) combined with fluorescent secondary antibody (Santa Cruz, CA, USA; 1:200). DAPI (Beyotime, China) served as nuclear stain. Stained muscle sections were photographed using a fluorescence microscope (Olympus, Tokyo, Japan) and 10 randomly selected areas were analyzed using Image J.
Western blot analysis of GMs
Frozen muscle samples were homogenized in Western blot lysis buffer (Beyotime, China) and centrifuged at 12,000×g for 5 min at 4°C. The total protein concentration of the supernatant was determined (BioRad, Hercules, CA, USA). Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene difluoride membranes, blocked with 5% milk powder, and incubated with primary antibodies against TGF-β1 (Santa Cruz Biotechnology), Smad3 (Santa Cruz Biotechnology), and HDAC4 (Abcam, Cambridge, UK) at 4°C overnight and. with horseradish peroxidase-conjugated anti-rat IgG secondary antibody (60 min, room temperature). Signals were detected using a chemiluminescence system (Pierce Biotechnology, Rockford, IL, USA) and quantified using an image analysis system (Bio-Rad).
Real-time quantitative reverse transcription PCR analysis of GMs
Total RNA was extracted from GMs using a Trizol-based extraction protocol (TaKaRa, Shiga, Japan), and cDNA was produced from 1 μg of total RNA using the RNA-to-cDNA kit (Bio-Rad). The cDNA was mixed with SYBR Green real-time PCR Master Mix and specific oligonucleotide primers (Smad3, TGF-β1, MyoD1, Pax7, or GAPDH), 1 μL each, and the real-time quantitative reverse transcription PCR (RT-qPCR) reaction was carried out using the TaqMan Assay (Applied Biosystems, Carlsbad, CA, USA) following the manufacturer’s instructions using rat-specific primers. (GAPDH was used to normalize messenger RNA [mRNA] concentrations; Table 1.) The mRNA levels were quantified on an ABI real time PCR machine (Applied Biosystems) and calculated by the 2−ΔΔCt method.
Table 1.
Rat-specific RT-PCR primers.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Smad3 | 5′-AGCACACAATAACTTGGACC-3′ | 5′-TAAGACACACTGGAACAGCGGATG-3′ |
| TGF-β1 | 5′-GACCGCAACAACGCAATCTA-3′ | 5′-AGGTGTTGAGCCCTTTCCA-3′ |
| MyoD | 5′-CACACTTCCCCACTACGGTGC-3′ | 5′-CACTGTAGTAGGCGGCGTCGTAG-3′ |
| Pax7 | 5′-GAAAGCCAAACACAGCATCGA-3′ | 5′-ACCCTGATGCATGGTTGATGG-3′ |
| GAPDH | 5′-TGCTGAGTATGTCGTGGAGTCTA-3′ | 5′-AGTGGGAGTTGCTGTTGAAATC-3′ |
Statistical analyses
All data were presented as means plus or minus standard deviation. Statistical significance was evaluated by 1-way analysis of variance for measurement data using statistical software SPSS, version 13.0 (SPSS, Chicago, IL, USA). P<0.05 was considered to be statistically significant.
Results
Denervation-induced muscle atrophy and protective effects of miR-206 treatment
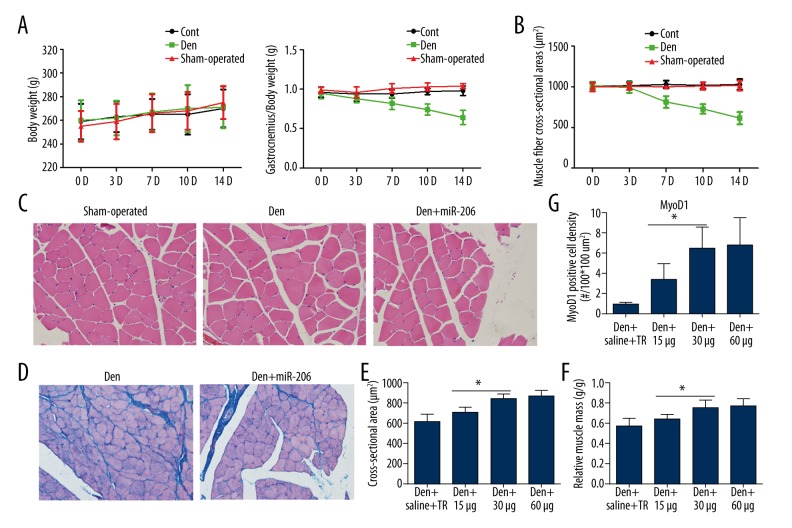
A denervation-induced muscle atrophy model for the GM was established in SD rats by sciatic nerve surgical transection. During the 14-day experimental period, the weight of SD rats did not change following denervation. However, the gastrocnemius relative muscle mass and the muscle fiber cross-sectional area reduced gradually in the denervated group compared with the control group and sham-operated group (Figure 1A, 1B; Table 2), indicating progressive muscle atrophy. Interestingly, in the miR-206 treatment group the morphology of muscle cells was restored on day 14 after surgery (Figure 1C). The process of muscle atrophy is often accompanied by the production of collagen fibers. Visualization of collagen fibers is one of the common evaluation indices for amyotrophy. Collagen fiber production as visualized by Masson’s trichrome staining was strongly inhibited in the Den+miR-206 group compared with the Den group (Figure 1D). These results indicated that miR-206 can effectively prevent fibrosis.
