Abstract
Transcranial direct current stimulation (tDCS) is an emerging, noninvasive technique of neurostimulation for treating pain. However, the mechanisms and pathways involved in its analgesic effects are poorly understood. Therefore, we investigated the effects of direct current stimulation (DCS) on thermal and mechanical nociceptive thresholds and on the activation of the midbrain periaqueductal gray (PAG) and the dorsal horn of the spinal cord (DHSC) in rats; these central nervous system areas are associated with pain processing. Male Wistar rats underwent cathodal DCS of the motor cortex and, while still under stimulation, were evaluated using tail-flick and paw pressure nociceptive tests. Sham stimulation and naive rats were used as controls. We used a randomized design; the assays were not blinded to the experimenter. Immunoreactivity of the early growth response gene 1 (Egr-1), which is a marker of neuronal activation, was evaluated in the PAG and DHSC, and enkephalin immunoreactivity was evaluated in the DHSC. DCS did not change the thermal nociceptive threshold; however, it increased the mechanical nociceptive threshold of both hind paws compared with that of controls, characterizing a topographical effect. DCS decreased the Egr-1 labeling in the PAG and DHSC as well as the immunoreactivity of spinal enkephalin. Altogether, the data suggest that DCS disinhibits the midbrain descending analgesic pathway, consequently inhibiting spinal nociceptive neurons and causing an increase in the nociceptive threshold. This study reinforces the idea that the motor cortex participates in the neurocircuitry that is involved in analgesia and further clarifies the mechanisms of action of tDCS in pain treatment.
Introduction
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique with promising clinical outcomes for various conditions, including Parkinson’s disease [1], stroke [2], multiple sclerosis [3], schizophrenia [4], major depression [5] and pain [6]. This approach is relatively safe, well tolerated, affordable and easily deployable and consists of the application of a weak electric current to the scalp via two electrodes (anode and cathode); its efficacy depends critically on parameters such as electrode position and current strength [7]. The clinical effects of tDCS have been attributed to modulation of neuronal plasticity and synaptic connections [8–11], an increase in cortical excitability beyond the stimulation period [7,12] and the consolidation of this neuroplasticity, which is regulated by several mechanisms including acetylcholine, dopamine, serotonin, GABA, Na+ channel and NMDA-receptor activity [8,13–17]. Additional studies have shown a prolonged facilitation or inhibition of subcortical neuron activation, both in humans and in animals [18,19].
The analgesia induced by tDCS when applied over the primary motor cortex (M1) has been attributed to modulation of areas associated with pain processing, including the anterior cingulate, insula, thalamic nuclei and upper brainstem [9,20–25], as well as to regulation of glutamate, GABA and opioid activity, which results in the activation of descending analgesic pathways [25–28]. Evidence suggests that anodal tDCS increases the excitability of the underlying motor cortex, whereas cathodal tDCS diminishes cortical excitability [12,29,30]. Anodal tDCS over M1 produces long-lasting therapeutic effects in chronic pain conditions, including fibromyalgia [31–33], central pain [34,35], and phantom limb pain [36]. Experimental anodal direct current stimulation (DCS) in rats has been shown to induce thermal antinociceptive effects [37] and to reverse inflammatory chronic pain [38] and chronic stress-induced pain via the inhibition of hippocampal TNFα and spinal BDNF levels [39,40]. In addition, anodal DCS has been found to reverse mechanical hyperalgesia and the behavioral alterations in neuropathic rats, which is accompanied by changes in the BDNF and cytokine levels in different areas of the central nervous system [41,42]. Cathodal tDCS over M1 also induces analgesia in patients with chronic pain [43,44]. Both anodal and cathodal tDCS are known to increase the nociceptive threshold in healthy subjects [22,44–47]; however, until now, no experimental studies have been conducted to evaluate the cathodal DCS-induced antinociception in naive rats to better understand the neurocircuitry that mediates this response.
The expression of inducible transcription factors (ITFs) or immediate early genes, including egr-1 (zif-268, krox-24, or zenk), c-fos and c-jun, has been widely used to map neuronal activation under different physiological and non-physiological conditions [48–50]. ITFs are induced in neurons in response to extracellular stimuli, including depolarization, growth factors and neurotransmitters such as glutamate and substance P [51–56]. ITF expression can be elicited by various types of noxious stimuli, including chemical, thermal and mechanical stimuli, in different areas of the brain and the spinal cord. These factors thus become important tools for the study of the neural circuitry underlying nociception [50,57–61]. A direct correlation between decrease in ITF expression and antinociception in the DHSC has been suggested [62–64]. Few studies have evaluated the relationship between cortical stimulation, analgesia and ITFs. Using Egr-1 and c-Fos immunoreactivity (IR), epidural motor cortex stimulation has been shown to induce analgesia in naive and neuropathic rats, inhibiting the spinal neurons and activating the PAG [65–67]. Although a top-down effect of tDCS has been hypothesized, which would lead to activation of the descending analgesic pathway and consequently an increase in the nociceptive threshold, its inhibitory action on spinal nociceptive neurons has yet to be demonstrated.
The present study was conducted to investigate the effects of cathodal DCS on the thermal and mechanical pain sensitivity of healthy rats and to evaluate the pattern of neuronal activation using Egr-1 detection in the midbrain periaqueductal gray (PAG), which is a key component of the descending analgesic pathway, and in the dorsal horn of the spinal cord (DHSC), which is the region where nociceptive information is received, integrated, and relayed to higher brain areas. In addition, to evaluate the involvement of the spinal opioid system, enkephalin labeling in the DHSC was also investigated.
Materials and Methods
Experimental design
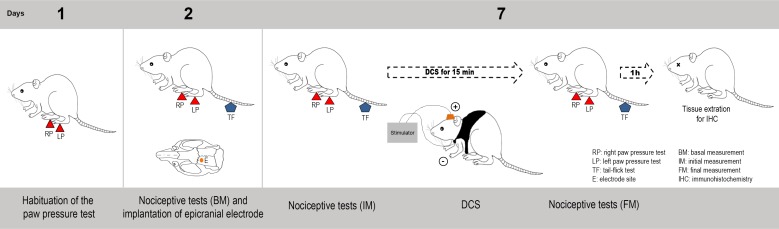
Adult rats were evaluated with nociceptive tests (described in the Measuring nociceptive threshold section below), and subsequently, under anesthesia, epicranial electrodes were implanted over their left motor cortex involving the functional area of the right hind limb. After 5 days, the nociceptive tests were performed again in the animals while they were awake. Next, a group of rats received a single session of 15 minutes of DCS, after which, they were re-evaluated on the nociceptive tests while still under stimulation. Rats that were submitted to the surgical procedures but were not electrically stimulated (sham) and rats that did not receive any surgical procedure (naive) were also evaluated. After 1 hour of the last nociceptive evaluation, the animals were anesthetized, perfused, and their tissue fragments were processed for immunohistochemical analysis to evaluate the neuronal activation pattern in the PAG and DHSC and enkephalin expression in the DHSC (Fig 1).
