Abstract
Background
Sulfonylureas are an effective and inexpensive treatment for type 2 diabetes. There is conflicting data about the safety of these drugs regarding mortality and cardiovascular outcomes. The objective of the present study was to evaluate the safety of the sulfonylureas most frequently used and to use trial sequential analysis (TSA) to analyze whether the available sample was powered enough to support the results.
Methods and Findings
Electronic databases were reviewed from 1946 (Embase) or 1966 (MEDLINE) up to 31 December 2014. Randomized clinical trials (RCTs) of at least 52 wk in duration evaluating second- or third-generation sulfonylureas in the treatment of adults with type 2 diabetes and reporting outcomes of interest were included. Primary outcomes were all-cause and cardiovascular mortality. Additionally, myocardial infarction and stroke events were evaluated. Data were summarized with Peto odds ratios (ORs), and the reliability of the results was evaluated with TSA. Forty-seven RCTs with 37,650 patients and 890 deaths in total were included. Sulfonylureas were not associated with all-cause (OR 1.12 [95% CI 0.96 to 1.30]) or cardiovascular mortality (OR 1.12 [95% CI 0.87 to 1.42]). Sulfonylureas were also not associated with increased risk of myocardial infarction (OR 0.92 [95% CI 0.76 to 1.12]) or stroke (OR 1.16 [95% CI 0.81 to 1.66]). TSA could discard an absolute difference of 0.5% between the treatments, which was considered the minimal clinically significant difference. The major limitation of this review was the inclusion of studies not designed to evaluate safety outcomes.
Conclusions
Sulfonylureas are not associated with increased risk for all-cause mortality, cardiovascular mortality, myocardial infarction, or stroke. Current evidence supports the safety of sulfonylureas; an absolute risk of 0.5% could be firmly discarded.
Review registration
PROSPERO CRD42014004330
In a meta-analysis of randomized clinical trials, Dimitris Rados and colleagues find that sulfonylurea use is not associated with an increased risk of all-cause or cardiovascular mortality.
Editors' Summary
Background
Worldwide, more than 400,000 people have diabetes, a chronic condition characterized by poor glycemic control—dangerously high levels of glucose (sugar) in the blood (hyperglycemia). Blood sugar levels are usually controlled by insulin, a hormone released by beta cells in the pancreas after meals (glucose levels in the blood increase when food is digested and glucose is absorbed). In people with type 2 diabetes (the most common type of diabetes), blood sugar control fails because the fat and muscle cells that normally respond to insulin by removing excess sugar from the blood become resistant to insulin. Type 2 diabetes can often be controlled initially with diet and exercise and with antidiabetic drugs such as metformin (which suppresses glucose production by the liver) and sulfonylureas (which stimulate the secretion of insulin by the pancreas). However, as the disease progresses, the pancreatic beta cells become impaired, and many patients eventually need insulin injections to prevent hyperglycemia. Long-term complications of diabetes, which include an increased risk of cardiovascular problems such as heart attacks (myocardial infarctions) and stroke, reduce the life expectancy of people with diabetes by about ten years compared to people without diabetes.
Why Was This Study Done?
Sulfonylureas have been used for decades to improve glycemic control in people with diabetes, but doubts about their safety were first raised in 1970. Since then, there have been conflicting reports about whether these inexpensive but effective drugs are associated with an increased risk of cardiovascular events and death. Because at least 20%–30% of people with diabetes in high-income countries take second- or third-generation sulfonylureas, such as glipizide and glimepiride, it is important to know whether sulfonylurea use increases the risk of a cardiovascular event or death by even a small amount. Here, the researchers evaluate the safety of the most widely used sulfonylureas by undertaking a systematic review and meta-analysis, with trial sequential analysis, of randomized clinical trials (RCTs) that evaluated second- or third-generation sulfonylureas for the treatment of adults with type 2 diabetes. A systematic review uses predefined criteria to identify all the research on a given topic, a meta-analysis combines the results of several trials, and trial sequential analysis is used to establish whether the sample size of a meta-analysis is sufficiently large to reach firm conclusions about the effect of interventions.
What Did the Researchers Do and Find?
The researchers identified 47 RCTs that met their criteria for inclusion in their study. In total, these RCTs involved 37,650 patients, 890 of whom died during follow-up. Meta-analysis of the results of these trials indicated that sulfonylurea use was not associated with an increased risk of all-cause mortality (death) or cardiovascular mortality. Moreover, sulfonylurea use was not associated with an increased risk of myocardial infarction or stroke. Trial sequential analysis indicated that the sample size in the meta-analysis was large enough that sufficient information was included in the analysis to conclude that fewer than one in 200 patients were likely to have been harmed by the use of sulfonylureas. That is, trial sequential analysis excluded the possibility that—compared to placebo (dummy drug), diet, or an active comparator drug—sulfonylurea use was associated with more than one death (or major cardiovascular event) in every 200 treated patients, which the researchers defined as the minimal clinically significant difference. Importantly, this finding did not change when sulfonylureas were used as an add-on to metformin treatment.
What Do These Findings Mean?
These findings suggest that sulfonylureas are not associated with a clinically significant increased risk for all-cause mortality, cardiovascular mortality, myocardial infarction, or stroke. The accuracy of these findings may be affected by some aspects of the researchers’ analyses, such as the inclusion of studies in their meta-analysis that were not designed to evaluate safety outcomes. Moreover, because this study was not designed to compare different sulfonylureas, further studies are needed to evaluate whether all second- and third-generation sulfonylureas are associated with similar all-cause and cardiovascular mortality risks. Overall, however, these findings are reassuring, and the researchers conclude that sulfonylureas should continue to be used in patients with type 2 diabetes provided their efficacy in controlling hyperglycemia outweighs the risks of weight gain and hypoglycemia (low blood sugar) that are known to be associated with these drugs.
Additional Information
This list of resources contains links that can be accessed when viewing the PDF on a device or via the online version of the article at http://dx.doi.org/10.1371/journal.pmed.1001992.
The US National Institute of Diabetes and Digestive and Kidney Diseases provides information about diabetes for patients, health-care professionals, and the general public, including information on treatments for diabetes (in English and Spanish)
The UK National Health Service Choices website provides information for patients and carers about type 2 diabetes, about treatments for type 2 diabetes, and about living with diabetes; it also provides people’s stories about diabetes
The charity Diabetes UK provides detailed information for patients and carers in several languages, including information on treatments for diabetes
The UK-based non-profit organization Healthtalkonline has interviews with people about their experiences of diabetes
MedlinePlus provides links to further resources and advice about diabetes and about medicines for diabetes; it also provides information about metformin and about glipizide, glimepiride, and other sulfonylurea drugs (in English and Spanish)
More information about this study is available from the PROSPERO International Prospective Register of Systematic Reviews; information about trial sequential analysis is also available
Introduction
Sulfonylureas are still used frequently in the treatment of patients with type 2 diabetes because they are effective in both improving glycemic control [1] and reducing the microvascular complications of diabetes [2]; in addition, they have the advantage of being inexpensive [3].
There are concerns regarding the safety of sulfonylureas that have persisted from the first randomized clinical trial (RCT) that evaluated sulfonylureas for diabetes treatment (University Group Diabetes Program) [4] until the present time [5–7]. In countries where first-generation sulfonylureas are still in use, they represent only 3% of all oral antihyperglycemic drug prescriptions [8]. Instead, second- and third-generation sulfonylureas are widely used, and it is estimated that 20%–30% of patients with diabetes in developed countries are on sulfonylureas [9,10]. Moreover, a higher proportion (40%–50%) of patients on such treatment have been described in recent multinational cardiovascular studies [11–13].
Observational studies have reported conflicting results regarding sulfonylurea safety [8,14–16], some of them disclosing an association of sulfonylurea use with increased risk of cardiovascular events [8,15]. However, observational studies have limitations because of selection and attrition bias, and from the results one can infer only association, and not causation [17]. There is still a current and intense debate surrounding these safety issues [5,6].