Figure 1.
Progression of denervation-induced muscle atrophy and improvement following miR-206 treatment. (A) Body weight and gastrocnemius relative muscle mass in the control, sham-operated, and Den groups 0 d, 3 d, 7 d, 10 d, and 14 d after surgery. (B) Changes of muscle fiber cross-sectional area in the control, sham-operated, and Den groups at 0 d, 3 d, 7 d, 10 d, and 14 d after sciatic nerve transection. (C) Histochemical staining (H&E) of GM for the sham-operated, Den, and Den+miR-206 groups. Normal muscle cell with round appearance and similar size are found in the sham-operated group. In the Den group, some cells show a polygonal shape, and a small number of hypertrophic and atrophic cells appear. In the Den+miR-206 group, the muscle cells show a less-rounded appearance. (D) Comparison of collagen fiber production between the Den and Den+miR-206 groups, as revealed by Masson’s trichrome staining. In the Den group muscle fibers were surrounded by collagen fibers, whereas in the Den+miR-206 group the production of collagen fiber was weakened. (E, F) Quantification of the cross-sectional area and gastrocnemius relative muscle mass (operated limb/normal limb) 14 d after surgery, as shown in Supplementary Figure 1. * P<0.05 in the miR-206 treatment group vs. Den+saline group. (G) Quantification of MyoD1 positive cell density, as shown in Supplementary Figure 2 in the Den+saline+RT (transfection reagent) and miR-206 treatment group (15 μg/rat, 30 μg/rat, and 60 μg/rat). * P<0.05.
Table 2.
Basic muscle characteristics.
| Ctrl | Sham-operated | Den | Den+ Saline | Den+miR-206 | |
|---|---|---|---|---|---|
| Bodyweight (g) | 270±20 | 273±14 | 275±13 | 267±15 | 269±11 |
| Relative muscle mass (g/g) | 0.95 ±0.05 | 0.93 ±0.07 | 0.56±0.08 | 0.58±0.09 | 0.78±0.05* |
| Cross-sectional area (μm2) | 1015.34±80.65 | 1008.41±49.12 | 615±71.53 | 672±57.22 | 863.16±65.61* |
Values for basic muscle characteristics are expressed as means ±SD. Ctrl – control group; Den – denervation group; Den+miR-206 – denervation plus miR-206 group; Den+Saline+RT – denervation plus saline and transfection reagent group.
Significantly different from the Den group at P<0.01.
In order to confirm appropriate treatment doses, injections of 15, 30, and 60 μg of miR-206 were administered. These improved the morphology of muscle cells and overall muscle condition and reduced the process of atrophy in denervated muscle (Supplementary Figure 1). Values for cross-sectional area and relative muscle mass were significantly increased between the 15-μg and 30-μg but not between the 30-μg and 60-μg miR-206 doses (Figure 1E, 1F). Similarly, when the expression of the myogenic factor MyoD1 was evaluated to measure the treatment effect of the 3 different doses of miR-206, significant differences were observed between the 15-μg and 30-μg but not between the 30-μg and 60-μg miR-206 doses (Figure 1G; Supplementary Figure 2).
