Abstract
Aim:
Immunoglobulin replacement therapy is required to reduce the frequency and severity of infections in patients with primary antibody deficiencies. Immunoglobulin G (IgG) can be administered intramuscularly, intravenously or subcutaneously. We aimed to evaluate the efficacy, dose adjustment and adverse events in subcutaneous immunoglobulin therapy by retrospectively presenting the records of 16 patients who received subcutaneous immunoglobulin therapy.
Material and Methods:
The demographic findings, clinical and laboratory findings, subcutaneous immunoglobulin dosage and dose frequency, infusion time, area and methods, adverse events and frequency of infections were obtained from patient files and recorded.
Results:
Sixteen patients (seven female, nine male) aged between 0–33 years who were diagnosed with primary immune deficiency and treated with subcutaneous immunoglobulin were enrolled. All patients had been receiving intravenous imunoglobulin (5–10%) at a dose of 0.33–1.25 gr/kg/dose with two-four week intervals before subcutaneous immunoglobulin. Subcutaneous immunoglobulin (10%) was administered at a dose of 0.03–0.43 gr/kg/dose with one-two week intervals. No significant difference was found between serum through IgG levels before administration of intravenous imunoglobulin and steady state IgG levels during subcutaneous immunoglobulin therapy. When five patients whose serum through IgG levels were below 600 mg/dL were evaluated, however, a significant increase was found in steady state IgG levels with subcutaneous immunoglobulin therapy (p=0.043). In a ten-month follow-up period, seven infections were observed in four patients (three upper respiratory infectons, two lower respiratory tract infections and three acute gastroenteritis). No acute severe bacterial infection was observed. Local advers reaction was reported in only 10 of 180 infusions (6%). No serious adverse events were reported. All 16 patients were willing to continue IgG replacement therapy by subcutaneous administration.
Conclusions:
Ig replacement therapy by subcutaneous route is an efficient, safe and easy option which is eligible for individual administration. Home therapy is feasible for patients with primary immune deficiency, if informed consent is obtained and sufficient education is ensured.
Keywords: Child, primary immune deficiency, subcutaneous immunoglobulin
Introduction
Primary antibody deficiencies are characterized with disruption in production or function of immunoglobulin (Ig) and tendency to infections (1). Respiratory and gastrointestinal system infections are typical findings. Life-long IgG replacement therapy may be needed to be protected from infections (2–4). IgG may be given by the intramuscular, intravenous and subcutaneous routes. Intravenous immunoglobulin (IVIG) is an efficient therapy which can be administered at a dose of 0.3–0.8 g/kg every 2–4 weeks (5, 6). However, it is a significant disadvantage that this therapy necessitates a healthcare center, nurse and vascular access and constitutes a risk in terms of systemic side effects (6). In addition, reduction in the trough serum IgG level between infusions increases both the risk of infection and leads to malaise, fatigue and flu-like symptoms in some patients (6). In subcutaneous IgG (SCIG) replacement treatment, the monthly IVIG dose may be given in divided doses with more frequent intervals (7). The usual application is administration in about two hours by infusion pump (6). The drug may be given into more than one sites during an application. Another alternative of subcutaneous IgG administration is subcutaneous rapid injection by way of butterfly needle without using pump (8). Depending on the method selected and recurrent doses, the highest amount of fluid and the shortest time are flexible in SCIG administration. The advantages of subcutaneous IgG treatment include safety (few side effects), absence of necessity of vascular access, easy use at home, more stable serum IgG levels and efficient prevention against infections (9). Although local complaints arising from infusion are frequent, they are mild and tend to decrease with recurrent infusions (6).
Here, the data of 16 patients in whom IgG replacement treatment was administered subcutaneously for the first time for primary immune deficiency in our country as a pilot application are presented retrospectively and discussed in accompaniment of the literature.