Fig 1. Experimental design.
Rats were habituated to the paw pressure test in the day 1. In the second day, they were evaluated in the paw pressure and tail flick tests, and subsequently, under anesthesia, epicranial electrodes were implanted over their left motor cortex. After 5 days (day 7), the nociceptive tests were performed again. A group of rats received a single session of 15 minutes of DCS (250 μA), after which, they were re-evaluated on the nociceptive tests while still under stimulation. Rats that were submitted to the surgical procedures but were not electrically stimulated (sham) and rats that did not receive any surgical procedure (naive) were also evaluated. After 1 hour of the last nociceptive evaluation, the animals were anesthetized, perfused, and their tissue fragments were processed for immunohistochemical analysis.
Animals
Twenty-five male Wistar rats (180–220 g) were housed in acrylic boxes (three rats per cage) for at least two days before the initiation of the experimental procedures. The boxes were kept at a constant ambient room temperature with a controlled temperature (22°±2°C) and a light/dark cycle (12/12 h), and they had wood shavings and free access to water and rat chow pellets. All procedures were in accordance with the guidelines on the ethical use of animals in research involving pain and nociception [68] and were approved by the Ethics Committee on the Use of Animals at Hospital Sírio Libanês (Sao Paulo, Brazil) under protocol number CEUA 2011/24.
Electrode implantation and electrical stimulation
Mimicking the clinical protocol that induces analgesia in healthy humans, we decided to use cathodal DCS on naive rats in our study. The electrode fixation and the stimulation protocol were applied according to an earlier study [69]. Briefly, rats were deeply anesthetized with ketamine/xylazine (50/20 mg/kg, intraperitoneal) as well as with a local scalp injection of 2% lidocaine (100 μL/animal, subcutaneous). Then, under stereotactic guidance using a map developed by our group [70], an epicranial electrode that consisted of a tubular plastic jacket was fixed onto the skull over the left primary motor cortex (M1) in the functional area of the right hind limb (1 mm caudal to bregma; 1 mm left to midline). Rats were treated with ketoprofen (10 mg/kg, subcutaneous) for three days after surgery. Five days after implantation of the electrode, the epicranial jacket was filled with a conductive gel (Electron Plus®, Hal, SP, Brazil), and the cathode was inserted. The animals were dressed in vests that placed a large rubber plate on the ventral thorax, which served as the counter electrode (anode). The electrodes were connected to an electrical stimulator (Striat®, IBRAMED, SP, Brazil) with wire leads, which allowed the animals to move freely in the cage (S1 Fig). The experimental group received a single DCS session of 15 minutes (250 μA over an area of 2.27 mm2), and the final measurements during the nociceptive tests were recorded while the rats were still under stimulation. The sham group was subjected to the same conditions but did not receive stimulation. The rats were randomly divided into the sham and stimulated groups. In total, the animals were divided into three groups: rats with no surgical procedure (naive, n = 7), rats with an epicranial electrode and false stimulation (sham, n = 7), and stimulated rats (n = 7). Four animals were excluded from the study because they removed their implants before the final nociceptive tests.
Measuring nociceptive threshold
Nociceptive tests were conducted prior to the electrode implantation and five days after, before (initial measurement) and during DCS (final measurement). Naive and sham rats were also evaluated. On the day of the test, the animals were brought into a separate quiet room 1 hour before the nociceptive tests to allow them to habituate to the environment. The thermal nociceptive threshold was determined using the tail-flick test, which has been previously described [71]. Briefly, radiant heat was focused on the lower third of their tail using an analgesiometer (EEF 300, Insight®, SP, Brazil). Movement of the tail activated a photocell, thereby turning off both the light and a reaction timer. The nociceptive threshold was defined as the time necessary to induce the tail-flick response. The mechanical nociceptive threshold was determined using a pressure apparatus on the right hind paw (EEF-440, Insight®), which has also been previously described [72]. Briefly, the mass (in grams) required to induce a withdrawal response represented the nociceptive threshold. The results were analyzed by comparing between the initial and final measurements. In order to reduce animal stress, the rats were handled by only one experimenter and were habituated to the paw pressure test the day preceding the electrode implantation. Both nociceptive tests were performed before electrode implantation (basal measurement) to evaluate if the nociceptive threshold could be changed after surgical procedure.
Immunohistochemistry
One hour after the last nociceptive test, the rats were deeply anesthetized with ketamine and xylazine and then subjected to transcardiac perfusion with saline solution followed by 4% paraformaldehyde (PFA) dissolved in 0.1 M phosphate buffer (PB). The animals were perfused 1 hour after the last nociceptive stimulus because the peak expressions of ITF proteins occur approximately 1 hour after the stimulus and fade by 3 to 4 hours post-stimulation [50]. The brain and lumbar spinal cord (L2-L5 segments) were collected and post-fixed in PFA for 4 hours, followed by incubation with 30% sucrose solution in PB for 48 hours at 4°C. Tissue sections (30 μm) were cut on a freezing microtome, washed in PB, and incubated for 12 to 16 hours at 4°C with rabbit anti-Egr-1 (Zif268, 1:1000, C-19; Santa Cruz Biotechnology®, CA, USA) or mouse anti-enkephalin (ENK, 1:1000, MAB350, Millipore®, CA, USA) primary antibodies that were diluted in 0.3% of Triton X-100 containing 5% normal donkey serum (Jackson ImmunoResearch®, ME, USA). Then, sections were incubated for 2 hours at room temperature with biotinylated secondary antibodies (Jackson ImmunoResearch®, ME, USA) and incubated with an avidin-biotin complex (1:100, ABC Elite kit, Vector Labs®, CA, USA) for 2 hours at room temperature. The sections were visualized with 0.05% diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich®, MO, USA) and 0.03% (final concentration) hydrogen peroxide in PB. The sections were then mounted on glass slides, air-dried, dehydrated, and coverslipped with Permount (Fisher Scientific®, PA, USA). The samples were washed between each step (3 x 10 min). The immunoreactivity was analyzed using a light microscope (E1000, Nikon®, NY, USA) and ImageJ software (National Institute of Health, MD, USA; http://rsbweb.nih.gov/ij/). Figures were assembled using Adobe Photoshop (Adobe Systems, CA, USA); the images were converted to black and white and optimized using contrast and brightness only. Quantitative analysis was performed to determine the density of nuclei showing positive immunoreactivity (IR) for Egr-1 in the rostral portion of the PAG (dorsomedial, dorsolateral, lateral and ventrolateral columns) and DHSC (laminae I-IV) and the IR of ENK in the DHSC (laminae I-VI). Measurements were taken from 7 different sections for each analyzed animal. The regions of interest were identified based on a stereotaxic atlas [73] and an atlas of the spinal cord [74].