MiR-206 reverses skeletal muscle atrophy by promoting muscle cell differentiation
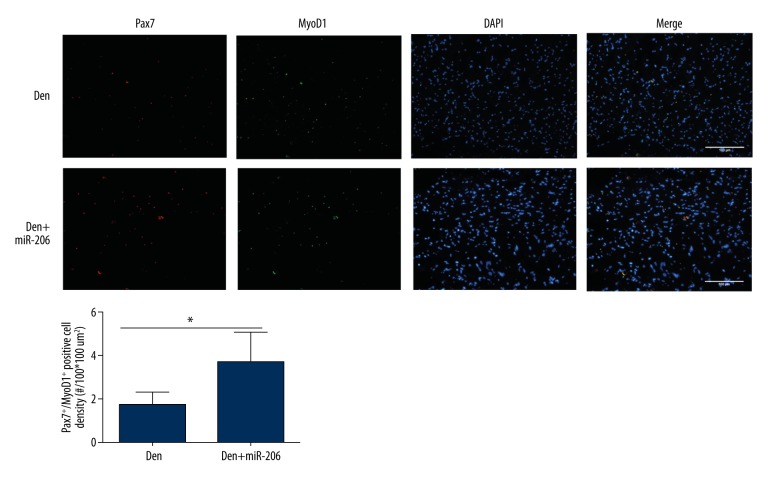
In order to further understand how miR-206 promotes muscle repair, we investigated its ability to regulate satellite cell function. Double immunofluorescence staining was performed using antibodies directed against Pax7, a satellite cell marker, and MyoD1, an important transcription factor for muscle cell differentiation[20,21]. Cells positive for Pax7 (Pax7+/MyoD1) were considered as quiescent satellite cells that were fusion incompetent and constitute the self-renewing lineage [22,23]. Cells positive for both Pax7 and MyoD1 (Pax7+/MyoD1+) were considered active satellite cells that are fusion competent and therefore differentiating cells. Quantification of MyoD1+/Pax7+ double immunofluorescence staining revealed significantly increased numbers of active satellite cells in the Den+miR1 group compared with the Den group (Figure 2A, 2B). These results indicated that miR-206 is able to regulate differentiation of satellite cells.
Figure 2.
Treatment with miR-206 promotes muscle cell differentiation following denervation-induced muscle atrophy. (A) Double immunofluorescence analysis of the GM in the Den and Den+miR-206 groups using the satellite cell marker Pax7 (red) and the muscle cell differentiation marker MyoD1 (green). DAPI (blue) was used as a nuclear stain. (B) Quantification of immunofluorescence staining showing the Pax7+/MyoD1+ positive cell density (#/100*100 um2). * P<0.05.
Expression levels of HDAC4, TGF-β1, and Smad3 in the GM following denervation-induced muscle atrophy
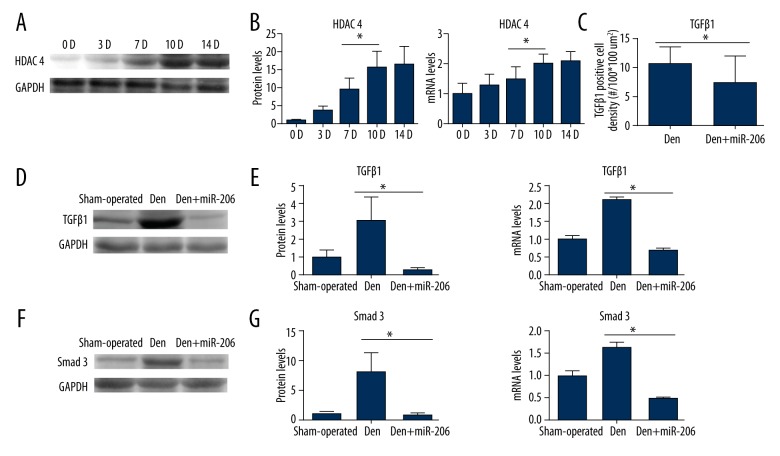
Although previous studies have shown that miR-206 promoted muscle cell differentiation by regulating HDAC4 expression, little is known about these regulatory mechanisms. When HDAC4 protein expression levels were analyzed, a significant and steady increase was observed 14 days after sciatic nerve transection (Figure 3A). Similar results were obtained for HDAC4 protein and mRNA expression levels following quantification of Western blot and RT-qPCR experiments (Figure 3B). Similar to HDAC4, TGF-β1 and the TGF-β1signaling protein Smad3 are also effective inhibitors of muscle cell differentiation. The expression level of TGF-β1 in the Den+miR-206 group declined compared with the Den group as assessed by immunofluorescence (Figure 3C; Supplementary Figure 3). RT-qPCR and Western blot analysis revealed that both TGF-β1 and Smad3 expression levels increased at day 14 following sciatic nerve denervation. MiR-206 treatment led to suppression of TGF-β1 and Smad3 expression 14 d after muscle denervation (Figure 3D–3G). These results indicate that HDAC4, TGFβ1, and Smad3 participate in the process of muscle atrophy, miR-206 regulates expression of HDAC4, and the TGF-β1/Smad3 axis protects against denervation-induced muscle atrophy.