Material and Methods
Data Collection:
The medical records of 16 patients who were being followed up because of primary immune deficiency in our Pediatric Allergy and Immunology Clinic and received subcutaneous immunoglobulin treatment were retrospectively evaluated. Approval was obtained from the ethics committee of Marmara University for this evaluation (number: 09.2015.153).
The demographic, clinical and laboratory findings included in the patient files were recorded in the data follow-up questionnaire. The follow-up period was 6 months-30 years and the diagnoses of the patients were rearranged according to the classification of the International Immune Deficiency Association updated in 2014 (10). In addition, the subcutaneous immunoglobulin dose and dose interval, duration, site and method of administration and side effects were recorded. A short questionnaire evaluating the patient’s satisfaction was applied additionally.
Subcutaneous administration:
The SCIG dose for the patients who previously used IVIG was calculated as about 1.37-fold of the IVIG doses. Afterwards, the official reports and prescriptions for the 10% immunoglobulin product approved for subcutaneous administration (Gamunex-C %10 IV/SC vial containing solution for injection, Dem İlaç San. Tic. Ltd. Şti. Istanbul) were prepared. After the patients were informed, verbal and written informed consent was obtained related with use of parenteral blood product and education was given to the patients and parents. The first subcutaneous administration was performed in the hospital under a nurse’s supervision (1.5–2.5 hours) at least two sites (upper arm, thigh or abdomen) with 25–50 mL for each site using 6 mm needle (Medtronic Quick-set, Medtronic MiniMed, USAD), infusion pump (Syringe Pump, SN-50F6, Sino Medical-Device Technology Co., Ltd., the people’s republic of China) and set. The following doses were administered at home. Vital findings and complaints if present were recorded before and after administration.
Statistical assessment:
All questionnaire data were analyzed using SPSS (Statistical Package for the Social Sciences) version 16.0 (SPSS Inc.; Chicago, IL, USA). The values were expressed as mean ±standard deviation (the lowest-the highest). The data were marked as scales for arithmetical measure and as nominal values for categorical data including present-absent (0–1). Mann Whitney U test was used based on the median values for comparisons of nonparametric data between two independent groups considering the number of patients. Chi-square test was used for comparison of categorical data between two groups and Fisher Exact test was used for investigating significant difference. A p value of <0.05 was considered statistically significant in both methods.
Results
1-. Demographic and clinical properties
The mean age of 16 patients (seven females, nine males) who had a diagnosis of primary immune deficiency was found to be 10.5±10.3 years (median: 7.5 and the lowest-the highest: 0–33 years). The mean body weight of the patients was found to be 28.3±19.8 kg (median: 24.5 and the lowest-the highest: 4–65 kg).
The distribution of the diagnoses was as follows: six combined immune deficiencies, three common variable immune deficiencies, three unclassified hypogammaglobulinemias, two DiGeorge, one Ataxia telangiectasia syndromes and one severe combined immune deficiency. The reason for switching to subcutaneous treatment was personal request in seven patients, difficult vascular access in five patients, systemic side effects with IVIG in two patients and serum IgG trough levels which did not increase because of gastrointestinal loss in two patients.
Fifteen patients were receiving 5–10% IVIG at a dose of 0.33–1.25 g/kg every 2–4 weeks before SCIG. IgG replacement treatment was initiated directly as SCIG in one patient. Subcutaneous IgG was administered at a dose of 0.03–0.43 g/kg every one-two weeks using 10% product. The subcutaneous IgG dose was about 1.44±0.46 –fold higher compared to the IVIG doses.
Here, the data related with 180 SCIG administrations performed in a total period of 10 months (6.1±2.4 months per patient, (median: 6 months and the lowest-the highest: 2–10 months)) are presented. 7±2 gr IgG replacement treatment was performed averagely per administration (median: 5 and the lowest-the highest: 5–10 kg). An interval of 13±6 days was present between two applications (median: 10 days and the lowest-the highest: 7–30 days). The mean number of applications per patient was 12±9 (median: 14 and the lowest-the highest: 1–35).