Recent meta-analyses evaluating the safety of sulfonylureas as a group [18–21] or in association with metformin [22] also reported contradictory results. Probably, this was due to the inclusion of observational studies [21,22], the inclusion of first-generation sulfonylureas [19,20], and the lack of evaluation of the risk of type II error [18,20,21]. Analyses that included second- or third-generation sulfonylureas did not report higher risk of mortality or cardiovascular events [18–21].
When dealing with negative results, it is important to evaluate the statistical reliability of the finding, i.e., the power of the analysis. Trial sequential analysis (TSA) is a tool that is increasingly being used [23] to assess whether optimal sample sizes—and benefit or harm boundaries—have been reached by an available sample of patients assuming a minimal clinically significant difference [24]. It has the potential to increase data reliability [24], and its use might be of great benefit in determining whether the currently evaluable evidence about the safety of sulfonylureas is enough to discard falsely positive or negative conclusions [25].
Therefore, the aim of this study was to evaluate the safety of second- and third-generation sulfonylurea use in patients with type 2 diabetes in terms of all-cause and cardiovascular mortality and cardiovascular events (myocardial infarction and stroke), and to quantify the statistical reliability of available data.
Methods
Protocol and Registration
We conducted this study using a preconceived protocol according to Cochrane Collaboration recommendations [26] and registered it in the PROSPERO registry (CRD42014004330). This report follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [27].
The ethical committee from the research board of Hospital de Clínicas de Porto Alegre exempts systematic reviews from ethical approval.
Data Sources and Searches
The present study was intended to evaluate the overall safety of the most frequently used sulfonylureas (both second and third generation) in type 2 diabetes through a review of RCTs. Therefore, the search strategy included the terms “type 2 diabetes” and “sulfonylureas” and used the recommended, highly sensitive Cochrane Collaboration strategy for RCT systematic reviews [26]. No outcome or comparator was added to the search terms.
We searched the online databases of MEDLINE (through PubMed), Embase, and the Cochrane Library, as well as conducting a manual review of reference lists of published studies from 1946 (Embase) and 1966 (MEDLINE) up to 31 December 2014. The terms used for searching PubMed are described in S1 Table. We also searched the ClinicalTrials.org registry and the 2014 abstract books of international diabetes meetings (American Diabetes Association and European Association for the Study of Diabetes) for unpublished studies. No time period restrictions were made. All potentially eligible studies were considered for review, limited to the English, Spanish, German, French, Japanese, and Portuguese languages. Three studies were written in languages other than these and were excluded [28–30].
Study Selection
We included RCTs that evaluated patients with type 2 diabetes who were randomized to receive a second- or third-generation sulfonylurea for at least 52 wk and that reported all-cause or cardiovascular mortality, myocardial infarction, or stroke data. As most of the studies were not specifically designed to evaluate these outcomes, absence of information was frequently observed. In these cases, we attempted to contact the corresponding authors before excluding any study due to lack of data.
We excluded studies comparing sulfonylureas with troglitazone as this medication was withdrawn from the market due to safety issues and is not currently available for clinical use; as rosiglitazone and pioglitazone are still available in some countries, they were included in the analyses. Duplicate reports and extensions of RCTs were also not considered for this review.
Data Extraction
Two investigators (D. V. R. and L. C. P.) independently evaluated the titles and abstracts of the articles retrieved using the search approach. Abstracts that did not meet the inclusion criteria or that met exclusion criteria were discarded. We selected the remaining studies for full-text evaluation and data extraction. Any disagreements regarding the inclusion or exclusion of a study were solved by consensus, and, if doubt persisted, a third reviewer (C. B. L) evaluated the reference.
We used a standardized form to extract the following details from retrieved studies: first author’s name, publication year and journal, study characteristics (comparator, co-intervention), patient characteristics (mean age, proportion of men/women, and proportion of patients with hypertension, with dyslipidemia, and who were active smokers), study methodology (intervention dosage, frequency, and duration), number of patients included and lost to follow-up, and number of patients with outcomes of interest (all-cause death, cardiovascular death, myocardial infarction, and stroke).
Quality Assessment
We assessed the included studies in six domains according to the Cochrane Collaboration’s tool for assessing risk of bias [26,31]: (i) random sequence generation, (ii) allocation concealment, (iii) blinding, (iv) incomplete outcome data, (v) selective reporting, and (vi) other bias; for other bias, we evaluated whether the study was conducted with funding support from the pharmaceutical industry. We evaluated the quality of the evidence for each meta-analysis using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The quality of evidence was classified as “high,” “moderate,” “low,” or “very low.”
Limitations of design or implementation (risk of bias), indirectness of evidence, inexplicable heterogeneity, inconsistent results, and presence of significant publication bias were assessed for each outcome and, if present, decreased the quality ranking of the results for that outcome. The following items were considered to increase the quality of the evidence: a large magnitude of effect, the presence of a dose–response gradient, and if plausible biases worked to decrease the confidence of the finding [32].
Data Synthesis and Analysis
We compared the outcomes of interest in patients treated with sulfonylureas versus a control group (diet, placebo, or other antihyperglycemic medication). We also performed a meta-analysis separating the controls in classes (diet or placebo and active comparators). We also assessed the use of sulfonylureas as first-line treatment (monotherapy), second-line treatment (in addition to some other medication), or unspecified treatment (when the study did not specify the line of treatment as an inclusion criterion). Because sulfonylureas are commonly used as a second agent in addition to metformin [1,33,34], we also assessed the effects of sulfonylureas when used as an add-on to metformin. We also did exploratory meta-analyses for each sulfonylurea (glibenclamide, glimepiride, glipizide, and gliclazide).
As recommended [26], if a study had more than two intervention groups using different comparators (e.g., rosiglitazone versus metformin versus sulfonylurea), we split the sulfonylurea group sample into two or more groups to avoid falsely increasing the sample size and thereby maintaining the randomization [26].
To evaluate whether the present meta-analysis had sufficient sample size to reach firm conclusions about the effect of interventions [24,25], we performed TSA for the major outcomes. Traditionally, interim analysis of a single trial evaluates whether the monitoring boundaries for a predefined estimated effect are reached before the whole trial population (optimal sample size) has been accrued [24,25]. Similarly, TSA performs a cumulative meta-analysis, which creates a Z curve of the summarized observed effect (the cumulative number of included patients and events) and the monitoring boundaries for benefit, harm, and futility, and it estimates the optimal sample size [24,25]. These boundaries and analyses are adjusted to account for the amount of available evidence and to control for repeated analyses, while maintaining type I error at 5% and the power at 80% [24,25]. Therefore, they are initially very wide, but as more information (trials, patients, and events) is included, they become narrower, converging to the unadjusted significance interval. If the Z curve of the cumulative meta-analysis crosses one of the boundaries, no further studies are required, and there is sufficient information to support the conclusions. Most importantly, when evaluating treatments that are expected to be not different, the futility boundary allows identification of the “no effect area” as early as possible. As the required number of observations (patients, events) is available, the Z curve crosses the futility boundary and identifies that further randomization is not necessary and that it can be affirmed that the intervention does not have the established effect [24,25]. We performed an initial analysis to evaluate the heterogeneity (I 2)–adjusted optimal sample size for confirming or discarding a harm of an absolute difference between groups of 0.5%, which would lead to a number needed to harm (NNH) of 200 patients.
The current study deals with rare event data and with studies reporting zero events in both arms (double-zero studies). Usual methods (Mantel–Haenszel odds ratio [OR]) used to summarize and aggregate dichotomous variables do not perform as expected in meta-analysis of rare events, and the risk of finding false positives is increased [26,35,36]. Therefore, the studies were summarized using the Peto OR method. This method seems to be better suited to these situations, especially when the incidence of events is near 1% and the effects of intervention are of a small magnitude [36]. As a sensitivity analysis, we performed the analysis with Mantel–Haenszel ORs.