Statistical analysis
Data are presented as the mean ± standard error of the mean. Statistical analyses were conducted with GraphPad Prism® 5.0 software (GraphPad Software Inc; La Jolla, CA, USA). The results of the nociceptive tests were analyzed using two-way repeated measures analysis of variance (ANOVA) followed by the Bonferroni post hoc test. Immunohistochemistry data were analyzed using one-way ANOVA followed by the Bonferroni post hoc test. In all cases, p < 0.05 was considered statistically significant.
Results
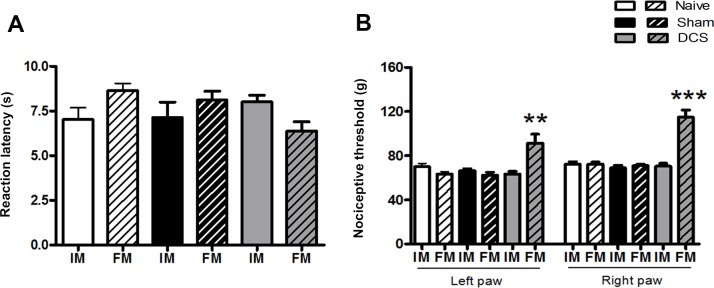
Cathodal DCS did not change the thermal nociceptive threshold in the tail of the animals (Factor Time x Factor Treatment, F(2,9) = 0.08030, p = 0.92) (Fig 2A). However, it was able to increase the mechanical nociceptive threshold of the left (Factor Time x Factor Treatment, F(2,27) = 15.37, p < 0.0001) and right (Factor Time x Factor Treatment, F(2,27) = 32.60, p < 0.0001) hind paws (53% and 73% increases, respectively) compared with that of the naive and sham animals (Fig 2B). The results obtained in the nociceptive tests, before electrode implantation in the basal measurement (data not showed), were equal to the data observed after 5 days of the surgical procedure, showing that the increase of nociceptive threshold after the DCS was a consequence of the electrical stimulation.
Fig 2. DCS and nociceptive response.
Nociceptive threshold was evaluated using the tail-flick test (A) and paw pressure test (B) before (initial measurement, IM) and during DCS (final measurement, FM) in naive (without surgical intervention), sham (with epicranial electrode without stimulation) and stimulated (DCS, 250 μA, 15 min) rats. Epicranial electrodes were placed in the left hemisphere. Values represent the mean ± SEM (n = 7 animals per group). *p < 0.05, when compared to the naive group. DCS: direct current stimulation.
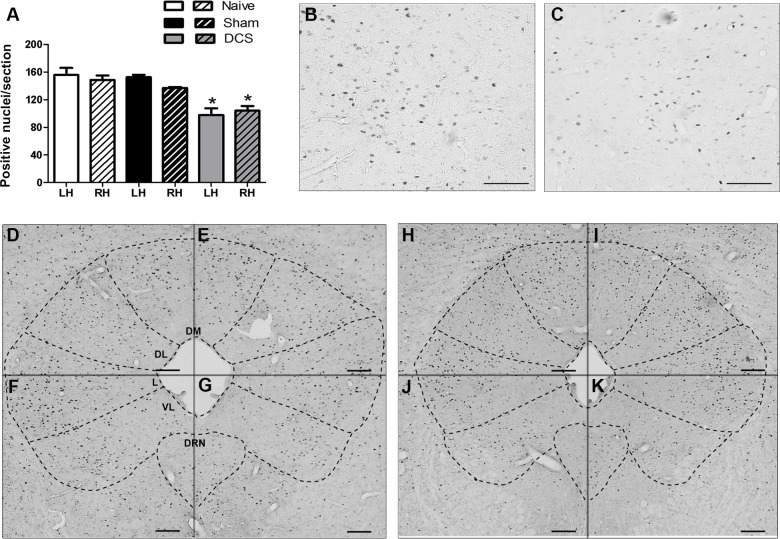
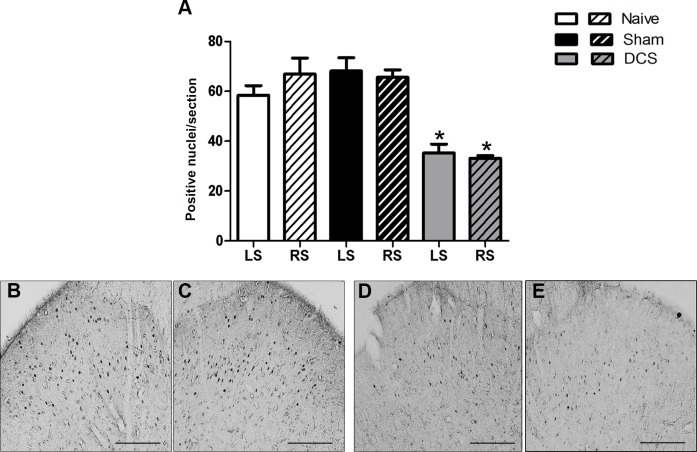
The immunohistochemistry analysis showed a bilateral decrease in Egr-1-positive neurons in the PAG after DCS (F(2,11) = 12.10, p = 0.0028, left hemisphere; F(2,12) = 15.30, p = 0.0009, right hemisphere), primarily in the ventrolateral PAG (Fig 3), compared with the results of the control groups. Furthermore, our results showed that DCS bilaterally decreased (F(2,9) = 17.05, p = 0.0020, left side; F(2,9) = 28.93, p = 0.0004, right side) the Egr-1-positive neurons in the DHSC (Fig 4) when compared with control animals.
Fig 3. DCS and neuronal activation in the PAG.
Quantification of the Egr-1-positive nuclei in the PAG of naive (without surgical intervention), sham (with epicranial electrode without stimulation) and stimulated (DCS, 250 μA, 15 min) rats. Values represent the mean ± SEM (n = 7 animals per group). *p < 0.05, when compared to the naive group (A). Photomicrographs show the Egr-1-positive nuclei staining in the PAG of naive (B, D-G) and stimulated (C, H-K) rats. Figs B and C represent higher magnification insets of the ventrolateral PAG of naive and stimulated rats, respectively. Sections of the left (B-D, F, H, J) and right (E, G, I, K) hemisphere of the PAG. Scale bars: 50 μm (B and C) and 100 μm (D-K). DCS: direct current stimulation; DL: dorsolateral PAG; DM: dorsomedial PAG; DRN: dorsal raphe nucleus; L: lateral PAG; LH: left hemisphere; PAG: midbrain periaqueductal gray; RH: right hemisphere; VL: ventrolateral PAG.
Fig 4. DCS and neuronal activation in the DHSC.