2-. Efficiency of subcutaneous treatment:
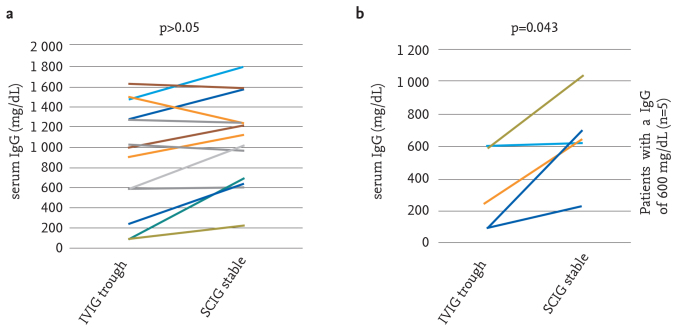
The serum mean trough IgG level before administration of the dose was found to be 976±564 mg/dL (median: 1 000 and the lowest-the highest: 90–1950 mg/dL) in administration of intravenous IgG and the mean stable serum IgG level was found to be 1 025±409 mg/dL (median: 1074 and the lowest-the highest: 230–1 791 mg/dL) during SCIG treatment (p>0.05). When five patients whose serum trough IgG levels were <600 mg/dL were evaluated, however, it was found that the serum mean trough IgG level before administration of the dose was found to be 322±251 mg/dL (median: 241 and the lowest-the highest: 90–598 mg/dL) in administration of IVIG and the mean stable serum IgG level was found to be 643±283 mg/dL (median: 647 and the lowest-the highest: 230–1028 mg/dL) during SCIG treatment (p=0.043). These data are presented in figure 1a and 1b, respectively. An example on a single patient can be given as follows: the serum trough IgG level was found to be 280 mg/dL while receiving IVIG at a dose of 0.8 g/kg/every 2 weeks, while the serum stable IgG level increased gradually with SCIG treatment and measured to be 1040 mg/dL in the 8th month of treatment in a patient who was being followed up with a diagnosis of combined immune deficiency and protein losing enteropathy.
Figure 1. a, b.
Comparison of serum trough IgG values and stable IgG values: (a) in all patients, (b) in five patients whose trough IgG values were <600 mg/dL with IVIG
IVIG: Intravenous immunoglobulin
On the other hand, the patients who received the total monthly SCIG dose in three or four divided doses (n=9) and the patients who received the total monthly dose in one or two divided doses (n=7) were compared and no significant difference was found between the two groups in terms of serum stable IgG levels, reaction and frequency of infection. However, the age, body weight and infusion volume were found to be significantly higher in the patients who received the total dose in three of four divided doses (p=0.003, p=0.001, p=0.023, respectively). These data are shown in Table 1.
Table 1.
Findings according to the number of monthly SCIG administration
| SCIG administration/month | p | ||
|---|---|---|---|
|
| |||
| 3–4 times | 1–2 times | ||
| Age (years)* | 16.1±10.3 | 3.3±3.9 | |
| 11 (7–33) | 2 (0–12) | 0.003 | |
| Body weight (kg)* | 40.7±16.7 | 12.3±9.1 | 0.001 |
| 45 (19–65) | 11 (4–30) | ||
| Infusion volume (mL) | 50 | 25 | 0.023 |
The data are presented as mean±standard deviation and median (the lowest-the highest) values. A p value of <0.05 was considered significant in both methods. SCIG: Subcutaneous immunoglobulin
Seven infections were observed in four patients in a follow-up period of ten months (three upper respiratory tract infections, two lower respiratory tract infections and two acute gastroenteritis). Acute severe bacterial infection was not observed in any patient. No significant difference was observed in terms of serum stable IgG levels when the four patients who had infection were compared with the 12 patients who did not have infection (p>0,05). However, the IgG levels tended to be higher in the group who did not have infection (1 157±383 mg/dL, median: 1219 and the lowest-the highest: 615–1 791 mg/dL vs 1 012±569 median: 1 119 and the lowest-the highest: 230–1 580).