The Peto OR method is not able to use the information from double-zero studies, and these studies are therefore excluded from the analysis. In this setting, it is suggested that a sensitivity analysis with continuity correction be performed [37]. However, TSA software does include double-zero trials in the analysis, using empirical continuity correction. This is performed by adding a constant in the number of events and non-events in both treatment arms. This constant is calculated for each trial and each arm, and this calculation is based on the OR of the meta-analysis (without the double-zero studies to be corrected) and the randomization ratio of the study that needs the empirical continuity correction [25]. Therefore, although our forest plots were constructed using Peto OR analysis (double-zero studies not plotted), double-zero studies were included in the TSA analysis and graphics.
We evaluated the heterogeneity using a Cochran Q test, with a threshold p-value of 0.1, and an I 2 test, with a value > 50% indicating high heterogeneity; 95% confidence intervals for I 2 values were calculated. We also analyzed heterogeneity by using τ2 (recommended for small events meta-analyses) [38].
We assessed small study bias by using a contour-enhanced funnel plot, and asymmetry by using Begg and Egger tests. A significant bias was considered if p < 0.10. A trim-and-fill computation was used to estimate the effect of missing studies on the interpretation of results.
The main analyses were conducted using Stata version 12.0 (StataCorp) and RevMan version 5.3 (Cochrane Collaboration). The Begg and Egger tests and the trim-and-fill tests were conducted using Stata version 12.0. The empirical continuity correction and TSA were conducted using TSA software version 0.9 (beta) (Copenhagen Trial Unit).
Results
Literature Search
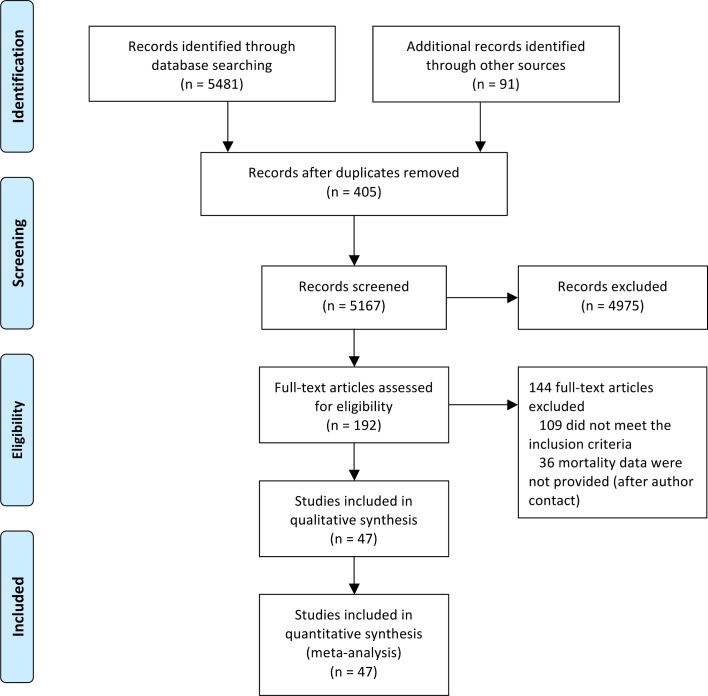
We identified 5,572 studies through both the literature and manual searches (Fig 1). After excluding duplicate references and reviewing titles and abstracts, we selected 192 references for full-text evaluation. Of these, 109 trials either did not meet the inclusion criteria or met the exclusion criteria. The main reasons for exclusion were short duration (40 references, 37%), duplicated records (24 references, 22%), and non-randomized study (17 references, 15%). In addition, 36 studies did not report outcome data. Contact information for authors was available from 28 of these studies (19 different authors). Five authors answered our request, but none of them provided additional data. These 36 studies with missing information represented only 10% of the total patient sample. The reviewers had a high agreement rate (κ = 0.917). The final number of studies included was 47 (with 55 pairwise comparisons) [2,39–84], representing 37,650 patients (16,037 randomized to sulfonylureas and 21,613 to comparators). There were 890 all-cause deaths, 354 cardiovascular deaths, 589 myocardial infarctions, and 275 strokes.
Fig 1. Study flowchart.
Study Characteristics and Risk of Bias
The included trials were published from 1986 to 2014. The duration of trials varied from 12 to 133 mo. The mean age of the patient population was 57.3 y, and mean baseline HbA1c was 7.2% (minimum 6.8%, maximum 12.2%). Most studies compared sulfonylureas with an active control group. Detailed information about included studies is provided in S2 Table.
We present details regarding the assessment of quality for individual studies and across studies in S1 and S2 Figs. Whether studies used random sequence generation, allocation concealment, and blinding of outcome assessment was unclear in most studies; most studies were considered to be at low risk of bias for the domains blinding of participants and personnel, incomplete outcome data, and selective reporting.
Sulfonylureas and All-Cause and Cardiovascular Mortality
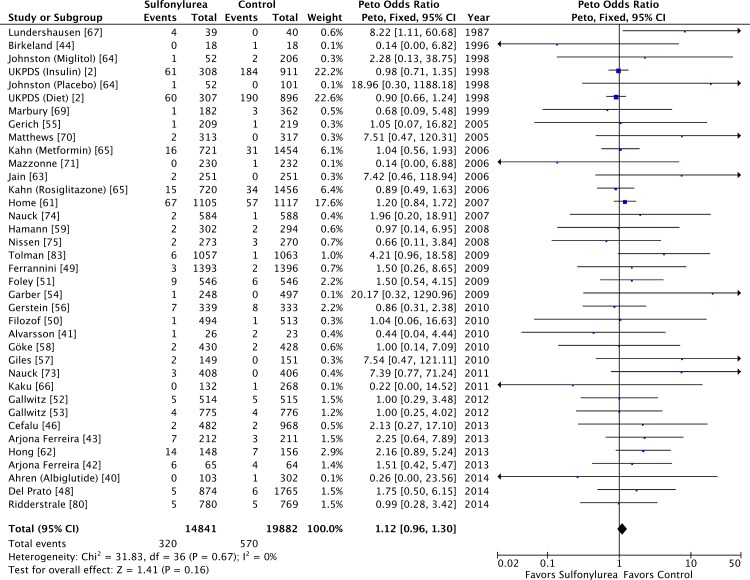
Our meta-analysis did not show a significant association between use of sulfonylureas and all-cause (OR 1.12 [95% CI 0.96 to 1.30]) (Fig 2) or cardiovascular mortality (OR 1.12 [95% CI 0.87 to 1.42]) (S3 Fig). Both analyses have low heterogeneity (all-cause mortality: I 2 = 0% [95% CI 0% to 17%], p for heterogeneity = 0.67; cardiovascular mortality: I 2 = 12% [95% CI 0% to 20%], p for heterogeneity = 0.30). The τ2 results were similar to the I 2 results. The inclusion of double-zero studies with empirical continuity correction analysis did not affect the results (OR 1.11 [95% CI 0.96 to 1.29] and OR 1.12 [95% CI 0.87 to 1.42] for all-cause and cardiovascular mortality, respectively). The sensitivity analyses with Mantel–Haenszel ORs also did not change the results.
Fig 2. Forest plot for all-cause mortality.
For studies with multiple treatment groups, the group being compared is presented in parentheses.
We intended to evaluate the long-term safety of sulfonylureas, so to address whether longer studies might show different results, we further restricted the analysis to studies with follow-up of at least 2 y. The results were similar for all-cause (OR 1.05 [95% CI 0.89 to 1.24]) and cardiovascular mortality (OR 1.07 [95% CI 0.83 to 1.39]). We identified small study bias for all-cause mortality. Despite this, the results were unaffected by the trim-and-fill computation: in reality, the point estimation after the computation of theoretical unpublished studies for all-cause mortality was smaller (OR 1.08 [95% CI 0.93 to 1.25]). There was no small study bias for cardiovascular mortality.