Quantification of the Egr-1-positive nuclei in the DHSC of naive (without surgical intervention), sham (with epicranial electrode without stimulation) and stimulated (DCS, 250 μA, 15 min) rats. Values represent the mean ± SEM (n = 7 animals per group). *p < 0.05, when compared to the naive group (A). Photomicrographs show the Egr-1-positive nuclei staining in the superficial laminae of the DHSC of naive (B, C) and stimulated (D, E) rats. Sections of the left (B, D) and right (C, E) DHSC. Scale bars: 50 μm. DCS: direct current stimulation; DHSC: dorsal horn of the spinal cord; LS: left side; RS: right side.
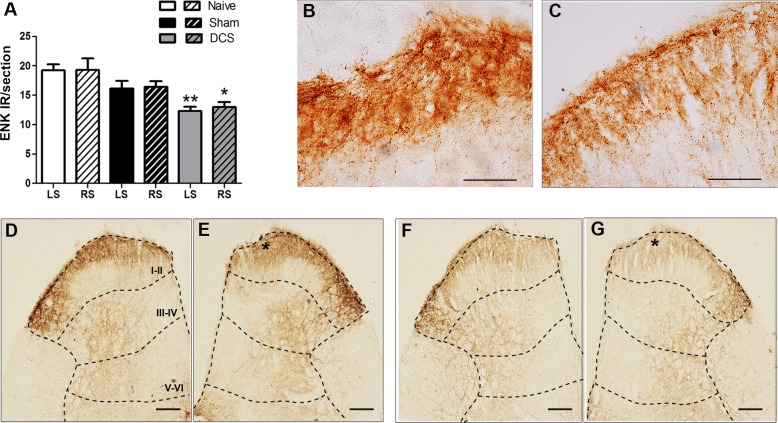
Cathodal DCS bilaterally decreased (F(2,11) = 11.06, p = 0.0038, left side; F(2,11) = 5.503, p = 0.0275, right side) the spinal ENK immunoreactivity compared with that of the naive group (Fig 5).
Fig 5. DCS and enkephalin immunostaining in the DHSC.
Quantification of the enkephalin (ENK) immunoreactivity (IR) in the DHSC of naive (without surgical intervention), sham (with epicranial electrode without stimulation) and stimulated (DCS, 250 μA, 15 min) rats. Values represent the mean ± SEM (n = 7 animals per group). *p < 0.05, when compared to the naive group (A). Photomicrographs show the ENK-IR in the DHSC of naive (B, D, E) and stimulated (C, F, G) rats. Figs B and C show higher magnification insets of laminae I-II of naive and stimulated animals, indicated by asterisks in figs E and G, respectively. Sections of the left (D, F) and right (B, C, E, G) DHSC. Scale bars: 50 μm (B and C) and 100 μm (D-G). Laminae I-VI of the DHSC are represented in figs D-G. DCS: direct current stimulation; DHSC: dorsal horn of the spinal cord; LS: left side; RS: right side.
Discussion
tDCS has been used in the treatment of various chronic pain disorders [31–36]; however, its mechanism of action remains poorly understood. We showed here that cathodal DCS, applied over the functional area of the hind limb, did not interfere with the thermal nociceptive threshold in the tail. Evidence indicates that the analgesic effect induced by cortical stimulation depends on the electrode position in the cortical somatotopy representation of pain perception [75,76]. In this regard, antinociception induced by epidural motor cortex stimulation in healthy rats was shown to have a topographic relationship with the stimulated area of the M1 [67]. Our results confirm this somatotopic analgesic effect induced by cortical stimulation, as cathodal DCS did not alter the nociceptive threshold in the tail but induced a bilateral increase of the mechanical nociceptive threshold in the hind paws of rats. In contrast with our findings, anodal DCS over the M1 has been shown to increase the nociceptive threshold in naive rats in the tail-flick test [37]. However, our results are consistent with findings obtained in healthy humans who were subjected to cathodal tDCS of the motor cortex; this procedure resulted in an increase in the cold and mechanical detection thresholds but not in the heat threshold, suggesting that cathodal tDCS temporarily reduces sensitivity to the A-fibers mediating the somatosensory inputs [46]. Interestingly, similar to the bilaterality that was observed in our results for the mechanical threshold, the aforementioned study also demonstrated bilateral changes in the cold detection threshold [46].
Another difference that could account for the differential antinociception observed in this study is the polarity-specific alteration in cortical blood flow (CBF) induced by tDCS. Cathodal tDCS results in a decrease in CBF in the cortical area, whereas anodal tDCS results in an increase [77]. In addition to this localized effect, the CBF decrease induced by cathodal tDCS spreads to target-adjacent areas [78]. This widespread modulation could, in turn, account for the widespread effect (namely, the bilateral antinociception) that was observed in our results. Additionally, another hypothesis considers diffuse noxious inhibitory control (DNIC) activation, which refers to the supraspinally mediated inhibitory process that occurs in the pain-signaling neurons of the DHSC in response to heterogenic noxious stimuli [79, 80]. In fact, tDCS displays synergistic effects when combined with a DNIC approach [22]. As suggested, a plausible mechanism could be that tDCS elicits activation of the same neural circuits that are associated with DNIC, particularly the endogenous descending analgesic pathways [22].
The most prominent neurocircuitry involved in the descending control of pain is the PAG, as activating the rostral ventromedial medulla and the locus ceruleus, which project their serotonergic and noradrenergic descending fibers, respectively, to the DHSC, results in the specific inhibition of the firing of spinal nociceptive neurons [81–83]. To evaluate whether the cathodal DCS-induced antinociception in naive rats was mediated by activation of the descending analgesic system, we evaluated the neuronal activation in the PAG and DHSC. DCS bilaterally decreased the Egr-1 immunolabeling in the PAG, primarily in the ventrolateral and lateral subdivision, and in the superficial laminae of the DHSC. Based on anatomic and functional patterns, the PAG has been subdivided into four columns: the dorsomedial, dorsolateral, lateral and ventrolateral [84]. Antinociception effects of different types are mediated by the lateral and ventrolateral PAG [85–87]; our findings corroborate this idea, as the changes in the activation pattern that occurred in these subdivisions were more evident. As previously shown, epidural motor cortex stimulation induces antinociception in healthy rats by enhancing the neuronal firing rate and Fos immunoreactivity within the PAG and, by simultaneously decreasing GABA and Egr-1 labeling; this suggests an inhibition of local GABAergic interneurons with subsequent activation of the excitatory neurons responsible for descending analgesic control [66]. Similarly, França et al. [67] also showed an increase in Fos immunolabeling in the different subdivisions of the PAG of naive rats after epidural stimulation; however, this increase was also observed in Egr-1. Further investigation of the PAG neurocircuitry involved in the tDCS-induced analgesic effect is needed to clarify which populations of neurons are modulated by this therapeutic intervention. Our results also suggest that cathodal DCS induces a disinhibition of the PAG with resulting activation of the descending analgesic pathways, increasing the nociceptive threshold through a top-down modulation mechanism, as previous proposed [22,26,40]. The immunohistochemistry results observed in this study demonstrate that the results of behavioral studies are consistent with both altered activation of the PAG, which is the main region of the midbrain descending analgesic pathway, and a decrease in the activation of spinal nociceptive neurons, which are the main input for ascending nociceptive information. As the expression of Egr-1 in the DHSC is directly related to the activity of nociceptive neurons [59], our results suggest that DCS leads to a decrease in the transmission of ascending nociceptive information. These results are supported by others who have demonstrated, after epidural cortical stimulation, a relationship between analgesia and decreased spinal Egr-1 in naive and neuropathic rats [65,67].