3-. Side effects
Subcutaneous IgG infusions were performed in a total of 180 applications in 10 months in the abdomen (n=3 patients), upper arm (n=12 patients) (Figure 2a, b) and thigh (n=12 patients). Side effects were reported only after 11 applications (6%). No side effect was observed in the infusions performed in the abdominal region, whereas pruritus (n=1) and erythema (n=3) were reported in the infusions performed in the thigh and pruritus (n=2), erythema (n=2) and pain (n=2) were reported in the infusions performed in the upper arm. The side effects described were limited only with the application site, whereas hypotension and headache were observed during application in one patient. In the same patient, the indication for SCIG was occurrence of systemic side effect with IVIG. Treatment was interrupted in this patient. On the other hand, systemic side effect was not observed with SCIG in another patient in whom anaphylaxis and/or anaphylactoid reaction was observed with IVIG for more than one time.
Figure 2. a, b.

Example of administration before (a) and after (b) subcutaneous immunoglobulin
4-. Satisfaction questionnaire results
When 16 patients were asked if they wanted to receive IgG replacement treatment at home or in hospital, all reported that they preferred home. While fourteen patients wished that the application be performed with the support of a nurse, two patients reported that they felt competent to perform the application themselves or with the assistance of their relatives. When the reasons for preferring home treatment were interrogated, the common views included feeling oneself better at home, absence of transportation problem, absence of time loss, convenience and being able to receive more education about the application from nurses. No patient wished to continue SCIG replacement treatment with IVIG.
Discussion
In our study, experiences of SCIG administration in 16 patients aged in a range changing from infancy to adulthood with a diagnosis of primary immune deficiency are presented. A tendency to increase in serum stable IgG levels was observed with subcutaneous IgG administration. Although the dose was specially increased, the serum stable IgG levels increased significantly in five patients whose serum trough levels did not increase above 600 mg/dL with IVIG. Acute severe bacterial infection was not observed in any patient in the ten month-follow-up period. Local side effects were reported rarely and tended to disappear with recurrent doses. It was notable that all patients reported they wished to continue their treatments with SCIG administration.
Primary immune deficiencies where immunoglobulin (IgG) replacement treatment is indicated and their levels of evidence have been described in previous publications (2–4). The primary aim of life-long IgG replacement treatment is to prevent organ damage by decreasing the frequency and severity of infection (6, 11, 12). Immunoglobulin replacement treatment can be efficiently administered by intramuscular, intravenous and subcutaneous routes (13, 14). The route of administration of immunoglobulin G (IgG) replacement treatment to be preferred depends on the patient’s individual decision, clinical status and availability of the product and infrastructure for the method selected. In recent years, infusion at home or rapid administration have become preferable methods for SCIG treatment in terms of cost. Replacement treatment is initiated directly with SCIG method in some patients, while some patients use IVIG and then continue with SCIG. IgG replacement treatment has been performed for a long time with rapid SCIG method especially in North European countries (15). Products with proven efficiency prepared at different concentrations (10%, 16%, 20%) convenient for use are available in the world (16). In our country, subcutaneous administration has been included in the instructions of the products with 10% concentration. However, no data related with use of SCIG have been found before the pilot application of our clinic.
Subcutaneous IgG is a safe, efficient and feasible treatment method especially in infants in whom it is difficult to obtain a vascular access, in patients who have had severe allergic side effects with IVIG and in patients with primary immune deficiencies in association with protein losing enteropathy (17). Other than these, SCIG is also administered because of the following factors: living in a place far from hospital, feeling severe malaise before the next IVIG dose, intense work or school life, frequent travelling and personal preference (17). In our clinic, SCIG was administered most commonly because of personal preference, problematic vascular access, gastrointestinal IgG loss and side effects with IVIG.