Sulfonylureas and Myocardial Infarction and Stroke
A smaller number of trials reported myocardial infarction and stroke data (23 studies each, comprising 26,521 and 26,175 patients for myocardial infarction and stroke, respectively). We found no significant difference for myocardial infarction in patients treated with sulfonylureas (OR 0.92 [95% CI 0.76 to 1.12]). Including double-zero studies with empirical continuity correction left the results unaffected (OR 0.92 [95% CI 0.76 to 1.12]). In addition, no significant association was observed between sulfonylureas and stroke (OR 1.16 [95% CI 0.81 to 1.66]), and the inclusion of double-zero studies with empirical continuity correction did not change these results (OR 1.16 [95% CI 0.89 to 1.63]). The sensitivity analyses with Mantel–Haenszel ORs also did not change the results for the myocardial infarction and stroke analyses. Small study bias was present for myocardial infarction, but the results were similar with the trim-and-fill computation (OR 0.90 [95% CI 0.74 to 1.09]). No small study bias was identified for stroke events.
All-Cause and Cardiovascular Mortality with Different Types of Comparators and According to Line of Treatment
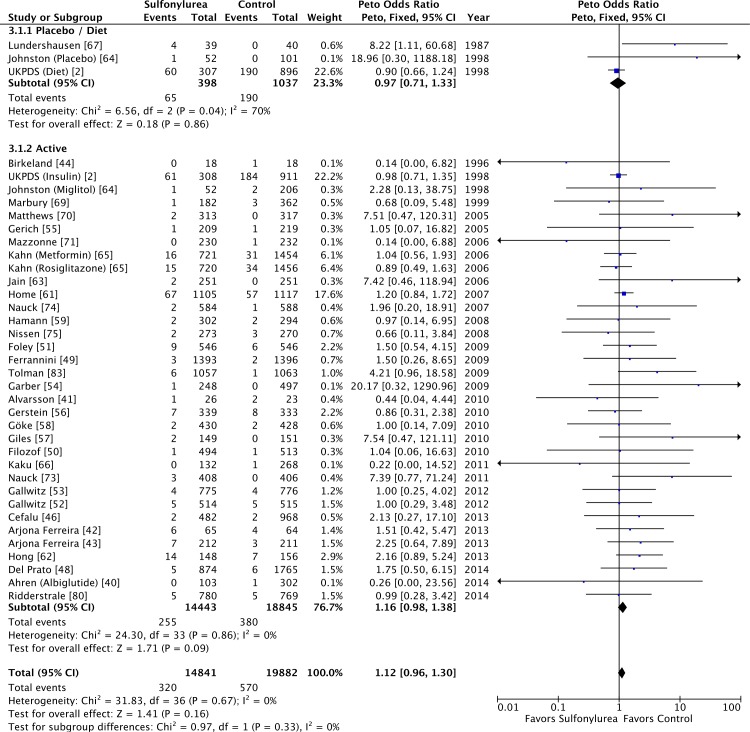
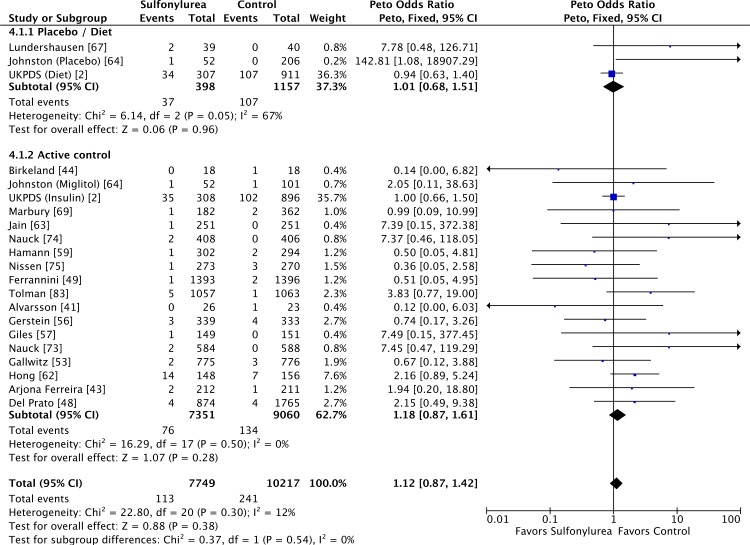
Sulfonylureas were not associated with significant all-cause mortality when compared to placebo or diet (OR 0.97 [95% CI 0.71 to 1.33]; I 2 = 70% [95% CI 43% to 84%], p for heterogeneity = 0.04) or to active comparators (OR 1.16 [95% CI 0.98 to 1.38]; I 2 = 0% [95% CI 0% to 18%], p for heterogeneity = 0.86) (Fig 3). The results for cardiovascular mortality were similar: placebo/diet OR 1.01 (95% CI 0.68 to 1.51; I 2 = 67% [95% CI 39% to 83%], p for heterogeneity = 0.05) and active comparator OR 1.18 (95% CI 0.87 to 1.61; I 2 = 0% [95% CI 0% to 21%], p for heterogeneity = 0.50) (Fig 4). We found also no significant difference in all-cause mortality across all comparator classes individually (S4 Fig).
Fig 3. Forest plots for all-cause mortality of sulfonylureas according to comparator (placebo/diet or active comparators).
For studies with multiple treatment groups, the group being compared is presented in parentheses.
Fig 4. Forest plots for cardiovascular mortality of sulfonylureas according to comparator (placebo/diet or active comparators).
For studies with multiple treatment groups, the group being compared is presented in parentheses.
When stratifying the analysis according to line of treatment, there was no difference in all-cause mortality between treatments irrespective of whether sulfonylureas were used as first-line treatment (OR 1.03 [95% CI 0.86 to 1.24]; I 2 = 0% [95% CI 0% to 31%], p for heterogeneity = 0.50), second-line treatment (OR 1.31 [95% CI 0.98 to 1.74]; I 2 = 0% [95% CI 0% to 30%], p for heterogeneity = 0.88) or treatment line unspecified (OR 1.30 [95% CI 0.63 to 2.67]; I 2 = 38% [95% CI 0% to 62%], p for heterogeneity = 0.17). For cardiovascular mortality, the results were also not affected: first-line treatment OR 1.06 (95% CI 0.81 to 1.39; I 2 = 14% [95% CI 0% to 40%], p for heterogeneity = 0.31), second-line treatment OR 1.42 (95% CI 0.71 to 2.85; I 2 = 2% [95% CI 0% to 51%], p for heterogeneity = 0.41), and treatment line unspecified OR 1.49 (95% CI 0.43 to 5.17; I 2 = 70% [95% CI 36% to 86%], p for heterogeneity = 0.07).
Sulfonylureas as Add-On to Metformin and All-Cause and Cardiovascular Mortality
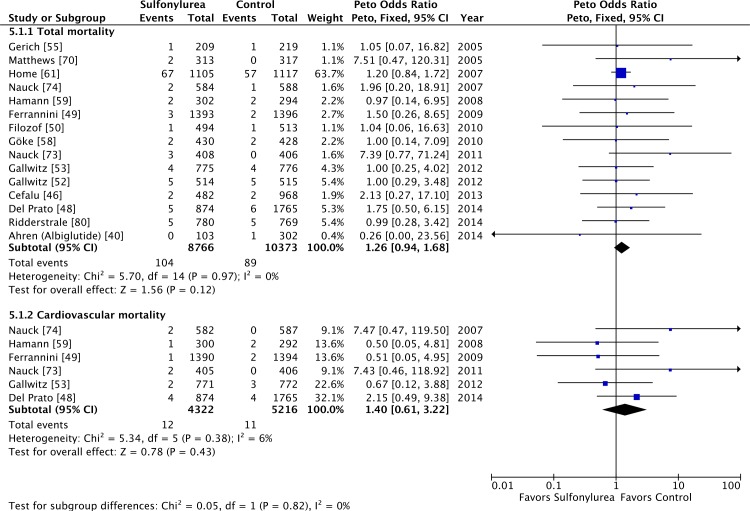
Sulfonylureas as an add-on to metformin were considered safe in terms of overall and cardiovascular mortality (Fig 5), with little heterogeneity: OR 1.26 (95% CI 0.94 to 1.68; I 2 = 0% [95% CI 0% to 31%], p for heterogeneity = 0.97) for all-cause mortality and OR 1.40 (95% CI 0.61 to 3.22; I 2 = 6% [95% CI 0% to 52%], p for heterogeneity = 0.38) for cardiovascular mortality. Including double-zero studies with empirical continuity correction in the analysis did not change these results. All studies in these analyses had active comparators against sulfonylureas.