A growing body of evidence has indicated the participation of the opioid system in the analgesia induced by cortical stimulation [27,28,88–91]. In an attempt to evaluate the involvement of the spinal opioid system in the antinociception induced by cathodal DCS in healthy rats, we evaluated enkephalin (ENK) labeling in the DHSC. Enkephalins are endogenous opiates that play an important role in the modulation of nociceptive information by mediating synaptic transmission in several areas of the central nervous system [92,93]. Most ENK present in the DHSC originates from local interneurons, although a few enkephalinergic fibers may descend from the brainstem [94–96] or originate from dorsal root ganglion neurons [97, 98]. In the DHSC, ENK is highly concentrated in the neurons located in the superficial laminae I and II and is sparsely distributed in the deep laminae V–VII and around the central canal [99, 100]. Additionally, ENK binds to both μ and δ opioid receptors with similar affinities but is inactive at κ opioid receptors [101]. Spinal ENK inhibits neurotransmitter release by the nociceptive primary afferents and the spinothalamic projection neurons in response to noxious peripheral stimuli [102–104]. Indeed, a positive correlation between an increase in spinal ENK and analgesia in chronic pain conditions has been shown [105–107]. Nevertheless, in the absence of persistent pain, spinal opioid release has been shown to be inhibited by activation of the descending analgesic system [108,109], which could serve to shut down the spinal opioid system whenever the descending serotonergic and noradrenergic pathways are active, thus preventing undesirable analgesia under normal conditions. Our findings are consistent with this process; our results show that during the DCS-induced antinociception in healthy rats, a decrease in spinal ENK concomitant with an inhibition of nociceptive neurons in the DHSC occurs, likely due to activation of the descending analgesic system.
The brainstem serotonergic and noradrenergic fibers are the main components of the descending analgesic pathways, as the release of their biogenic amines in the DHSC result in inhibition of spinal nociceptive neurons [82]. However, although the majority of serotonergic fibers act directly on the spinal nociceptive neurons, the noradrenergic fibers have been shown to act indirectly through the secondary release of opiate-like substances [110,111]. As DCS is a modulatory intervention that targets the endogenous neural circuits, it could induce a differential activation of such parallel pathways, resulting in enhanced serotonergic fiber-mediated and reduced noradrenergic fiber-mediated effects and a subsequent decrease in ENK release. Despite this differential modulation, the net effect is still the activation of the descending analgesic system culminating with inhibition of the spinal nociceptive neurons. This hypothesis also explains our results, which showed a decrease in both ENK and Egr-1 immunoreactivity in the DHSC.
Altogether, the present findings suggest that DCS induces disinhibition of the midbrain descending analgesic pathway with a subsequent inhibition of spinal nociceptive neurons, leading to an increase in the nociceptive threshold. This study reinforces the idea that the motor cortex is involved in the neural circuits that control the nociceptive response and highlights the importance of further studies evaluating the clinical use of tDCS as an alternative for pain control.
Supporting Information
The epicranial electrode was fixed onto the skull over the left primary motor cortex (1 mm caudal to bregma; 1 mm left to midline). Before DCS, the epicranial jacket was filled with a conductive gel and the cathode was inserted. The animals were dressed in vests that placed a large rubber plate on the ventral thorax, which served as the counter electrode (anode). The electrodes were connected to an electrical stimulator with wire leads, which allowed the animals to move freely in the cage. Afterward, rats received a single DCS session of 15 minutes.
(TIF)
Acknowledgments
This research was supported by the Hospital Sírio Libanês and São Paulo State Foundation (FAPESP). LFD, RLP and ARB were funded by FAPESP (11/21631-3, 09/50772-4 and 12/20911-5, respectively). ARB is supported by NARSAD Young Investigator from the Brain & Behavior Research Foundation (Grant Number 20493) and Brazilian National Council for Scientific and Technological Development (CNPq, Grant Numbers 303197 and 470904).
Abbreviations
- DCS
direct current stimulation
- DHSC
dorsal horn of the spinal cord
- M1
primary motor cortex
- PAG
midbrain periaqueductal gray
- tDCS
transcranial direct current stimulation
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Hospital Sírio Libanês and São Paulo State Foundation (FAPESP). LFD, RLP and ARB were funded by FAPESP (11/21631-3, 09/50772-4 and 12/20911-5, respectively). ARB is supported by NARSAD Young Investigator from the Brain & Behavior Research Foundation (Grant Number 20493) and Brazilian National Council for Scientific and Technological Development (CNPq, Grant Numbers 303197 and 470904). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, et al. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010; 81:1105–11. 10.1136/jnnp.2009.202556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn MK, Jee SJ, Kim YW. Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann Rehabil Med. 2013; 37:759–65. 10.5535/arm.2013.37.6.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrucci R, Vergari M, Cogiamanian F, Bocci T, Ciocca M, Tomasini E, et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation. 2012; 34:121–7. [DOI] [PubMed] [Google Scholar]
- 4.Brunoni AR, Shiozawa P, Truong D, Javitt DC, Elkis H, Fregni F, et al. Understanding tDCS effects in schizophrenia: a systematic review of clinical data and an integrated computation modeling analysis. Expert Rev Med Devices. 2014; 11:383–94. 10.1586/17434440.2014.911082 [DOI] [PubMed] [Google Scholar]
- 5.Shiozawa P, Fregni F, Benseñor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014; 17:1443–52. 10.1017/S1461145714000418 [DOI] [PubMed] [Google Scholar]
- 6.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with noninvasive brain stimulation techniques. Lancet Neurol. 2007; 6:188–91. [DOI] [PubMed] [Google Scholar]
- 7.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003; 553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002; 125:2238–47. [DOI] [PubMed] [Google Scholar]
- 9.Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005; 22:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005; 568:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008; 1:206–23. 10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 12.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000; 527:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex. 2004; 14:1240–5. [DOI] [PubMed] [Google Scholar]