The dose should be determined after the IVIG and SCIG alternatives are discussed with the patient and the option appropriate for the clinical status is specified. The approaches are different in Europe and America in terms of specifying the monthly SCIG dose. In Europe, the IVIG dose used currently is used subcutaneously (14, 18). On the other hand, the American sources recommend to multiply the current dose with a coefficient which varies by the concentration of the product and which is derived from the area under the curve pharmacokinetically (×1.37 for 16% and 1.53 for 20%) (16). In our country, studies have recommended to use the coefficient 1.37 for the products with 10% concentration (19). In the practice of our clinic, the total monthly dose was established by using the same coefficient. After the monthly dose was established, it was divided into 5 or 10 g portions and the interval and frequency of administration were established. Therefore, administration is performed four times a month in some patients and once a month in some other patents. When these patients were compared, no difference was found in terms of serum stable IgG levels and the frequency of infection and local side effects.
In terms of clinical efficiency, the frequencies of acute severe bacterial infection of the two approaches which did and did not use the coefficient mentioned above were not found to be different (18, 20). The number of other infections was found to be lower when the coefficient was used (21). On the other hand, the characteristic of protection from infection was also a valid principle for administration of IVIG when the IgG replacement dose was increased (22). In a study conducted by Bonagura et al. (23) which compared the two methods, it was advised that the dose could be increased by 10–25% once a month for SCIG independent of the coefficient, when necessary, in order to obtain the individual serum stable IgG level protecting the person from infection. Another recommendation for establishing the dose was specifying the SCIG dose by constant intervals according to the previous IVIG dose and history of infection (24). In this approach, dose recommendation was also offered for replacement treatment with direct SCIG method in newly diagnosed patients. In the light of all these data, it is clear that the biological IgG level which would protect the individual from infection will be ensured by establishing the individual dose by close clinical monitoring independent of use of the coefficient. In our study, acute severe bacterial infection was not observed in the patients who used SCIG. On the other hand, the patients who had other infections tended to have lower serum stable IgG levels compared to the ones who did not have other infections, though the difference was not significant. In these patients, follow-up was continued by increasing the SCIG doses by 10%.
Another marker for evaluation of the clinical efficiency is measurement of stable serum IgG levels. However, an increase up to 20% should be expected in IgG levels, even if the dose used for IVIG administration is administered exactly as SCIG without using any coefficient (16). The reason for this is trough and stable IgG measurements arising from the pharmacokinetic and dynamic differences of the two parenteral applications. In the light of this information, it is clear that serum IgG measurement alone is not a sufficient marker for efficiency. In the patients presented here, a similar insignificant increase in IgG levels was also observed following SCIG. However, clinical follow-up of infections and specifying the severity of infections were the most important determinants for changing the SCIG dose.
Local side effects including pain, erythema and swelling may be observed in the site of administration during subcutaneous infusion. The frequency of complaints vary according to the site selected, time of administration and the volume and concentration used, but they tend to decrease as the administration is continued (25). Decreasing the rate of infusion, increasing the number of the sites, increasing the frequency of infusion, applying local anesthetics, changing the application site, selecting a fatty site, selection of a 9–15 mm and 23–25 G needle in adults and a 4–6 mm and 24–27 G needle in children, reviewing educations, use of hyaluronidase or changing the product have been recommended to decrease these side effects (25). It was found that the complaints were eliminated with these measures in our patients.
Different studies have investigated satisfaction of patients and increase in the quality of life using questionnaires. Especially studies which performed cost analysis reported the positive effects of prevention of school and labor loss in terms of both cost and satisfaction (26). When direct and indirect costs were calculated, it was found that rapid SCIG administration at home was more efficient compared to infusion or administration of IVIG with assistance of a nurse (27). No cost analysis was performed in our clinical observation. However, all patients stated that they wanted to continue their treatments with SCIG method.