Fig 5. Forest plots for all-cause and cardiovascular mortality of sulfonylureas as an add-on to metformin.
For studies with multiple treatment groups, the group being compared is presented in parentheses.
Individual Sulfonylurea Agents and Mortality
As an exploratory evaluation, all-cause mortality analysis for each individual sulfonylurea is shown in S5 Fig. Results are similar for cardiovascular mortality. In both analyses, heterogeneity was small. Glipizide was associated with increased all-cause (OR 1.68 [95% CI 1.06 to 2.66]) and cardiovascular mortality (OR 2.1 [95% CI 1.09 to 3.72]), but these analyses are based on a small number of patients and studies.
A sensitivity analysis excluding glipizide trials from the main analyses was performed. We observed a reduction in ORs for all-cause (OR 1.03 [95% CI 0.86 to 1.23]) and cardiovascular mortality (OR 1.00 [95% CI 0.77 to 1.30]). Of note, the futility boundary was still reached in this situation.
Trial Sequential Analysis
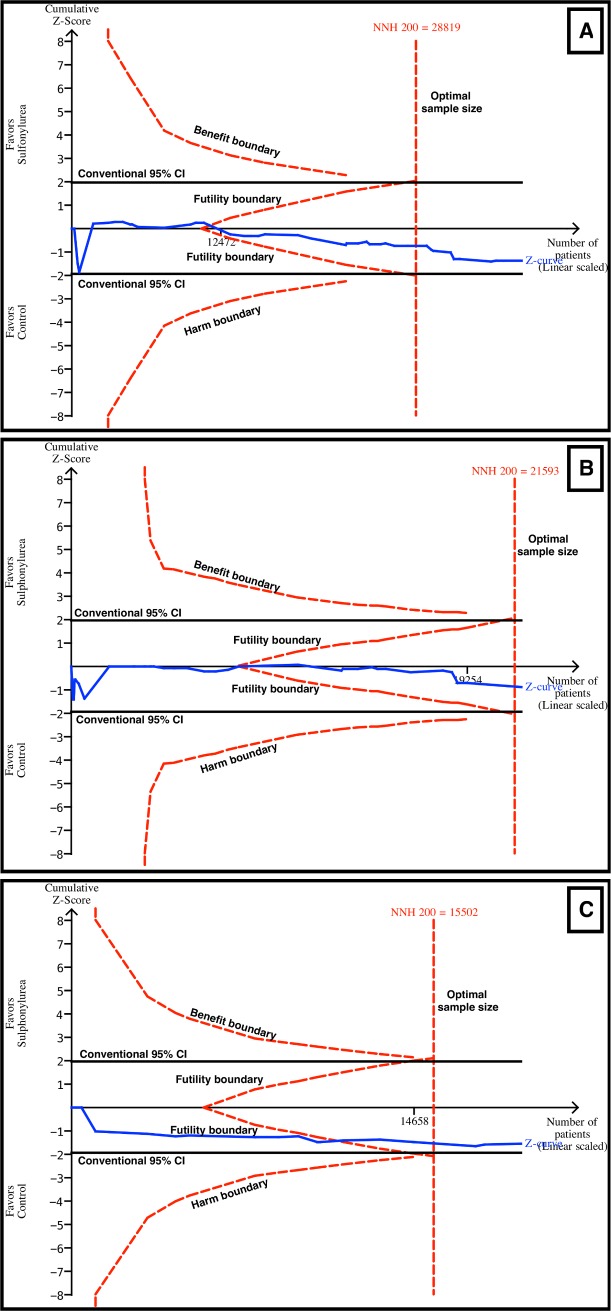
TSA evaluates whether there is enough information to reach firm conclusions, and this analysis was performed for the main outcomes in this review. For all-cause and cardiovascular mortality, TSA showed that a NNH of 200 could be discarded, as the number of patients evaluated for all-cause (n = 37,650) and cardiovascular mortality (n = 21,893) surpassed the optimal sample sizes (n = 29,819 for all-cause mortality and n = 21,593 for cardiovascular mortality) (Fig 6A and 6B). The combination of sulfonylureas and metformin was evaluated with TSA as well. The Z curve surpassed the optimal sample size boundary, and a NNH of 200 could be discarded for all-cause mortality (Fig 6C) but not for cardiovascular mortality. Similarly, for myocardial infarction and stroke, the futility boundaries were reached.
Fig 6. TSA for all-cause and cardiovascular mortality.
TSA discarded harm with sulfonylurea use with an α of 5%, a β of 80%, and an absolute difference of 0.5% between the groups (sulfonylurea and comparator). The blue line represents the Z curve (cumulative effect size), the red dashed lines represent the harm, benefit, and futility boundaries and the estimated optimal sample size adjusted to sample size and repeated analysis, and the black lines represent the conventional confidence intervals. The black number and marking in the x-axis represent the number of patients accrued until that point. (A) Sulfonylureas overall for all-cause mortality. Futility and optimal sample size boundaries were crossed. (B) Sulfonylureas overall for cardiovascular mortality. Futility and optimal sample size boundaries were crossed. (C) Sulfonylureas as add-on to metformin for all-cause mortality. Futility and optimal sample size boundaries were crossed.
Meta-Analysis Quality Evaluation and Summary of Findings
The GRADE quality of evidence for all-cause and cardiovascular mortality was high. The identified small study bias does not appear to have skewed the results of the meta-analysis. Financial support from the pharmaceutical industry is a conservative bias, as it might have increased the chance of benefit for the comparator drug [85].
We graded the myocardial infarction and stroke meta-analysis as being of moderate quality, because these outcomes are at greater risk of being skewed due to underreporting and misdiagnosis.
Discussion
The data presented here suggest that the most frequently used sulfonylureas (second and third generation) are not associated with increased all-cause and cardiovascular mortality in patients with type 2 diabetes. By using TSA, we were able to discard harm at a rate of 1 in every 200 treated patients (i.e., 0.5% of absolute risk) for mortality (all-cause and cardiovascular) and major events (myocardial infarction and stroke). We defined this rate as the minimal clinically significant difference based on a previous study [86]. Furthermore, this finding did not change when sulfonylureas were compared with almost every drug class currently available for the treatment of type 2 diabetes or when sulfonylureas were used as an add-on to metformin.
Other systematic reviews have also evaluated this topic [18–22]. Although some of these studies identified an increased risk of occurrence of mortality or cardiovascular events with sulfonylurea use [19,20,22], others did not find an increased risk [18,21]. These contradictory results may be explained by the inclusion, or not, of first-generation sulfonylureas [19,20], observational studies [21,22], and short-term studies [18–21]. Furthermore, most systematic reviews did not evaluate whether the data presented had enough power to support the conclusions [18,20,21]. We included only RCTs evaluating second- or third-generation sulfonylureas, as monotherapy or in combination. We chose to include only these sulfonylureas, because they are more frequently used than first-generation sulfonylureas [8], alone or in combination with metformin [10].
The current position of regulatory agencies for new drug approval for type 2 diabetes is based on a “safety” approach, with a request that non-inferiority trials be performed to show that a new antihyperglycemic drug has no increased cardiovascular risk compared to placebo [87]. Most recent published large trials have such “no harm” results [11–13]. Although our study might be seen as a non-inferiority safety trial, most of the included studies compared sulfonylureas with active comparators. Therefore, another interpretation of our data is that sulfonylureas are not different from alternative therapies currently available in terms of mortality and cardiovascular outcomes. Furthermore, the US Food and Drug Administration (FDA) states that, for a new drug to treat hyperglycemia to be considered save, the upper limit of the confidence interval of the OR for cardiovascular events must be below 1.30 [87], and the TSA discarded a risk smaller than that. In other words, our study shows that current knowledge can discard a risk small enough to settle the concerns on the safety of sulfonylureas—at least according to the FDA requirements [87].