Figure 3.
Expression of TGF-β1, Smad3, and HDAC4 in the GM following denervation-induced muscle atrophy. (A) Western blot analysis results showing the expression of HDAC4 protein levels 0 d, 3 d, 7 d, 10 d, and 14 d after sciatic nerve transection in comparison to GAPDH controls. (B) Quantification of Western blot and RT-PCR results showing protein and mRNA expression levels of HDAC4 at 0 d, 3 d, 7 d, 10 d, and 14 d after sciatic denervation surgery. (C) Quantification of immunofluorescence staining, as shown in Supplementary Figure 3, displaying TGF-β1 positive cell densities (#/100*100 um2). * P<0.05. (D–G) Representative Western blot for TGF-β1 and Smad3 expression in the sham-operated, Den, and Den+miR-206 groups. Quantification of Western blot and RT-qPCR results show relative protein and mRNA level of TGF-β1 and Smad3 expression in the sham-operated, Den, and Den+miR-206 groups. n=4, per group; * P<0.05.
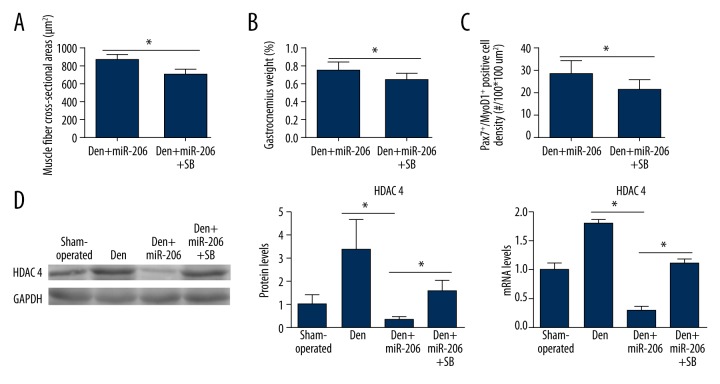
TGFβ-1 receptor antagonist SB431542 attenuated the effect of miR-206 on denervation-induced muscle atrophy
In order to further verify the importance of miR-206 in regulating the TGF-β1/Smad3 axis for protection against denervation-induced muscle atrophy, the TGFβ1 receptor inhibitor SB431542 was employed in combination with miR-206 treatment in the rat muscle atrophy model. Interestingly, treatment with the TGFβ-1 antagonist SB431542 reduced muscle fiber cross-sectional area, muscle wet weight, and the percentage of the Pax7+/MyoD1+ differentiating satellite cell population in the Den+miR-206+SB group compared with the Den+miR-206 (Figure 4A–4C; Supplementary Figure 4). Therefore, the beneficial effects of miR-206 treatment in the denervation-induced muscle atrophy rat model were greatly reduced by coadministration with SB431542. At the molecular level, HDAC4 mRNA and protein levels were significantly enhanced again in the Den+miR-206+SB group compared with the Den+miR-206 group (Figure 4D). These results demonstrated that the beneficial effect of miR-206 on denervation-induced muscle atrophy was dependent on the TGFβ1/Smad3 axis signaling via HDAC4 expression.
Figure 4.