- 14.Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology. 2004; 29:1573–8. [DOI] [PubMed] [Google Scholar]
- 15.Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Frommann K, et al. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci. 2004; 19:2720–6. [DOI] [PubMed] [Google Scholar]
- 16.Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007; 27:14442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terney D, Bergmann I, Poreisz C, Chaieb L, Boros K, Nitsche MA, et al. Pergolide increases the efficacy of cathodal direct current stimulation to reduce the amplitude of laser-evoked potentials in humans. J Pain Symptom Manage. 2008; 36:79–91. 10.1016/j.jpainsymman.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 18.Di Lazzaro V, Ziemann U, Lemon RN. State of the art: physiology of transcranial motor cortex stimulation. Brain Stimul. 2008; 1:345–62. 10.1016/j.brs.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 19.Brunoni AR, Fregni F, Pagano RL. Translational research in transcranial direct current stimulation (tDCS): a systematic review of studies in animals. Rev Neurosci. 2011; 22:471–81. 10.1515/RNS.2011.042 [DOI] [PubMed] [Google Scholar]
- 20.Holsheimer J, Nguyen JP, Lefaucheur JP, Manola L. Cathodal, anodal or bifocal stimulation of the motor cortex in the management of chronic pain? Acta Neurochir Suppl. 2007; 97:57–66. [DOI] [PubMed] [Google Scholar]
- 21.Zaghi S, Heine N, Fregni F. Brain stimulation for the treatment of pain: a review of costs, clinical effects and mechanisms of treatment for three different central neuromodulatory approaches. J Pain Manag. 2009; 2:339–52. [PMC free article] [PubMed] [Google Scholar]
- 22.Reidler JS, Mendonca ME, Santana MB, Wang X, Lenkinski R, Motta AF, et al. Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. J Pain. 2012; 13:450–8. 10.1016/j.jpain.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 23.DaSilva AF, Mendonca ME, Zaghi S, Lopes M, DosSantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012; 52:1283–95. 10.1111/j.1526-4610.2012.02141.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DaSilva AF, Truong DQ, DosSantos MF, Toback RL, Datta A, Bikson M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front Neuroanat. 2015; 9:89 10.3389/fnana.2015.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foerster BR, Nascimento TD, Deboer M, Bender MA, Rice IC, Truong DQ, et al. Excitatory and inhibitory brain metabolites as targets of motor cortex transcranial direct current stimulation therapy and predictors of its efficacy in fibromyalgia. Arthritis Rheumatol. 2015; 67:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008; 2329:37–70. [DOI] [PubMed] [Google Scholar]
- 27.DosSantos MF, Love TM, Martikainen IK, Nascimento TD, Fregni F, Cummiford C, et al. Immediate effects of tDCS on the m-opioid system of a chronic pain patient. Front Psychiatry. 2012; 3:93 10.3389/fpsyt.2012.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DosSantos MF, Martikainen IK, Nascimento TD, Love TM, DeBoer MD, Schambra HM, et al. Building up analgesia in humans via the endogenous m-opioid system by combining placebo and active tDCS: a preliminary report. PLoS One. 2014; 9:e102350 10.1371/journal.pone.0102350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001; 57:1899–901. [DOI] [PubMed] [Google Scholar]
- 30.Wassermann EM, Grafman J. Recharging cognition with DC brain polarization. Trends Cogn Sci. 2005; 9:503–5. [DOI] [PubMed] [Google Scholar]
- 31.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheumatol. 2006; 54:3988–98. [DOI] [PubMed] [Google Scholar]
- 32.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. 2015; 156:62–71. 10.1016/j.pain.0000000000000006 [DOI] [PubMed] [Google Scholar]
- 33.Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, Brandao R, Coutinho L, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: Phase II open-label dose optimization. J Pain. 2016; 17:14–26. 10.1016/j.jpain.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006; 122:197–209. [DOI] [PubMed] [Google Scholar]
- 35.Mehta S, McIntyre A, Guy S, Teasell RW, Loh E. Effectiveness of transcranial direct current stimulation for the management of neuropathic pain after spinal cord injury: a meta-analysis. Spinal Cord. 2015; 53:780–5. 10.1038/sc.2015.118 [DOI] [PubMed] [Google Scholar]
- 36.Bolognini N, Spandri V, Ferraro F, Salmaggi A, Molinari AC, Fregni F, et al. Immediate and sustained effects of 5-day transcranial direct current stimulation of the motor cortex in phantom limb pain. J Pain. 2015;16:657–65. 10.1016/j.jpain.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 37.Nekhendzy V, Fender CP, Davies MF, Lemmens HJ, Kim MS, Bouley DM, et al. The antinociceptive effect of transcranial electrostimulation with combined direct and alternating current in freely moving rats. Anesth Analg. 2004; 98:730–7. [DOI] [PubMed] [Google Scholar]
- 38.Laste G, Caumo W, Adachi LN, Rozisky JR, de Macedo IC, Filho PR, et al. After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Exp Brain Res. 2012; 221:75–83. 10.1007/s00221-012-3149-x [DOI] [PubMed] [Google Scholar]
- 39.Spezia Adachi LN, Caumo W, Laste G, Fernandes Medeiros L, Ripoll Rozisky J, de Souza A, et al. Reversal of chronic stress-induced pain by transcranial direct current stimulation (tDCS) in an animal model. Brain Res. 2012; 1489:17–26. 10.1016/j.brainres.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 40.Spezia Adachi LN, Quevedo AS, de Souza A, Scarabelot VL, Rozisky JR, de Oliveira C, et al. Exogenously induced brain activation regulates neuronal activity by top-down modulation: conceptualized model for electrical brain stimulation. Exp Brain Res. 2015; 233:1377–89. 10.1007/s00221-015-4212-1 [DOI] [PubMed] [Google Scholar]
- 41.Cioato SG, Medeiros LF, Marques Filho PR, Vercelino R, de Souza A, Scarabelot VL, et al. Long-lasting effect of transcranial direct current stimulation in the reversal of hyperalgesia and cytokine alterations induced by the neuropathic pain model. Brain Stimul. 2015; pii: S1935-861X(15)01232–2. [DOI] [PubMed] [Google Scholar]
- 42.Filho PR, Vercelino R, Cioato SG, Medeiros LF, de Oliveira C, Scarabelot VL, et al. Transcranial direct current stimulation (tDCS) reverts behavioral alterations and brainstem BDNF level increase induced by neuropathic pain model: Long-lasting effect. Prog Neuropsychopharmacol Biol Psychiatry. 2016; 64:44–51. 10.1016/j.pnpbp.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 43.Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, et al. Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. J Pain. 2013; 14:371–83. 10.1016/j.jpain.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 44.Vaseghi B, Zoghi M, Jaberzadeh S. A meta-analysis of site-specific effects of cathodal transcranial direct current stimulation on sensory perception and pain. PLoS One. 2015; 10:e0123873 10.1371/journal.pone.0123873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. 2008; 15:1124–30. 10.1111/j.1468-1331.2008.02270.x [DOI] [PubMed] [Google Scholar]