A particular aspect of our meta-analysis was the use of TSA. This analysis explores the possibility of a false negative result and evaluates the statistical reliability of the present data. To perform this analysis, it is necessary to establish a minimal clinically significant difference in the outcomes between the groups. We chose an absolute difference of 0.5%, which means a NNH of 200. This is half of the risk reported in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study [86], where an absolute difference of 1% (a NNH of 100) in mortality was found for intensive glucose lowering. We believe using this minimal clinically significant difference for mortality and cardiovascular outcomes is clinically meaningful and provides useful information. This approach allowed us to exclude a risk as small as one death in every 200 treated patients for the evaluated outcomes. Ideally, it would be desirable to be able to exclude a smaller risk, for example a NNH of 500. However, this approach would require a sample of almost 195,000 patients to be randomized. Such a number of individuals will probably never be enrolled, as it is more than five times the number of patients enrolled in sulfonylurea trials in the last 30 years.
Some limitations of the present study must be acknowledged. Unfortunately, we were not able to include all the identified studies in the meta-analyses because the mortality outcomes were not available, even after trying to contact the authors. However, these excluded studies represented only 10% of the study population. It seems unlikely that these data would change the results, as optimal sample size was reached for most analyses. We also could not include three studies because of language restrictions. Some of our analyses were explorative ones (individual sulfonylureas, individual comparators), and the results should be interpreted and used in clinical practice with caution. To assess whether eligible studies were published in the last year, we updated the review of databases (MEDLINE, Embase, and Cochrane Library) with the original strategy up to 9 February 2016. We identified one new study that would fulfill the inclusion criteria of this review [88]. This study included 720 patients randomized to glimepiride or saxagliptin. There was only one death in each group; hence, these additional data did not change our result. Finally, most studies were not designed for assessing long-term safety endpoints, but all of them had a duration of 52 wk or more, which partially controls for this limitation. Although 52 wk may be a short time frame to identify mortality and cardiovascular outcomes, restricting the analysis to longer studies (at least 2 y) did not change the results. Finally, as most of the ORs in our results had a lower limit lower than 1 but close to it, different analysis methods may lead to different results. However, we decreased the uncertainty by performing sensitivity analyses and also explored the consistency of the results by using TSA.
Our study findings are reassuring, as we could discard there being a significantly increased risk with the use of a frequently prescribed antihyperglycemic medication. The sensitivity analyses disclosed that glipizide was associated with an increased risk of mortality; however, it must be stressed that our study was not designed to compare different sulfonylureas, and this result is only exploratory and was based on only a few studies, with a small number of events. A recent network meta-analysis—the preferable approach for comparing drugs that have not been directly compared—showed that second- and third-generation sulfonylureas had similar all-cause and cardiovascular mortality risks [89]. Therefore, this finding regarding glipizide must be further evaluated in future studies. Whether second- and third-generation sulfonylureas should be considered as a class or as individual agents has yet to be determined.
Another important unresolved question is which drug should be added to patients who are failing metformin monotherapy. To date, no antihyperglycemic agent used in association with metformin has reduced mortality or cardiovascular events. Even the recent published trials of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes and high cardiovascular risk did not show a reduction in cardiovascular events [11–13], but there was a concern regarding heart failure incidence in two of the trials [12,90]. Our data show that second- and third-generation sulfonylureas are a safe option, but we hope that newer drugs will do better than that and will be able to decrease cardiovascular events and mortality risks compared to sulfonylureas. Although the EMPA-REG study did not directly explore this issue, the results suggest that empagliflozin might be such a drug, as it was able to reduce the risk of cardiovascular events and mortality (all cause and cardiovascular) [91]. To clarify the question of which should be the preferred drug for patients failing metformin, the Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes (CAROLINA) and the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) results are awaited [92,93], as they will further evaluate sulfonylureas against newer drug classes in the long term.
In conclusion, the present study suggests that the use of second- and third-generation sulfonylureas in patients with type 2 diabetes is not associated with increased cardiovascular risk and all-cause mortality, irrespective of comparator or background medication. Sulfonylureas should therefore still be used; however, it is important to weigh their efficacy in controlling hyperglycemia and low cost against the risks of hypoglycemia and weight gain.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
(DOCX)
(PDF)
(DOC)
Abbreviations
- NNH
number needed to harm
- OR
odds ratio
- RCT
randomized clinical trial
- TSA
trial sequential analysis
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). CNPq had no role in the design and conduct of the study; the extraction, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
References
- 1. Hirst JA, Farmer AJ, Dyar A, Lung TW, Stevens RJ (2013) Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta-analysis. Diabetologia 56: 973–984. 10.1007/s00125-013-2856-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853. [PubMed] [Google Scholar]
- 3. Klarenbach S, Cameron C, Singh S, Ur E (2011) Cost-effectiveness of second-line antihyperglycemic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. CMAJ 183: E1213–E1220. 10.1503/cmaj.110178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meinert CL, Knatterud GL, Prout TE, Klimt CR (1970) A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes 19 (Suppl): 789–830. [PubMed] [Google Scholar]
- 5. Genuth S (2015) Should sulfonylureas remain an acceptable first-line add-on to metformin therapy in patients with type 2 diabetes? No, it’s time to move on! Diabetes Care 38: 170–175. 10.2337/dc14-0565 [DOI] [PubMed] [Google Scholar]
- 6. Abrahamson MJ (2015) Should sulfonylureas remain an acceptable first-line add-on to metformin therapy in patients with type 2 diabetes? Yes, they continue to serve us well! Diabetes Care 38: 166–169. 10.2337/dc14-1945 [DOI] [PubMed] [Google Scholar]
- 7. Nissen SE (2012) Cardiovascular effects of diabetes drugs: emerging from the dark ages. Ann Intern Med 157: 671–672. 10.7326/0003-4819-157-9-201211060-00016 [DOI] [PubMed] [Google Scholar]
- 8. Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, et al. (2009) Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK General Practice Research Database. BMJ 339: b4731 10.1136/bmj.b4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kontopantelis E, Springate DA, Reeves D, Ashcroft DM, Rutter M, et al. (2015) Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study. Diabetologia 58: 505–518. 10.1007/s00125-014-3473-8 [DOI] [PubMed] [Google Scholar]
- 10. Turner LW, Nartey D, Stafford RS, Singh S, Alexander GC (2014) Ambulatory treatment of type 2 diabetes in the U.S., 1997–2012. Diabetes Care 37: 985–992. 10.2337/dc13-2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, et al. (2015) Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 373: 232–242. 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- 12. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, et al. (2013) Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 369: 1317–1326. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 13. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, et al. (2013) Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 369: 1327–1335. 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- 14. Roumie CL, Greevy RA, Grijalva CG, Hung AM, Liu X, et al. (2014) Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all-cause mortality among patients with diabetes. JAMA 311: 2288–2296. 10.1001/jama.2014.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, et al. (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 32: 1900–1908. 10.1093/eurheartj/ehr077 [DOI] [PubMed] [Google Scholar]