The effect of the TGF-β1 inhibitor SB431542 combined with miR-206 treatment following denervation-induced muscle atrophy. (A, B) Measurement of GM cross-sectional area and relative muscle mass in the Den+miR-206 and Den+miR-206+SB groups. n=4, per group; * P<0.05. (C) Quantification of Pax7+/MyoD1+ positive cell density (#/100*100 um2) in the Den+miR-206 and Den+miR-206+SB groups, as shown in Supplementary Figure 4. n=4, per group; * P<0.05. (D) Representative Western blot showing relative HDAC4 protein level in the sham-operated, Den, Den+miR-206, and Den+miR1+SB groups. Quantification of Western blot and RT-qPCR results show relative protein and mRNA level of HDAC4 expression in the sham-operated, Den, Den+miR-206, and Den+miR-206+SB groups. n=4 per group, * P<0.05.
Discussion
The main findings to emerge from the present study are that miR-206 treatment significantly preserved muscle function and reduced the process of atrophy by enhancing satellite cell differentiation after skeletal muscle denervation-induced muscle atrophy. The underlying molecular mechanisms of the beneficial effects of miR-206 included suppression of the TGFβ1/Smad3 signaling axis and the associated regulation of HDAC4 expression.
Peripheral nerve injury leads to progressive atrophy of muscle fibers and to rapid decline of their functional capacity[24]. A previous study showed that disordered muscle differentiation results in further deterioration of the denervated muscle because skeletal fibers have only low numbers of differentiation-competent satellite cells that are available for muscle repair[25]. It has been suggested that miR-206 plays a crucial role in the differentiation of satellite cells[6,10,26]. Our data are in agreement with these reports in that the population of MyoD1+/Pax7+ differentiating satellite cells increased significantly following miR-206 injection in vivo, and in turn muscle condition was maintained better in miR-206-treated rats compared with the Den group. Therefore, it appears that miR-206 promotes muscle regeneration by stimulation of muscle satellite cell differentiation.
In regard to molecular mechanisms, it has been previously shown that miR-206 promoted differentiation of satellite cells by suppression of HDAC4, which is considered an inhibitor of cell differentiation that works by inhibiting myogenic regulatory factors, such as Myf5, MRF4, and myogenin[27]. In addition, the TGF-β1/Smad3 signaling axis has been shown to be required for tissue repair and also plays important roles in myogenic differentiation [11,12]. It has previously been demonstrated that inhibition of TGF-β1/Smad3 signaling promoted myogenic differentiation of mouse satellite cells induced by testosterone [28]. Smad3-null satellite cells showed increased myostatin expression and reduced propensity for self-renewal [22]. Furthermore, TGF-β1 regulates HDAC4 to control myogenic differentiation through Smad3 [11]. In light of the critical role of the TGF-β1/Smad3 axis in myogenic differentiation, we hypothesized that TGF-β1/Smad3 may participate in miR-206-mediated regulation of HDAC4 expression in order to promote satellite cell differentiation necessary for muscle tissue repair. In line with this hypothesis, our results revealed that the expression levels of TGF-β1, Smad3, and HDAC4 increased above basal levels after sciatic nerve denervation surgery, but miR-206 injections significantly reduced their expression levels back to control levels. Thus, the TGF-β1/Smad3 axis and HDAC4 are major players in the molecular mechanism involving miR-206 in muscle regeneration. These molecular changes resulted in the enhancement of satellite cell differentiation and the maintenance of muscle fiber cross-sectional area and muscle wet weight.
Notably, growing evidence implicates TGF-β1 as a factor involved in collagen formation during muscle atrophy, which impedes satellite cell differentiation, fusion of differentiated satellite cells, and revascularization of damaged muscle area. This results in the acceleration of muscle damage and reduced motor function [29]. We found that miR-206 treatment markedly reduced collagen formation after muscle denervation, thereby providing a favorable environment for differentiation, revascularization, and regeneration of muscles.
To further confirm that the TGF-β1/Smad3 axis is the prominent signal pathway for the beneficial effects observed with miR-206, the TGF-β1 receptor inhibitor SB431542 was applied to counteract miR-206 treatment. Combined treatment led to the blockage of miR-206’s beneficial effects on muscle regeneration following sciatic nerve denervation. These experiments further suggested that the ability of miR-206 to regulate HDAC4 was dependent on the TGF-β1/Smad3 axis. These results suggest that the TGF-β1/Smad3 axis may be one of the important signal pathways by which miR-206 regulates HDAC4 expression. We showed that the beneficial effect of miR-206 in denervated muscles achieved via down-regulation of HDAC4 was partly attenuated by SB431542.