- 46.Bachmann CG, Muschinsky S, Nitsche MA, Rolke R, Magerl W, Treede RD, et al. Transcranial direct current stimulation of the motor cortex induces distinct changes in thermal and mechanical sensory percepts. Clin Neurophysiol. 2010; 121:2083–9. 10.1016/j.clinph.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 47.Vaseghi B, Zoghi M, Jaberzadeh S. Differential effects of cathodal transcranial direct current stimulation of prefrontal, motor and somatosensory cortices on cortical excitability and pain perception—a double-blind randomized sham-controlled study. Eur J Neurosci. 2015; 42:2426–37. 10.1111/ejn.13043 [DOI] [PubMed] [Google Scholar]
- 48.Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996; 50:83–107. [DOI] [PubMed] [Google Scholar]
- 49.Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochem Int. 1997; 31:477–510. [DOI] [PubMed] [Google Scholar]
- 50.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev 1998; 28:370–490. [DOI] [PubMed] [Google Scholar]
- 51.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990; 4:477–85. [DOI] [PubMed] [Google Scholar]
- 52.Chapman V, Buritova J, Honoré P, Besson JM. Physiological contributions of neurokinin 1 receptor activation, and interactions with NMDA receptors, to inflammatory-evoked spinal c-Fos expression. J Neurophysiol. 1996; 76:1817–27. [DOI] [PubMed] [Google Scholar]
- 53.Tao YX, Wei F, Zhao ZQ. A contribution of neurokinin-1 receptor to formalin-induced c-fos expression in the rat spinal dorsal horn. Neurosci Lett. 1997; 221:105–8. [DOI] [PubMed] [Google Scholar]
- 54.Hiscock JJ, Mackenzie L, Medvedev A, Willoughby JO. Kainic acid and seizure-induced Fos in subtypes of cerebrocortical neurons. J Neurosci Res. 2001; 66:1094–100. [DOI] [PubMed] [Google Scholar]
- 55.Szakács R, Weiczner R, Mihály A, Krisztin-Péva B, Zádor Z, Zádor E. Non-competitive NMDA receptor antagonists moderate seizure-induced c-fos expression in the rat cerebral cortex. Brain Res Bull. 2003;59:485–93. [DOI] [PubMed] [Google Scholar]
- 56.Soyguder Z. Multiple neurotransmitter receptors contribute to the spinal Fos expression." Brain Res. 2005; 1033:202–9. [DOI] [PubMed] [Google Scholar]
- 57.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987; 328:632–4. [DOI] [PubMed] [Google Scholar]
- 58.Herdegen T, Walker T, Leah JD, Bravo R, Zimmermann M. The KROX-24 protein, a new transcription regulating factor: expression in the rat central nervous system following afferent somatosensory stimulation. Neurosci Lett. 1990; 120:21–4. [DOI] [PubMed] [Google Scholar]
- 59.Herdegen T, Kovary K, Leah J, Bravo R. Specific temporal and spatial distribution of JUN, FOS, and KROX-24 proteins in spinal neurons following noxious transsynaptic stimulation. J Comp Neurol. 1991; 313:178–91. [DOI] [PubMed] [Google Scholar]
- 60.Lanteri-Minet M, Isnardon P, de Pommery J, Menetrey D. Spinal and hindbrain structures involved in visceroception and visceronociception as revealed by the expression of Fos, Jun and Krox-24 proteins. Neuroscience 1993; 55:737–53. [DOI] [PubMed] [Google Scholar]
- 61.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998; 45:1–8. [DOI] [PubMed] [Google Scholar]
- 62.Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989; 12:459–62. [DOI] [PubMed] [Google Scholar]
- 63.Gogas KR, Presley RW, Levine JD, Basbaum AI. The antinociceptive action of supraspinal opioids results from an increase in descending inhibitory control: correlation of nociceptive behavior and c-fos expression. Neuroscience. 1991; 42:617–28. [DOI] [PubMed] [Google Scholar]
- 64.Buritova J, Honoré P, Besson JM. Indomethacin reduces both Krox-24 expression in the rat lumbar spinal cord and inflammatory signs following intraplantar carrageenan. Brain Res. 1995; 674:211–20. [DOI] [PubMed] [Google Scholar]
- 65.Pagano RL, Assis DV, Clara JA, Alves AS, Dale CS, Teixeira MJ, et al. Transdural motor cortex stimulation reverses neuropathic pain in rats: a profile of neuronal activation. Eur J Pain. 2011; 15:268.e1–14. 10.1016/j.ejpain.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 66.Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain. 2012; 153:2359–69. 10.1016/j.pain.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 67.França NR, Toniolo EF, Franciosi AC, Alves AS, de Andrade DC, Fonoff ET, et al. Antinociception induced by motor cortex stimulation: somatotopy of behavioral response and profile of neuronal activation. Behav Brain Res. 2013; 250:211–21. 10.1016/j.bbr.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 68.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16:109–10. [DOI] [PubMed] [Google Scholar]
- 69.Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006; 47:1216–24. [DOI] [PubMed] [Google Scholar]
- 70.Fonoff ET, Pereira JP Jr, Camargo LV, Dale CS, Pagano RL, Ballester G, et al. Functional mapping of the motor cortex of rat using transdural electrical stimulation. Behav Brain Res. 2009; 202:138–41. 10.1016/j.bbr.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 71.D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharm Exp Ther. 1941; 72:9. [Google Scholar]
- 72.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957; 111:409–19. [PubMed] [Google Scholar]
- 73.Paxinos GW, Watson C. The rat brain in stereotaxic coordinates San Diego: Academic Press [Google Scholar]
- 74.Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984; 230:133–41. [DOI] [PubMed] [Google Scholar]
- 75.Canavero S, Bonicalzi V. Cortical stimulation for central pain. Journal of Neurosurgery 1995;83:1117 [DOI] [PubMed] [Google Scholar]
- 76.Nguyen JP, Lefaucheur JP, Decq P, Uchiyama T, Carpentier A, Fontaine D, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999; 82:245–51. 2005. [DOI] [PubMed] [Google Scholar]
- 77.Wachter D, Wrede A, Schulz-Schaeffer W, Taghizadeh-Waghefi A, Nitsche MA, Kutschenko A, et al. Transcranial direct current stimulation induces polarity-specific changes of cortical blood perfusion in the rat. Exp Neurol. 2011; 227:322–7. 10.1016/j.expneurol.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 78.Mielke D, Wrede A, Schulz-Schaeffer W, Taghizadeh-Waghefi A, Nitsche MA, Rohde V, et al. Cathodal transcranial direct current stimulation induces regional, long-lasting reductions of cortical blood flow in rats. Neurol Res. 2013; 35:1029–37. 10.1179/1743132813Y.0000000248 [DOI] [PubMed] [Google Scholar]