- 16. Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, et al. (2012) Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 157: 601–610. 10.7326/0003-4819-157-9-201211060-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sedgwick P (2013) Prospective cohort studies: advantages and disadvantages. BMJ: 347: f6726. [Google Scholar]
- 18. Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM (2007) A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 30: 389–394. [DOI] [PubMed] [Google Scholar]
- 19. Hemmingsen B, Schroll JB, Lund SS, Wetterslev J, Gluud C, et al. (2013) Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. Cochrane Database Syst Rev 4: CD009008 10.1002/14651858.CD009008.pub2 [DOI] [PubMed] [Google Scholar]
- 20. Monami M, Genovese S, Mannucci E (2013) Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 15: 938–953. 10.1111/dom.12116 [DOI] [PubMed] [Google Scholar]
- 21. Phung OJ, Schwartzman E, Allen RW, Engel SS, Rajpathak SN (2013) Sulphonylureas and risk of cardiovascular disease: systematic review and meta-analysis. Diabet Med 30: 1160–1171. 10.1111/dme.12232 [DOI] [PubMed] [Google Scholar]
- 22. Rao AD, Kuhadiya N, Reynolds K, Fonseca VA (2008) Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: a meta-analysis of observational studies. Diabetes Care 31: 1672–1678. 10.2337/dc08-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bolland MJ, Grey A, Gamble GD, Reid IR (2014) The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol 2: 364–365. [DOI] [PubMed] [Google Scholar]
- 24. Wetterslev J, Thorlund K, Brok J, Gluud C (2008) Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 61: 64–75. [DOI] [PubMed] [Google Scholar]
- 25. Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, et al. (2011) User manual for Trial Sequential Analysis (TSA). Copenhagen: Copenhagen Trial Unit, Centre for Clinical Intervention Research. 115 p. [Google Scholar]
- 26. Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions London: Cochrane Collaboration. [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bu S, Xing XY, Wang N, Zhao WH, Yang WY (2004) [Evaluation of the efficacy and safety of once daily injection of glargine combined with glipizide GITS in the treatment of type 2 diabetes mellitus]. Chin J Evid Based Med 4: 464–467. [Google Scholar]
- 29. Li MK, Liu Q, Yu ZZ, Cui SM, Shi P, et al. (1999) [Observation of the effect of combination of glipizide and insulin for type2 diabetic patients with secondary failure to oral sulfonylureas]. J Clin Int Med 15: 38. [Google Scholar]
- 30. Onuchin SG, Elsukova OS, Solov’ev OV, Onuchina EL (2010) [Capabilities of hypoglycemic therapy in women with decompensated type 2 diabetes mellitus]. Ter Arkh 82: 34–41. [PubMed] [Google Scholar]
- 31. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, et al. (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guyatt GH, Oxman AD, Kunz R, Jaeschke R, Helfand M, et al. (2008) Incorporating considerations of resources use into grading recommendations. BMJ 336: 1170–1173. 10.1136/bmj.39504.506319.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phung OJ, Scholle JM, Talwar M, Coleman CI (2010) Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 303: 1410–1418. 10.1001/jama.2010.405 [DOI] [PubMed] [Google Scholar]
- 34. Gross JL, Kramer CK, Leitao CB, Hawkins N, Viana LV, et al. (2011) Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med 154: 672–679. 10.7326/0003-4819-154-10-201105170-00007 [DOI] [PubMed] [Google Scholar]
- 35. Diamond GA, Bax L, Kaul S (2007) Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med 147: 578–581. [DOI] [PubMed] [Google Scholar]
- 36. Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 26: 53–77. [DOI] [PubMed] [Google Scholar]
- 37. Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23: 1351–1375. [DOI] [PubMed] [Google Scholar]
- 38. Rucker G, Schwarzer G, Carpenter JR, Schumacher M (2008) Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 8: 79 10.1186/1471-2288-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abbatecola AM, Rizzo MR, Barbieri M, Grella R, Arciello A, et al. (2006) Postprandial plasma glucose excursions and cognitive functioning in aged type 2 diabetics. Neurology 67: 235–240. [DOI] [PubMed] [Google Scholar]
- 40. Ahren B, Johnson SL, Stewart M, Cirkel DT, Yang F, et al. (2014) HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 37: 2141–2148. 10.2337/dc14-0024 [DOI] [PubMed] [Google Scholar]
- 41. Alvarsson M, Berntorp K, Fernqvist-Forbes E, Lager I, Steen L, et al. (2010) Effects of insulin versus sulphonylurea on beta-cell secretion in recently diagnosed type 2 diabetes patients: a 6-year follow-up study. Rev Diabet Stud 7: 225–232. 10.1900/RDS.2010.7.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arjona Ferreira JC, Corry D, Mogensen CE, Sloan L, Xu L, et al. (2013) Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis 61: 579–587. 10.1053/j.ajkd.2012.11.043 [DOI] [PubMed] [Google Scholar]
- 43. Arjona Ferreira JC, Marre M, Barzilai N, Guo H, Golm GT, et al. (2013) Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care 36: 1067–1073. 10.2337/dc12-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Birkeland KI, Rishaug U, Hanssen KF, Vaaler S (1996) NIDDM: a rapid progressive disease. Results from a long-term, randomised, comparative study of insulin or sulphonylurea treatment. Diabetologia 39: 1629–1633. [DOI] [PubMed] [Google Scholar]
- 45. Campbell IW, Menzis DG, Chalmers J, McBain AM, Brown IRF (1994) One year comparative trial of metformin and glipizide in type 2 diabetes mellitus. Diabete Metab 20: 394–400. [PubMed] [Google Scholar]
- 46. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, et al. (2013) Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382: 941–950. 10.1016/S0140-6736(13)60683-2 [DOI] [PubMed] [Google Scholar]
- 47. Clauson P, Karlander S, Steen L, Efendic S (1996) Daytime glibenclamide and bedtime NPH insulin compared to intensive insulin treatment in secondary sulphonylurea failure: a 1-year follow-up. Diabet Med 13: 471–477. [DOI] [PubMed] [Google Scholar]
- 48. Del Prato S, Camisasca R, Wilson C, Fleck P (2014) Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year study. Diabetes Obes Metab 16: 1239–1246. 10.1111/dom.12377 [DOI] [PubMed] [Google Scholar]
- 49. Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahrén B, et al. (2009) Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab 11: 157–166. 10.1111/j.1463-1326.2008.00994.x [DOI] [PubMed] [Google Scholar]
- 50. Filozof C, Gautier JF (2010) A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study. Diabet Med 27: 318–326. 10.1111/j.1464-5491.2010.02938.x [DOI] [PubMed] [Google Scholar]
- 51. Foley JE, Sreenan S (2009) Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naive patients with type 2 diabetes. Horm Metab Res 41: 905–909. 10.1055/s-0029-1234042 [DOI] [PubMed] [Google Scholar]
- 52. Gallwitz B, Guzman J, Dotta F, Guerci B, Simó R, et al. (2012) Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 379: 2270–2278. 10.1016/S0140-6736(12)60479-6 [DOI] [PubMed] [Google Scholar]
- 53. Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, et al. (2012) 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 380: 475–483. 10.1016/S0140-6736(12)60691-6 [DOI] [PubMed] [Google Scholar]
- 54. Garber A, Henry RR, Ratner R, Hale P, Chang CT, et al. (2011) Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabet Obes Metab 13: 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gerich J, Raskin P, Jean-Louis L, Purkayastha D, Baron MA (2005) PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care 28: 2093–2099. [DOI] [PubMed] [Google Scholar]
- 56. Gerstein HC, Ratner RE, Cannon CP, Serruys PW, García-García HM, et al. (2010) Effect of rosiglitazone on progression of coronary atherosclerosis in patients with type 2 diabetes mellitus and coronary artery disease: the assessment on the prevention of progression by rosiglitazone on atherosclerosis in diabetes patients with cardiovascular history trial. Circulation 121: 1176–1187. 10.1161/CIRCULATIONAHA.109.881003 [DOI] [PubMed] [Google Scholar]