Conclusions
Our results showed that the overexpression of miR-206 in muscle accelerated the differentiation process of satellite cells by regulating HDAC4 expression, and that the TGF-β1/Smad3 signaling pathway was an important pathway by which miR-206 regulated HDAC4. Thus, the TGF-β1/Smad3/HDAC4 axis plays an important role in the progression of muscle atrophy. Overexpression of miR-206 might represent a possible strategy to attenuate denervation-induced skeletal muscle atrophy through suppression of TGF-β1 and HDAC4 signaling and by promoting satellite cell differentiation.
Supplementary materials
Effect of miR-206 treatment (15 μg/rat, 30 μg/rat, and 60 μg/rat) on muscle cell morphology in comparison with the Den+saline+RT (transfection reagent) group (H&E).
The expression of MyoD1 (red) in the Den+ miR-206 treatment group (15 μg/rat, 30 μg/rat, and 60 μg/rat). Immunofluorescence staining for MyoD1 (red), nuclear stain (blue).
Immunofluorescence analysis of the GM using TGF-β1 antibodies (red) in the Den and Den+miR-206 groups. DAPI (blue) was used as a nuclear stain.
Double immunofluorescence analysis of the GM using Pax7 (red), the muscle cell differentiation marker MyoD1 (green), and the nuclear stain DAPI (blue) in the Den+miR-206 and Den+miR-206+SB groups.
Footnotes
Competing interests
The author(s) declare that they have no competing interests.
Source of support: Supported by a National Natural Science Foundation of China (Grant No 81171722) and by Postgraduate Innovation Foundation Project of the Shanxi Province of China (Grant No 20143012)
References
- 1.Soldado F, Fontecha CG, Marotta M, et al. The role of muscle imbalance in the pathogenesis of shoulder contracture after neonatal brachial plexus palsy: A study in a rat model. J Shoulder Elbow Surg. 2014;23:1003–9. doi: 10.1016/j.jse.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 2.Gwag T, Park K, Park J, et al. Celastrol overcomes HSP72 gene silencing-mediated muscle atrophy and induces myofiber preservation. J Physiol Pharmacol. 2015;66:273–83. [PubMed] [Google Scholar]
- 3.Bongers KS, Fox DK, Ebert SM, et al. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab. 2013;305:E907–15. doi: 10.1152/ajpendo.00380.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li QT, Zhang PX, Yin XF, et al. Functional recovery of denervated skeletal muscle with sensory or mixed nerve protection: A pilot study. PLoS One. 2013;8:e79746. doi: 10.1371/journal.pone.0079746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batt J, Bain J, Goncalves J, et al. Differential gene expression profiling of short and long term denervated muscle. FASEB J. 2006;20:115–17. doi: 10.1096/fj.04-3640fje. [DOI] [PubMed] [Google Scholar]
- 6.Wang YM, Ding XB, Dai Y, et al. Identification and bioinformatics analysis of miRNAs involved in bovine skeletal muscle satellite cell myogenic differentiation. Mol Cell Biochem. 2015;404:113–22. doi: 10.1007/s11010-015-2371-9. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MB, Woodworth-Hobbs ME, Zheng B, et al. miR-23a is decreased during muscle atrophy by a mechanism that includes calcineurin signaling and exosome-mediated export. Am J Physiol Cell Physiol. 2014;306:C551–58. doi: 10.1152/ajpcell.00266.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirby TJ, McCarthy JJ. MicroRNAs in skeletal muscle biology and exercise adaptation. Free Radic Biol Med. 2013;64:95–105. doi: 10.1016/j.freeradbiomed.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan H, Zhang Y, Cai B, et al. Upregulation of microRNA-1 and microRNA-133 contributes to arsenic-induced cardiac electrical remodeling. Int J Cardiol. 2013;167:2798–805. doi: 10.1016/j.ijcard.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Rau CS, Jeng JC, Jeng SF, et al. Entrapment neuropathy results in different microRNA expression patterns from denervation injury in rats. BMC Musculoskelet Disord. 2010;11:181. doi: 10.1186/1471-2474-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winbanks CE, Wang B, Beyer C, et al. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J Biol Chem. 2011;286:13805–14. doi: 10.1074/jbc.M110.192625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KO, Sampson ER, Maynard RD, et al. Ski inhibits TGF-beta/phospho-Smad3 signaling and accelerates hypertrophic differentiation in chondrocytes. J Cell Biochem. 2012;113:2156–66. doi: 10.1002/jcb.24089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24:2543–55. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto A, Fujita N, Arakawa T, et al. Influence of electrical stimulation on calpain and ubiquitin-proteasome systems in the denervated and unloaded rat tibialis anterior muscles. Acta Histochem. 2014;116:936–42. doi: 10.1016/j.acthis.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Quy PN, Kuma A, Pierre P, Mizushima N. Proteasome-dependent activation of mammalian target of rapamycin complex 1 (mTORC1) is essential for autophagy suppression and muscle remodeling following denervation. J Biol Chem. 2013;288:1125–34. doi: 10.1074/jbc.M112.399949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakasa T, Ishikawa M, Shi M, et al. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med. 2010;14:2495–505. doi: 10.1111/j.1582-4934.2009.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Sun L, Liang H, et al. Overexpression of microRNA-1 causes atrioventricular block in rodents. Int J Biol Sci. 2013;9:455–62. doi: 10.7150/ijbs.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stipursky J, Francis D, Gomes FC. Activation of MAPK/PI3K/SMAD pathways by TGF-beta(1) controls differentiation of radial glia into astrocytes in vitro. Dev Neurosci. 2012;34:68–81. doi: 10.1159/000338108. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Wang YA, Meng A, et al. Inhibiting TGFbeta1 has a protective effect on mouse bone marrow suppression following ionizing radiation exposure in vitro. J Radiat Res. 2013;54:630–36. doi: 10.1093/jrr/rrs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joanisse S, Gillen JB, Bellamy LM, et al. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J. 2013;27:4596–605. doi: 10.1096/fj.13-229799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olguin HC, Patzlaff NE, Olwin BB. Pax7-FKHR transcriptional activity is enhanced by transcriptionally repressed MyoD. J Cell Biochem. 2011;112:1410–17. doi: 10.1002/jcb.23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge X, McFarlane C, Vajjala A, et al. Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res. 2011;21:1591–604. doi: 10.1038/cr.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoyama T, Nishijo K, Prajapati SI, et al. Rb1 gene inactivation expands satellite cell and postnatal myoblast pools. J Biol Chem. 2011;286:19556–64. doi: 10.1074/jbc.M111.229542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik B, Nirmalananthan N, Gray AL, et al. Co-induction of the heat shock response ameliorates disease progression in a mouse model of human spinal and bulbar muscular atrophy: Implications for therapy. Brain. 2013;136:926–43. doi: 10.1093/brain/aws343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauerslev S, Vissing J, Krag TO. Muscle atrophy reversed by growth factor activation of satellite cells in a mouse muscle atrophy model. PLoS One. 2014;9:e100594. doi: 10.1371/journal.pone.0100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JF, Tao Y, Li J, et al. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190:867–79. doi: 10.1083/jcb.200911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharathy N, Taneja R. Methylation muscles into transcription factor silencing. Transcription. 2012;3:215–20. doi: 10.4161/trns.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braga M, Bhasin S, Jasuja R, et al. Testosterone inhibits transforming growth factor-beta signaling during myogenic differentiation and proliferation of mouse satellite cells: Potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol. 2012;350:39–52. doi: 10.1016/j.mce.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birbrair A, Zhang T, Wang ZM, et al. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. 2014;6:245. doi: 10.3389/fnagi.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of miR-206 treatment (15 μg/rat, 30 μg/rat, and 60 μg/rat) on muscle cell morphology in comparison with the Den+saline+RT (transfection reagent) group (H&E).
The expression of MyoD1 (red) in the Den+ miR-206 treatment group (15 μg/rat, 30 μg/rat, and 60 μg/rat). Immunofluorescence staining for MyoD1 (red), nuclear stain (blue).
Immunofluorescence analysis of the GM using TGF-β1 antibodies (red) in the Den and Den+miR-206 groups. DAPI (blue) was used as a nuclear stain.
Double immunofluorescence analysis of the GM using Pax7 (red), the muscle cell differentiation marker MyoD1 (green), and the nuclear stain DAPI (blue) in the Den+miR-206 and Den+miR-206+SB groups.