Immunoglobulin replacement treatment by way of subcutaneous method is an efficient, safe and easy option which is suitable for individual administration. Subcutaneous immunoglobulin administration can be performed at home for eager patients with primary antibody deficiency who do not have contraindications after necessary training is given and informed consent is obtained. The dose to prevent infection should be established individually and dynamically with evaluation of close clinical monitoring and intermittent measurement of serum stable IgG levels.
Acknowledgments
We all memorialize our mentor Prof Dr Isil Barlan with respect and deep missing who is one of the founders of Marmara University Pediatric Allergy and Immunology Division.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study.
Informed Consent: Written informed consent was not obtained from patients due to the retrospective nature of this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.K.A., A.K., S.B., A.Ö., I.B.; Design - E.K.A., A.K., I.B.; Supervision - S.B., A.Ö.; Materials -E.K.A., A.K.; Data Collection and/or Processing - E.K.A., A.K.; Analysis and/or Interpretation - E.K.A., A.K.; Literature Review - E.K.A., A.K., S.B., A.Ö., I.B.; Writing - E.K.A., A.K., I. B.; Critical Review - S.B., A.Ö., I.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Castigli E, Geha RS. Molecular basis of common variable immunodeficiency. J Allergy Clin Immunol. 2006;117:740–6. doi: 10.1016/j.jaci.2006.01.038. http://dx.doi.org/10.1016/j.jaci.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 2.Chapel HM. Consensus on diagnosis and management of primary antibody deficiencies. Consensus panel for the diagnosis and management of primary antibody deficiencies. BMJ. 1994;308:581–5. doi: 10.1136/bmj.308.6928.581. http://dx.doi.org/10.1136/bmj.308.6928.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Primary immunodeficiency diseases Report of an IUIS Scientific Committee. International Union of Immunological Societies. Clin Exp Immunol. 1999;1:1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Notarangelo L, Casanova JL, Fischer A, et al. International union of immunological societies primary immunodeficiency diseases classification committee. Primary immunodeficiency diseases: an update. J Allergy Clin Immunol. 2004;114:677–87. doi: 10.1016/j.jaci.2007.08.053. http://dx.doi.org/10.1016/j.jaci.2004.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore ML, Quinn JM. Subcutaneous immunoglobulin replacement therapy for primary antibody deficiency: advancements into the 21st century. Ann Allergy Asthma Immunol. 2008;101:114–21. doi: 10.1016/S1081-1206(10)60197-4. http://dx.doi.org/10.1016/S1081-1206(10)60197-4. [DOI] [PubMed] [Google Scholar]
- 6.Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin North Am. 2008;28:413–37. doi: 10.1016/j.iac.2008.01.008. http://dx.doi.org/10.1016/j.iac.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112:1–7. doi: 10.1016/j.clim.2004.02.002. http://dx.doi.org/10.1016/j.clim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol. 2010;30:301–7. doi: 10.1007/s10875-009-9352-2. http://dx.doi.org/10.1007/s10875-009-9352-2. [DOI] [PubMed] [Google Scholar]
- 9.Gardulf A. Immunoglobulin treatment for primary antibody deficiencies: advantages of the subcutaneous route. BioDrugs. 2007;21:105–16. doi: 10.2165/00063030-200721020-00005. http://dx.doi.org/10.2165/00063030-200721020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Al-Herz W, Bousfiha A, Casanova JL, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2014;5:1–33. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Allergy Asthma and Immunology . Eight guiding principles for effective use of IVIG for patients with primary immunodeficiency. USA: Milwaukee; 2011. [Google Scholar]
- 12.Gardulf A, Nicolay U, Math D, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114:936–42. doi: 10.1016/j.jaci.2004.06.053. http://dx.doi.org/10.1016/j.jaci.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Aebersold P. Intravenous immunoglobulins in the 21st century: progress and challenges in efficacy, safety and paths to licensure. Bethesda (MD): FDA Workshop; 2005. [Google Scholar]