- 79.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurons in the rat. Pain. 1979; 6:283–304. [DOI] [PubMed] [Google Scholar]
- 80.Dickenson AH, Le Bars D. Diffuse noxious inhibitory controls (DNIC) involve trigeminothalamic and spinothalamic neurons in the rat. Exp Brain Res. 1983; 49:174–80. [DOI] [PubMed] [Google Scholar]
- 81.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978; 4:451–62. [DOI] [PubMed] [Google Scholar]
- 82.Millan MJ. Descending control of pain. Prog Neurobiol. 2002; 66:355–74. [DOI] [PubMed] [Google Scholar]
- 83.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009; 60:214–25. 10.1016/j.brainresrev.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994; 17:379–89. [DOI] [PubMed] [Google Scholar]
- 85.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995; 46:575–605. [DOI] [PubMed] [Google Scholar]
- 86.Keay KA, Bandler R. Distinct central representations of inescapable and escapable pain: observations and speculation. Exp Physiol. 2002; 87:275–9. [DOI] [PubMed] [Google Scholar]
- 87.Benarroch EE. Periaqueductal gray: an interface for behavioral control. Neurology. 2012; 78:210–7. 10.1212/WNL.0b013e31823fcdee [DOI] [PubMed] [Google Scholar]
- 88.Fonoff ET, Dale CS, Pagano RL, Paccola CC, Ballester G, Teixeira MJ, et al. Antinociception induced by epidural motor cortex stimulation in naive conscious rats is mediated by the opioid system. Behav Brain Res. 2009; 196:63–70. 10.1016/j.bbr.2008.07.027 [DOI] [PubMed] [Google Scholar]
- 89.de Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids. Pain. 2011; 152:320–6. 10.1016/j.pain.2010.10.032 [DOI] [PubMed] [Google Scholar]
- 90.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain. 2007; 127:183–94. [DOI] [PubMed] [Google Scholar]
- 91.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain. 2013; 154:2563–8. 10.1016/j.pain.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 92.Hökfelt T, Elde R, Johansson O, Terenius L, Stein L. The distribution of enkephalin-immunoreactive neuron bodies in the rat central nervous system. Neurosci Lett. 1977, 5:25–31. [DOI] [PubMed] [Google Scholar]
- 93.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984; 7:309–38. [DOI] [PubMed] [Google Scholar]
- 94.Hökfelt T, Terenius L, Kuypers HG, Dann O. Evidence for enkephalin immunoreactive neurons in the medulla oblongata projecting to the spinal cord. Neurosci Lett. 1979; 14:55–60. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Zhao S, Rodriguez E, Takatoh J, Han BX, Zhou X, et al. Identifying local and descending inputs for primary sensory neurons. J Clin Invest. 2015; 125:3782–94. 10.1172/JCI81156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bowman BR, Goodchild AK. GABA and enkephalin tonically alter sympathetic outflows in the rat spinal cord. Auton Neurosci. 2015; 193:84–91. 10.1016/j.autneu.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 97.Senba E, Shiosaka S, Hara Y, Inagaki S, Sakanaka M, Takatsuki K, et al. Ontogeny of the peptidergic system in the rat spinal cord: immunohistochemical analysis. J Comp Neurol. 1982; 208:54–66. [DOI] [PubMed] [Google Scholar]
- 98.Pohl M, Collin E, Bourgoin S, Conrath M, Benoliel JJ, Nevo I, et al. Expression of preproenkephalin A gene and presence of Met-enkephalin in dorsal root ganglia of the adult rat. J. Neurochem. 1994; 63:1226–34. [DOI] [PubMed] [Google Scholar]
- 99.Bennett GJ, Ruda MA, Gobel S, Dubner R. Enkephalin immunoreactive stalked neurons and lamina IIb islet neurons in cat substantia gelatinosa. Brain Res. 1982; 240:162–6. [DOI] [PubMed] [Google Scholar]
- 100.Ruda MA, Bennett GJ, Dubner R. Neurochemistry and neural circuitry in the dorsal horn. Prog Brain Res. 1986; 66:219–68. [DOI] [PubMed] [Google Scholar]
- 101.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, et al. Pharmacological characterization of the cloned κ-, δ-, and μ-opioid receptors. Molecular Pharmacology. 1993; 45:330–4. [PubMed] [Google Scholar]
- 102.Beaudry H, Dubois D, Gendron L. Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci. 2011; 31:13068–77. 10.1523/JNEUROSCI.1817-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruda MA. Opiates and pain pathways: demonstration of enkephalin synapses on dorsal horn projection neurons. Science. 1982; 215:1523–5. [DOI] [PubMed] [Google Scholar]
- 104.Basbaum AI, Levine JD. Opiate analgesia. How central is a peripheral target? N Engl J Med. 1991; 325:1168–9. [DOI] [PubMed] [Google Scholar]
- 105.George A, Marziniak M, Schäfers M, Toyka KV, Sommer C. Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain. 2000; 88:267–75. [DOI] [PubMed] [Google Scholar]
- 106.Buritova J, Le Guen S, Fournié-Zaluski MC, Roques BP, Besson JM. Antinociceptive effects of RB101(S), a complete inhibitor of enkephalin-catabolizing enzymes, are enhanced by (+)-HA966, a functional NMDA receptor antagonist: a c-Fos study in the rat spinal cord. Eur J Pain. 2003; 7:241–9. [DOI] [PubMed] [Google Scholar]
- 107.Giuliani A, Fernandez M, Farinelli M, Baratto L, Capra R, Rovetta G, et al. Very low level laser therapy attenuates edema and pain in experimental models. Int J Tissue React. 2004; 26:29–37. [PubMed] [Google Scholar]
- 108.Song B, Chen W, Marvizon JC. Inhibition of opioid release in the rat spinal cord by serotonin 5-HT(1A) receptors. Brain Research. 2007;1158:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen W, Song B, Marvizón JC. Inhibition of opioid release in the rat spinal cord by alpha2C adrenergic receptors. Neuropharmacology. 2008;54:944–53. 10.1016/j.neuropharm.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu RJ, Zhang RX, Qiao JT, Dafny N. Interrelations of opioids with monoamines in descending inhibition of nociceptive transmission at the spinal level: an immunocytochemical study. Brain Res. 1999; 830:183–90. [DOI] [PubMed] [Google Scholar]
- 111.Ma J, Qiao JT, Dafny N. Opiate-like substances mediate norepinephrine-induced but not serotonin-induced antinociception at spinal level: reevaluation by an electrophysiological model of formalin test in rats. Life Sci. 2001; 69:969–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The epicranial electrode was fixed onto the skull over the left primary motor cortex (1 mm caudal to bregma; 1 mm left to midline). Before DCS, the epicranial jacket was filled with a conductive gel and the cathode was inserted. The animals were dressed in vests that placed a large rubber plate on the ventral thorax, which served as the counter electrode (anode). The electrodes were connected to an electrical stimulator with wire leads, which allowed the animals to move freely in the cage. Afterward, rats received a single DCS session of 15 minutes.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.