- 57. Giles TD, Elkayam U, Bhattacharya M, Perez A, Miller AB (2010) Comparison of pioglitazone vs glyburide in early heart failure: insights from a randomized controlled study of patients with type 2 diabetes and mild cardiac disease. Congest Heart Fail 16: 111–117. 10.1111/j.1751-7133.2010.00154.x [DOI] [PubMed] [Google Scholar]
- 58. Göke B, Gallwitz B, Eriksson JG, Hellqvist A, Gause-Nilsson I (2013) Saxagliptin vs. glipizide as add-on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long-term (52-week) extension of a 52-week randomised controlled trial. Int J Clin Pract 67: 307–316. [DOI] [PubMed] [Google Scholar]
- 59. Hamann A, Garcia-Puig J, Paul G, Donaldson J, Stewart M (2008) Comparison of fixed-dose rosiglitazone/metformin combination therapy with sulphonylurea plus metformin in overweight individuals with type 2 diabetes inadequately controlled on metformin alone. Exp Clin Endocrinol Diabetes 116: 6–13. [DOI] [PubMed] [Google Scholar]
- 60. Hanefeld M, Patwardhan R, Jones NP (2007) A one-year study comparing the efficacy and safety of rosiglitazone and glibenclamide in the treatment of type 2 diabetes. Nutr Metab Cardiovasc Dis 17: 13–23. [DOI] [PubMed] [Google Scholar]
- 61. Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, et al. (2007) Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med 357: 28–38. [DOI] [PubMed] [Google Scholar]
- 62. Hong J, Zhang Y, Lai S, Lv A, Su Q, et al. (2013) Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 36: 1304–1311. 10.2337/dc12-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jain R, Osei K, Kupfer S, Perez AT, Zhang J, et al. (2006) Long-term safety of pioglitazone versus glyburide in patients with recently diagnosed type 2 diabetes mellitus. Pharmacotherapy 26: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 64. Johnston PS, Lebovitz HE, Coniff RF, Simonson DC, Raskin P, et al. (1998) Advantages of alpha-glucosidase inhibition as monotherapy in elderly type 2 diabetic patients. J Clin Endocrinol Metab 83: 1515–1522. [DOI] [PubMed] [Google Scholar]
- 65. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, et al. (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355: 2427–2443. [DOI] [PubMed] [Google Scholar]
- 66. Kaku K, Rasmussen MF, Nishida T, Seino Y (2011) Fifty-two-week, randomized, multicenter trial to compare the safety and efficacy of the novel glucagon-like peptide-1 analog liraglutide vs glibenclamide in patients with type 2 diabetes. J Diabetes Investig 2: 441–447. 10.1111/j.2040-1124.2011.00128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lundershausen R, Orban S, Pissarek D, Panzram G (1987) [Long-term effect of combination glibenclamide-insulin treatment in the secondary failure of sulfonylurea therapy—results of a one-year double blind study]. Wien Klin Wochenschr 99: 603–608. [PubMed] [Google Scholar]
- 68. Madsbad S, Kilhovd B, Lager I, Mustajoki P, Dejgaard A (2001) Comparison between repaglinide and glipizide in type 2 diabetes mellitus: a 1-year multicentre study. Diabet Med 18: 395–401. [DOI] [PubMed] [Google Scholar]
- 69. Marbury T, Huang WC, Strange P, Lebovitz H (1999) Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract 43: 155–166. [DOI] [PubMed] [Google Scholar]
- 70. Matthews DR, Dejager S, Ahren B, Fonseca V, Ferrannini E, et al. (2010) Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab 12: 780–789. 10.1111/j.1463-1326.2010.01233.x [DOI] [PubMed] [Google Scholar]
- 71. Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, et al. (2006) Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 296: 2572–2581. [DOI] [PubMed] [Google Scholar]
- 72. Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Koide H (2006) Effect of pioglitazone on urinary liver-type fatty acid-binding protein concentrations in diabetes patients with microalbuminuria. Diabetes Metab Res Rev 22: 385–389. [DOI] [PubMed] [Google Scholar]
- 73. Nauck MA, Del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, et al. (2011) Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 34: 2015–2022. 10.2337/dc11-0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP (2007) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 9: 194–205. [DOI] [PubMed] [Google Scholar]
- 75. Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, et al. (2008) Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299: 1561–1573. 10.1001/jama.299.13.1561 [DOI] [PubMed] [Google Scholar]
- 76. Perriello G, Pampanelli S, Brunetti P, di Pietro C, Mariz S (2007) Long-term effects of pioglitazone versus gliclazide on hepatic and humoral coagulation factors in patients with type 2 diabetes. Diab Vasc Dis Res 4: 226–230. [DOI] [PubMed] [Google Scholar]
- 77. Petrica L, Petrica M, Vlad A, Dragos Jianu C, Gluhovschi G, et al. (2009) Nephro- and neuroprotective effects of rosiglitazone versus glimepiride in normoalbuminuric patients with type 2 diabetes mellitus: a randomized controlled trial. Wien Klin Wochenschr 121: 765–775. 10.1007/s00508-009-1279-3 [DOI] [PubMed] [Google Scholar]
- 78. Petrica L, Vlad A, Petrica M, Jianu CD, Gluhovschi G, et al. (2011) Pioglitazone delays proximal tubule dysfunction and improves cerebral vessel endothelial dysfunction in normoalbuminuric people with type 2 diabetes mellitus. Diabetes Res Clin Pract 94: 22–32. 10.1016/j.diabres.2011.05.032 [DOI] [PubMed] [Google Scholar]
- 79. Quatraro A, Consoli G, Ceriello A, Giugliano D (1986) Combined insulin and sulfonylurea therapy in non-insulin-dependent diabetics with secondary failure to oral drugs: a one year follow-up. Diabete Metab 12: 315–318. [PubMed] [Google Scholar]
- 80. Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, et al. (2014) Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2: 691–700. 10.1016/S2213-8587(14)70120-2 [DOI] [PubMed] [Google Scholar]
- 81. Ristic S, Collober-Maugeais C, Cressier F, Tang P, Pecher E (2007) Nateglinide or gliclazide in combination with metformin for treatment of patients with type 2 diabetes mellitus inadequately controlled on maximum doses of metformin alone: 1-year trial results. Diabetes Obes Metab 9: 506–511. [DOI] [PubMed] [Google Scholar]
- 82. Rosenstock J, Wilson C, Fleck P (2013) Alogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: a prospective, double-blind, randomized, 1-year study. Diabetes Obes Metab 15: 906–914. 10.1111/dom.12102 [DOI] [PubMed] [Google Scholar]
- 83. Tolman KG, Freston JW, Kupfer S, Perez A (2009) Liver safety in patients with type 2 diabetes treated with pioglitazone: results from a 3-year, randomized, comparator-controlled study in the US. Drug Saf 32: 787–800. [DOI] [PubMed] [Google Scholar]
- 84. Vahatalo M, Ronnemaa T, Viikari J (2007) Recognition of fasting or overall hyperglycaemia when starting insulin treatment in patients with type 2 diabetes in general practice. Scand J Prim Health Care 25: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, et al. (2004) Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ 170: 477–480. [PMC free article] [PubMed] [Google Scholar]
- 86. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. US Food and Drug Administration (2008) Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes Silver Spring (Maryland): US Food and Drug Administration. [Google Scholar]
- 88. Schernthaner G, Duran-Garcia S, Hanefeld M, Langslet G, Niskanen L, et al. (2015) Efficacy and tolerability of saxagliptin compared with glimepiride in elderly patients with type 2 diabetes: a randomized, controlled study (GENERATION). Diabetes Obes Metab 17: 630–638. 10.1111/dom.12461 [DOI] [PubMed] [Google Scholar]
- 89. Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS, et al. (2015) Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol 3: 43–51. 10.1016/S2213-8587(14)70213-X [DOI] [PubMed] [Google Scholar]
- 90. Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 385: 2067–2076. 10.1016/S0140-6736(14)62225-X [DOI] [PubMed] [Google Scholar]
- 91. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2127–2128. [DOI] [PubMed] [Google Scholar]
- 92. Rosenstock J, Marx N, Kahn SE, Zinman B, Kastelein JJ, et al. (2013) Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 10: 289–301. 10.1177/1479164112475102 [DOI] [PubMed] [Google Scholar]
- 93. Nathan DM, Buse JB, Kahn SE, Krause-Steinrauf H, Larkin ME, et al. (2013) Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 36: 2254–2261. 10.2337/dc13-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
(DOCX)
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.