- 14.EMEA Committee for Proprietary Medicinal Products (CPMP) Note for guidance on the clinical investigation of human normal immunoglobulin for subcutaneous and intramuscular use (CPMP/BPWG/283/00) London: European Agency for Evaluation of Medicinal Products; 2002. [Google Scholar]
- 15.Šedivá A, Chapel H, Gardulf A, European Immunoglobulin Map Group (35 European Countries) for European Society for Immunodeficiencies (ESID) Primary Immunodeficiencies Care in Development Working Party Europe immunoglobulin map. Clin Exp Immunol. 2014;178:141–3. doi: 10.1111/cei.12546. http://dx.doi.org/10.1111/cei.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger M. Choices in IgG replacement therapy for primary immune deficiency diseases: subcutaneous IgG vs. intravenous IgG and selecting an optimal dose. Curr Opin Allergy Clin Immunol. 2011;11:532–8. doi: 10.1097/ACI.0b013e32834c22da. http://dx.doi.org/10.1097/ACI.0b013e32834c22da. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro RS. Subcutaneous immunoglobulin therapy given by subcutaneous rapid push vs infusion pump: a retrospective analysis. Ann Allergy Asthma Immunol. 2013;111:51–5. doi: 10.1016/j.anai.2013.04.015. http://dx.doi.org/10.1016/j.anai.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies a prospective, multi-national study. J Clin Immunol. 2006;26:177–85. doi: 10.1007/s10875-006-9002-x. http://dx.doi.org/10.1007/s10875-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 19.Wasserman RL, Irani A-M, Tracy J, et al. Pharmacokinetics and safety of subcutaneous immune globulin (human), 10% caprylate/chromatography purified in patients with primary immunodeficiency disease. Clin Exp Immunol. 2010;161:518–26. doi: 10.1111/j.1365-2249.2010.04195.x. http://dx.doi.org/10.1111/j.1365-2249.2010.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M, Subcutaneous IgG Study Group Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–73. doi: 10.1007/s10875-006-9021-7. http://dx.doi.org/10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 21.Haddad E, Berger M, Wang ECY, Jones CA, Bexon M, Baggish JS. Higher Doses of Subcutaneous IgG Reduce Resource Utilization in Patients with Primary Immunodeficiency. J Clin Immunol. 2012;32:281–9. doi: 10.1007/s10875-011-9631-6. http://dx.doi.org/10.1007/s10875-011-9631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orange JS, Belohradsky BH, Berger M, et al. Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy. Clin Exp Immunol. 2012;169:172–81. doi: 10.1111/j.1365-2249.2012.04594.x. http://dx.doi.org/10.1111/j.1365-2249.2012.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008;122:210–2. doi: 10.1016/j.jaci.2008.04.044. http://dx.doi.org/10.1016/j.jaci.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Fadeyi M, Tran T. Calculating the dose of subcutaneous immunoglobulin for primary immunodeficiency disease in patients switched from intravenous to subcutaneous immunoglobulin without the use of a dose-adjustment coefficient. PT. 2013;38:768–70. [PMC free article] [PubMed] [Google Scholar]
- 25.Jolles S, Orange JS, Gardulf A, et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. 2015;179:146–60. doi: 10.1111/cei.12485. http://dx.doi.org/10.1111/cei.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abolhassani H, Sadaghiani MS, Aghamohammadi A, Ochs HD, Rezaei N. Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: systematic review and meta analysis. J Clin Immunol. 2012;32:1180–92. doi: 10.1007/s10875-012-9720-1. http://dx.doi.org/10.1007/s10875-012-9720-1. [DOI] [PubMed] [Google Scholar]
- 27.Beauté J, Levy P, Millet V, et al. Economic evaluation of immunoglobulin replacement in patients with primary antibody deficiencies. Clin Exp Immunol. 2010;160:240–5. doi: 10.1111/j.1365-2249.2009.04079.x. http://dx.doi.org/10.1111/j.1365-2249.2009.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]